DOI: 10.11817/j.issn.1672-7207.2017.10.004
PbO2/Ti阳极氧化苯酚的有效电流表征及阶段反应动力学
王立章1,伍波1,李鹏1,赵玉1,李哲楠1,杨胜翔1,赵跃民2
(1. 中国矿业大学 环境与测绘学院,江苏 徐州,221116;
2. 中国矿业大学 化工学院,江苏 徐州,221116)
摘要:采用X线衍射(X-ray diffraction, XRD)和扫描电镜(scanning electron microscope, SEM)对以电镀法制备的PbO2/Ti阳极进行表征,以阳极氧化电流占比系数γ描述苯酚氧化与析氧反应之间的影响机制,并基于法拉第定律建立描述有机物氧化进程的通用阶段反应动力学模型;在不同电流密度和进水流量条件下进行苯酚阳极氧化实验。研究结果表明:电极表面晶相主要为β-PbO2,晶粒平均直径为50.23 nm,形成的团聚体粒径在5~10 μm之间。PbO2/Ti阳极析氧电位约为1.78 V;液相中苯酚的存在可抑制氧气析出,且析氧反应电阻由无苯酚情况下的148.3 Ω增大至195.9 Ω。回归分析和F检验结果证明理论预测值与实验数据之间具有一致性。
关键词:PbO2/Ti;阳极氧化;有效电流;阶段反应动力学;苯酚
中图分类号:X703.1;O646 文献标志码:A 文章编号:1672-7207(2017)10-2583-07
Quantitative characterization of effective current and step-reaction kinetics toward electro-oxidation of phenol on PbO2/Ti anode
WANG Lizhang1, WU Bo1, LI Peng1, ZHAO Yu1, LI Zhenan1, YANG Shengxiang1, ZHAO Yuemin2
(1. School of Environment Science and Spatial Informatics,
China University of Mining and Technology, Xuzhou 221116, China;
2. School of Chemical Engineering and Technology, China University of Mining and Technology, Xuzhou 221116, China)
Abstract: The electroplating method was employed for preparation of PbO2/Ti anode, which was determined by the X-ray diffraction (XRD) analysis and scanning electron microscope (SEM) characterization. A novel parameter γ, defined as fractional current applied to effective oxidation was proposed to describe the relation between the oxygen evolution and anodic oxidation of organic compounds. Then, step-reaction kinetics was achieved via Faraday’s law by introducing the parameter γ. Phenol electro-oxidation experiment was carried out at different applied current densities and flow rates. The results show that the main content of the coating material on Titanium (Ti) is β-PbO2 with average crystal size is about 50.23 nm, and these grains can effectively grow to form aggregate with particle size of 5 μm to 10 μm even dispersed on the Ti plate. The oxygen evolution potential of the prepared PbO2/Ti anode is 1.78 V or so, and phenol can decrease oxygen evolution rate by increasing the resistance of water decomposition to 195.9 Ω, which is much higher than that in Na2SO4 solution (148.3 Ω). The regressive analysis and F-test results confirm that the model prediction can satisfactorily match the experimental data.
Key words: PbO2/Ti; anodic oxidation; effective current; step-reaction kinetics; phenol
电极是电化学反应的核心;具体到水处理范畴,阳极氧化和析氧反应同时进行,高析氧电位(oxygen evolution potential, OEP)阳极的优选成为有机物高效降解的前提。基于此,具有极高OEP(2.3 V vs. SCE) 的掺硼金刚石电极(boron doped diamond,BDD)被广泛研究[1-3],但高昂的造价限制了此类电极的工程应用;与BDD电极相比,廉金属氧化物PbO2阳极在中性溶液中OEP可达1.75 V(vs. SCE)以上,但因其成本低、制备条件要求不高而备受工业界青睐,因而对其进行研究极具工程参考价值[4]。另一方面,为准确调控阳极氧化进程,建立精确描述有机物氧化进程动力学模型至关重要。当前多数研究仍采用一级反应动力学或响应面法进行实验数据处理[5-6],这种基于结果的研究方法不能预测反应过程,更不能充分指导电化学反应器的精确设计。为改变这种状况,DOMINGUEZ等[1-2]基于电化学反应本征动力学提出了“反应控制-扩散控制”两阶段相结合的有机物阳极氧化机制,其模型认为在反应控制条件下有机物浓度可实现线性降低(即有机物氧化所需理论电流和输入电流相等);该理论特别适合于描述BDD阳极氧化过程,但对其他电极材料并不能完全适用:如通过对王雅琼等[4]、ZHAO等[7]分别采用高析氧电位阳极PbO2和SnO2氧化苯酚、苯甲酸所得的实验结果进行分析发现:即使在反应控制阶段,按照法拉第定律计算所得的理论电流与输入电流数值有较大偏差,但其比值一直为常数。这表明,电极过程中输入电流并非完全用于氧化有机物,应该存在与阳极本征析氧特性相关联的宏观参数决定这一进程;因而,充分考虑阳极析氧和有机物氧化之间的关联性成为建立准确描述有机物阳极氧化进程动力学通用模型的关键。本文作者采用X线衍射(X-ray diffraction,XRD)和扫描电镜(scanning electron microscope,SEM)对电沉积法制备的PbO2/Ti阳极进行物相表征;然后通过循环伏安法(cyclic voltammetry,CV)和极化测试手段分析所制备阳极在浓度为0.211 mol/L Na2SO4溶液中的催化活性和析氧能力;同时,对电化学阻抗谱(electrochemical impedance spectroscopy,EIS)进行拟合分析,得到Nyquist等效电路各元件具体数值用以反映体系中有机物氧化和析氧活性之间的关联性。同时,根据提出的表征电极催化活性的阳极氧化电流占比系数γ,结合法拉第定律和电化学反应本征动力学建立通用于各种阳极材料的有机物氧化阶段反应动力学,以期为电氧化进程预测提供理论依据。最后,以在多种实验条件下所获得的苯酚氧化实验结果对建立的动力学模型进行详细的验证。
1 材料和方法
1.1 PbO2/Ti阳极制备
PbO2/Ti阳极制备主要步骤如下:1) 把钛板放入10%(质量分数)NaOH溶液中加热微沸2 h除油;2) 除油后的钛板先在10%(质量分数)H2SO4中浸泡1 h,然后用10%(质量分数)草酸溶液微沸酸蚀至溶液呈红棕色;3) 经预处理的钛板作为阳极,不锈钢为阴极,电镀电位为2.25 V,电镀时间为4 h,混合镀液成分组成为0.5 mol/L Pb(NO3)2,0.2 mol/L Cu(NO3)2,0.1 mol/L HNO3和0.04 mol/L NaF。电镀反应如下。
阳极反应:Pb2++2H2O→PbO2+4H+;
阴极反应:Cu2++2e→Cu。
1.2 PbO2/Ti阳极物相表征及性能测试
阳极表面形貌采用美国FEI公司Quanta 250型环境扫描电子显微镜系统在高真空下测定。电极表面晶体结构表征在德国Bruker公司D8 Advance X线衍射仪上完成;测试电压和电流分别为40 kