


Trans. Nonferrous Met. Soc. China 22(2012) 1127-1132
Preparation and gas sensing properties for acetone of amorphous Ag modified NiFe2O4 sensor
JIAO Wan-li, ZHANG Lei
School of Materials Science and Engineering, Shandong University of Technology, Zibo 255049, China
Received 23 May 2011; accepted 11 January 2012
Abstract: Nickel ferrite nano-powders were prepared by microwave radiating low-temperature solid-state reaction method, and then modified with Ag by dipping method. The crystal structure and morphology of the samples were characterized by means of X-ray diffraction(XRD), scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS). The gas sensing properties of the samples were also investigated. The results reveal that the Ag, as amorphous structure, can efficiently prevent the reuniting and growing-up of nanosized NiFe2O4 grains, and 1.5% Ag modified NiFe2O4 sensor has a better sensitivity, up to 43, for acetone gas than 1.5%Ag mixed NiFe2O4 sensor prepared by low-temperature solid-state reaction, at an optimal working voltage of 4.5 V. The quick response time (1 s) and fast recovery time (~10 s) are the main characteristics of this sensor.
Key words: nickel ferrite; low temperature solid state reaction; dipping method; silver; acetone
1 Introduction
Semiconducting metal oxides such as zinc oxide, tin oxide and tungsten oxide have been widely studied for their gas sensing applications; however, presently many other oxides have also been explored for the gas sensing devices. Recently, few reports have appeared on the ferrites as gas sensors. Different spinel ferrites, such as NiFe2O4, CdFe2O4, ZnFe2O4 and CuFe2O4, have been studied for various gas-sensing applications [1-6]. Many investigators consider that noble metal, such as Ag and Pd, doped into semiconductor, can influence the compounding of electron-cavity [7], and enhance the gas adsorption and redox, thereby the gas sensing performance should be improved [8,9]. DARSHANE et al [10,11] discovered that Pd-doped NiFe2O4 and MgFe2O4 exhibited the highest sensitivity towards liquefied petroleum gas (LPG) and the optimal working current was 125 mA which was lower than that of general SnO2 gas sensor for LPG gas. FAN et al [12,13] discovered that Ag-doped ZnO nanowires exhibited the highest sensitivity towards alcohol gas. A number of wet-chemical methods [14-16] have been used to synthesize nanocrystalline nickel ferrite. However, these cases are general complicated, expensive and far from being environmentally friendly. A novel preparation technique of nanomaterials, convenient and environment friendly solid state reaction synthesis at ambient conditions, has been developed to prepare nanosized compounds. But the heat control problem existing in the low temperature solid state reaction is not solved efficiently. Use of microwave energy for synthesis and processing of materials is an exciting new field in material science with enormous potential for synthesizing new materials and novel microstructures [17].
In the present work, NiFe2O4 nano-powders were prepared by microwave radiating low-temperature solid-state reaction method, using FeSO4·7H2O, NiSO4·6H2O and NaOH as reactors. NiFe2O4 nano-powders were dipped into AgNO3 solution, then calcined. The crystal structure and gas sensing performance of the samples were characterized, and compared with those of NiFe2O4 mixed with Ag prepared by low-temperature solid-state reaction.
2 Experimental
2.1 Preparation of Ag modified NiFe2O4 nanopowders
Nickel ferrite was prepared by a simple solid-state reaction route. Analytical grade NiSO4·6H2O, FeSO4·7H2O and NaOH were mixed in the molar ratio of 1:2:6 and ground together in an agate mortar for about 20 min. In this mixing process, the reaction took place exothermally, and with a gradual change in color from greenish red to brown. This mixture was subjected to radiation at home microwave oven of 700 W for 10 min. The radiated powder was washed with deionized water to remove sodium sulfate until the content of Na+ in filtrate was less than 0.5% tested by FP640 flame photometer. The washed powder was dried at 100 °C for 1 h to get the final polycrystalline NiFe2O4 nanoparticles. The NiFe2O4 nanopowder was dipped into AgNO3 solution and the mass ratios of Ag to NiFe2O4 were 1.5%, 2% and 2.5%, respectively. The mixed solution was churn up for 2 h,then dried at room temperature followed by calcining at 550 °C for 1 h to form Ag modified NiFe2O4 nanopowders. Adding homemade Ag2O nanopowders into the mixture of FeSO4·7H2O, NiSO4·6H2O and NaOH, the mass ratios of Ag to NiFe2O4 also was 1.5%, then the mixture was ground and microwave heat treated to form Ag mixed NiFe2O4 compound nano-powders.
2.2 Characterization of Ag modified NiFe2O4 nanopowders
The crystal structure of samples was characterized by X-ray diffractometer (XRD) (model: D/max-RB with an accelerating voltage of 40 kV) with Cu Kα radiation (λ=1.54059 nm) and a scan rate of 8 (°)/min at room temperature. The morphology of the synthesized powder was scanned with FEI Sirion 200 scanning electron microscope (SEM). The X-ray photoelectron spectroscopy (XPS) was characterized by an America Thermo VG company ESCALAB250 multi technical surface analysis system. The XPS spectra were recorded using a Al Kα (1486.6 eV) X-ray source. The binding energy of the C1s core level electron was taken to be 284.6 eV for energy calibration.
2.3 Fabrication of sensor and measurement of gas sensing characteristics
The paste formed from a mixture of Ag modified NiFe2O4 nano-powders with deionized water and alcohol mixed solution was coated onto an Al2O3 tube on which two gold leads were installed at each end. The Al2O3 tube was about 4 mm in length, 1.5 mm in external diameter and 1.2 mm in internal diameter. The gas sensors were sintered at 500 °C for 1 h to evaporate the adhesive and eliminate the effect of the surface absorbed water. A heater using Ni–Cr wire was inserted into the Al2O3 tube to supply working temperature which could be adjusted in the range of 100-500 °C. The schematic diagram of gas sensor is shown in Fig. 1.
To improve the stability and repeatability, the sensors were aged at 300 °C for 4 d in air prior to use. The gas sensing characteristics were measured on WS-30A gas sensor test apparatus. The electrical resistance of a sensor was measured in air and in test gases, respectively. The test gases or liquilts were injected into the glass enclosure with a syring through the inlet. Alcohol, C2H5OH, methane, CH4, acetone, CH3COCH3, liquefied petroleum gas (LPG), toluene, CH3C6H5, and CO were used. The sensitivity (S) was defined as the ratio of the electrical resistance in air (R0) to that in a test gas (Rg).
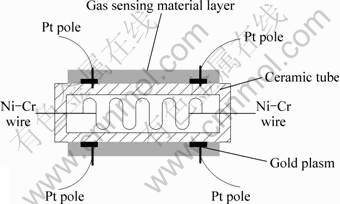
Fig. 1 Schemic diagram of gas sensor
3 Results and discussion
3.1 Structure analysis
Nickel ferrite was prepared by a low-temperature solid-state reaction route [11]. The desired NiFe2O4 spinel phase was confirmed by powder X-ray diffraction analysis. Figure 2 shows the XRD pattern at room temperature for 1.5% Ag mixed NiFe2O4 sample and 2% Ag modified NiFe2O4 sample. The diffraction peaks of these two samples all match well with JCPDS reported data of cubic NiFe2O4 spinel structure at 2θ=30.58°, 35.56°, 43.52°, 54.06°, 57.66° and 62.84°. In addition, the diffraction peaks at 2θ=38.16°, 44.36° and 64.44°, match with Ag in Fig. 2(a), and the intensities of Ag diffraction peaks are strong, which indicates that the crystallization of Ag is fine. But in Fig. 2(b), no peaks correspond to Ag, AgNO3, Ag2O or other oxides of silver. The ionic radius of Ag+ is 1.26 nm, whereas those of Ni2+ and Fe3+ are 0.69 nm and 0.64 nm, respectively, so Ag+ cannot be doped into the spinel lattice of Ni ferrite. In the case, significant changes in lattice parameters are not obtained. This phenomenon indicates that the mechanism of NiFe2O4 sample modified with Ag is different from that of NiFe2O4 sample mixed with Ag.
Therefore, X-ray photoelectron spectroscopy (XPS) was characterized to prove the Ag phase structure. Figure 3 shows the XPS spectra of Ag 3d of sample modified by 1.5% Ag.
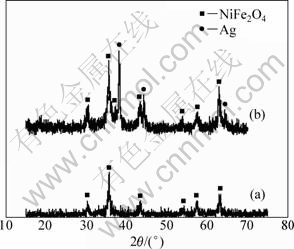
Fig. 2 XRD patterns of samples: (a) 2%Ag modified NiFe2O4; (b) 1.5%Ag mixed NiFe2O4
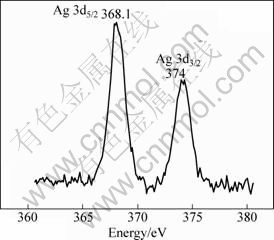
Fig. 3 XPS spectrum of Ag 3d of 1.5%Ag modified NiFe2O4
The results show that the Ag 3d5/2 spectrum is at approximately 368.1 eV, and the energy difference between the Ag 3d3/2 and 3d5/2 states is approximately 6 eV, which agrees well with the character value of pure Ag (Ag3d5/2, 368.3 eV), and as distinguished from that of Ag2O and AgO, the binding energies are 367.8 eV and 367.4 eV, respectively. Hence, the XPS results show that Ag exists as pure Ag phase, but this fact was not detected by the X-ray diffraction, so Ag phase is amorphous. Figure 4 represents the XPS spectra of O 1s with a single peak at 530 eV corresponding to O 1s bonded with Ni-ions and Fe-ions in the lattice of material.
The contents of O, Fe, Ni, and Ag for the Ag modified NiFe2O4 sample calculated from the area of O 1s, Fe 2p, Ni 2p and Ag 3d XPS peaks are shown in Table 1.
The XPS results indicate that the mole ratio of Ag to Ni is 4% which is higher than that of the content in their corresponding target 3%, so the Ag, with amorphous structure, is mainly residing on the surface of NiFe2O4 particles.
The morphology of the samples can be visualized from scanning electron micrograph of the NiFe2O4 samples before and after being modified with 1.5% Ag, as shown in Fig. 5.
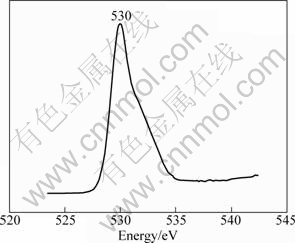
Fig. 4 XPS spectrum of O 1s of sample modified with 1.5% Ag
Table 1 Surface compositions of NiFe2O4 sample modified with 1.5%Ag analyzed by XPS peak area

Figure 5 shows that the NiFe2O4 particles are of anomalous plate-like structure. Amorphous structure Ag resides on the surface and intergranular regions of NiFe2O4 particles shown in Fig. 5(b), which make NiFe2O4 particles boundary dim or indistinct and can efficiently prevent the reuniting and growing-up of nanosized NiFe2O4 grains, so the effective surface area is thus expected to increase largely. This may be the reason of giving a maximum gas response.
3.2 Gas sensing characteristics of Ag modified NiFe2O4 gas sensors
Figure 6 shows the gas sensitivities of NiFe2O4 samples modified by different contents of Ag in the presence of 1000×10-6 acetone vapor, at various working voltages.
The gas sensitivity depends on the working voltage and Ag content. The sensitivity increases with increasing working voltage and reaches the maximum value corresponding to an optimum working voltage. For the pure NiFe2O4 sample, there is a slow increase in the sensitivity to the maximum value of 10 at the optimum working voltage of 4.5 V, whereas for 1.5% Ag modified NiFe2O4 sample, the sensitivity significantly increases with the increase of the working voltage up to 4.5 V. When the sensitivity decreases above 4.5 V, the maximum sensitivity of 43 is 4 times higher than that of pure NiFe2O4 sample, and is twice that of NiFe2O4 sample mixed with 1.5% Ag. The sensitivity decreases with the increasing content of Ag.
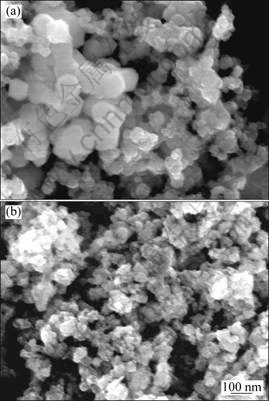
Fig. 5 SEM images of samples before and after being modified with 1.5%Ag: (a) NiFe2O4; (b) NiFe2O4+1.5% Ag
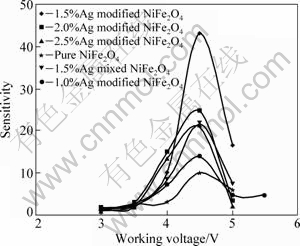
Fig. 6 Gas sensitivities of NiFe2O4 gas sensors modified with Ag at different working voltages
Figure 7 shows the gas sensitivity of Ag modified NiFe2O4 samples in various acetone gas contents, at working voltage of 4.5 V.
The sensitivity increases with increasing gas content significantly until the acetone vapor content reaches 500×10-6, and then there is a slow increase in the sensitivity from 500×10-6 to 1000×10-6 of acetone vapor content. At a lower gas content, the unimolecular layer of gas molecules would be expected to form at the interface, which would interact with the interface more actively, giving larger response. There would be multilayers of gas molecules at the interface of junction at the higher gas content resulting in the saturation in gas response. It also can be seen from Fig.7, for Ag modified NiFe2O4 samples, at lower gas content, 100×10-6, the sensitivities are all up to 5.
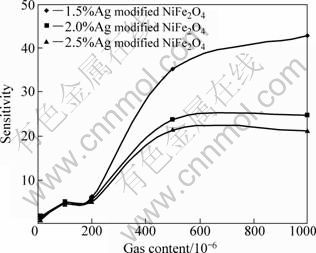
Fig. 7 Sensitivity vs acetone gas content for Ag modified NiFe2O4 samples
The sensitivities of the 1.5% Ag modified NiFe2O4 sample to other reducing gases, such as methane, alcohol, toluene, LPG and CO, were also investigated at the optimum working voltage, respectively. As can be seen from Fig. 8, the sensitivity of this sensor is remarkably higher to acetone than to other gases.
Because —OH group is present on the surface of NiFe2O4 nanoparticles, which can form hydrogen bond with the isolated electron pair at O atom in carbonyl group (C=O) of acetone, so the physical adsorption to acetone gas of the NiFe2O4 nanoparticles modified by1.5% Ag can be reinforced.
The cyclic response-recovery curve was also investigated and the response characteristic to 500×10-6 acetone gas of the 1.5% Ag modified NiFe2O4 sample is shown in Fig. 9. One can see that the time taken by the sensor reaching 90% of the maximum sensitivity at the optimum working voltage of 4.5 V is about 1 s. The time taken by the sensor to come back once the studied gas is removed is found to be 10 s. And the sensitivity value is stable. The response and recovery time of this sensor for other gases (500×10-6 ethanol and LPG) shows similar results.
3.3 Gas sensing mechanism
When a semiconductor sensor is exposed to a gas, the change in resistance is mainly due to the reaction between the reducing gas and the oxygen species adsorbed on the surface of the semiconductor. The adsorption of gas, which depends on both the type of test gas and the sensor material, may affect the response characteristic. Better response would be expected if a large amount of gas is adsorbed and subsequently the reaction between the adsorbed reducing gases and oxygen species is more favorable. The extent of adsorbed oxygen ions and existence of their different chemical forms (O-, O2-, O2-, etc.) on the sensor surface are controlled by the sensor operating temperature. The gas sensing mechanism of oxide semiconductors to reduce gases can be understood as follows [14,16]:
O2(g)+e→O2-(ads) (1)
O2-(ads)+e→2O-(ads) (2)
O-(ads) +e→O2-(ads) (3)
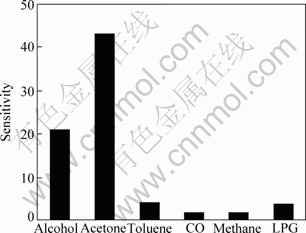
Fig. 8 Sensitivity of different gases to 1.5%Ag NiFe2O4 modified gas sensor
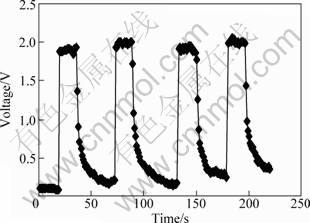
Fig. 9 Cyclic response-recovery curve of NiFe2O4 gas sensor modified with 1.5%Ag
The oxidation reactions of CH3COCH3 could be represented as follows:
CH3COCH3+5O-(ads)→3CO+3H2O+5e (4)
CH3COCH3+8O-(ads)→3CO2+3H2O+8e (5)
CH3COCH3+5O2-(ads)→3CO+3H2O+10e (6)
CH3COCH3+8O2-(ads)→3CO2+3H2O+16e (7)
In reactions, acetone gas reacts with adsorbed oxygen to form CO or CO2, inducing electron donation into the n-NiFe2O4. In these cases, electrons are drawn from the oxide, which releases the electrons and thus increases the charge in the conduction band of the n-type oxide and hence the conductivity increases.
From our study, it is observed that the 1.5% Ag modified NiFe2O4 sample shows improved sensing response in comparison with pure NiFe2O4 sample and 1.5% Ag modified NiFe2O4 sample is probably due to Ag amorphous structure, preventing nanosized NiFe2O4 particles from reuniting and growing-up, and results in larger specific area and high surface activity.
4 Conclusions
1) Amorphous Ag modified NiFe2O4 nano-powders were prepared by microwave radiating low-temperature solid-state reaction method followed by dipping technology.
2) The Ag, with amorphous structure, residing on the surface and intergranular regions of NiFe2O4 particles, can efficiently prevent nanosized NiFe2O4 particles from reuniting and growing-up.
3) The sensitivity of 1.5% Ag modified NiFe2O4 gas sensor to acetone gas reaches 43, which is 4 times as high as that of pure NiFe2O4 sample, and is twice that of NiFe2O4 sample mixed with 1.5% Ag, at the optimum working voltage of 4.5 V.
4) The sensitivity of 1.5% Ag modified NiFe2O4 gas sensor is remarkably higher to acetone than to other gases, and the response and recovery time of this sensor is 1 s and 10 s, respectively.
References
[1] GOPAL C V, MANORAMA S V, RAO V J. Semiconducting gas sensor for chlorine based on inverse spinel nickel ferrite [J]. Sensors and Actuators B: Chemical, 1999, 55(1): 90-95.
[2] YANG Liu-fang, XIE Yong-an, ZHAO He-yun, WU Xing-hui, WANG Yu-de. Preparation and gas-sensing properties of NiFe2O4 semiconductor materials [J]. Solid State Electronics, 2005, 49(8): 1029-1033.
[3] GE Xiu-tao. Effect of Ag+-doped on the conductance and gas-sensing properties of CdFe2O4 [J]. Chin J Chem Phys, 2001, 14(4): 485-490.
[4] ZHANG Guo-ying, LI Chun-sheng, CHENG Fang-yi, CHEN Jun. ZnFe2O4 tubes: Synthesis and application to gas sensors with high sensitivity and low-energy consumption [J]. Sensors and Actuators B: Chemical, 2007, 120: 403-410.
[5] SUN Zhi-peng, LIU Lang, JIA Dian-zeng, PAN Wei-yu. Simple synthesis of CuFe2O4 nanoparticles as gas-sensing materials [J]. Sensors and Actuators B: Chemical, 2007,125 (1): 144-148.
[6] KAPSE V D, GHOSH S A, RAGHUWANSHI F C, KAPSE S D. Nanocrystalline spinel Ni0.6Zn0.4Fe2O4: A novel material for H2S sensing [J]. Materials Chemistry and Physics, 2009, 113(6): 638-644.
[7] WANG Jin-ying, HUANG Miao-liang, ZHONG Qi-quan, LIN Jian-ming, WU Ji-huai. Photocatalysis and preparation of nano-sized ZnWO4/Ag by hydrothermal process [J]. Journal of Functional Materials, 2009, 40(9): 1442-1444.
[8] SATYANARAYANA L, REDDY K M, MANORAMA S V. Nanosized spinel NiFe2O4: A novel material for the detection of liquefied petroleum gas in air [J]. Materials Chemistry and Physics, 2003, 82(1): 21-26.
[9] ZHAO Meng, WANG Jin-xing, FENG Cai-hui, ZOU Bo, CHEN Cheng, WANG Zhu-yi, WU Feng-qing, ZOU Le-hui. Preparation and gas sensing properties to formaldehyde of TiO2/Ag2O nanocrystalline [J]. Acta Phys Chim Sin, 2007, 23(7): 1003-1006.
[10] DARSHANE S L, SURYANVANSHI S S, MULLA I S. Nanostructured nickel ferrite: A liquid petroleum gas sensor [J]. Ceramics International, 2009, 35(5): 1793-1797.
[11] DARSHANE S L, MULLA I S. Influence of palladium on gas-sensing performance of magnesium ferrite nanoparticles [J]. Materials Chemistry and Physics, 2010, 119(1-2): 319-323.
[12] FAN Xin-hui, LIN He, MA Xue-hong, YAN Wen. Effect of Ag dopants on gas sensitivity of ZnO nanowires [J]. Nanoscience&Nanotechnology, 2009, 6(2): 61-65.
[13] YU Ling-min, ZHU Chang-chun, YUE Miao, FAN Xin-hui, QI Li-jun. Study on the gas sensitivity property to alcohol of Ag doped ZnO nanowires [J]. Journal of Functional Materials, 2008, 39(5): 867-869.
[14] REZELSCU N, IFTIMIE N, REZELSCU E, DOROFTEI C, POPA P D. Semiconducting gas sensor for acetone based on the fine grained nickel ferrite [J]. Sensors and Actuators B: Chemical, 2006, 114(1): 427-432.
[15] BARATI M R, SEYYED EBRAHIMI S A, BADIEI A. The role of surfactant in synthesis of magnetic nanocrystalline powder of NiFe2O4 by sol–gel auto-combustion method [J]. Journal of Non- Crystalline Solids, 2008, 354: 5184-5185.
[16] LIU Yan-li, WANG Hua, YANG Yu, LIU Zhi-min, YANG Hai-feng, SHEN Guo-li, YU Ru-qin. Hydrogen sulfide sensing properties of NiFe2O4 nanopowder doped with noble metals [J]. Sensors and Actuators B: Chemical, 2004, 102(1): 148-154.
[17] ZHANG Lei, JIAO Wan-li. Formation of nanosized nickel ferrite plate-like crystal assisted with microwave radiation [J]. J Chin Ceram Soc, 2008, 36(11): 1505-1508.
非晶态Ag表面修饰NiFe2O4的制备及对
丙酮气体的气敏性能
焦万丽,张 磊
山东理工大学 材料科学与工程学院,淄博 255049
摘 要:采用微波诱导低温固相反应法合成NiFe2O4纳米粉体,并通过浸渍法在其表面修饰Ag,通过X射线衍射分析(XRD)、扫描电镜(SEM) 和X射线光电子能谱(XPS)研究修饰前、后粉体的结构及形貌,并对修饰不同含量Ag的旁热式气敏元件的气敏性能进行测试。结果表明:表面修饰的Ag为非晶态,能够有效阻止NiFe2O4纳米颗粒的团聚及长大,当表面修饰1.5%Ag后,NiFe2O4气敏元件的最佳工作电压为4.5 V,对丙酮气体具有较高的灵敏度,达到43,且响应(1 s)和恢复(~10 s)时间快,优于直接固相反应法掺杂1.5%Ag所得的NiFe2O4气敏元件的气敏性能。
关键词:镍铁氧体;低温固相反应;浸渍法;银;丙酮
(Edited by LI Xiang-qun)
Foundation item: Project (2006BS04035) supported by the Youth Scientific Research Foundation of Shandong Province, China
Corresponding author: JIAO Wan-li; Tel: +86-533-2787151; E-mail: jiaowanli1977@163.com
DOI: 10.1016/S1003-6326(11)61294-6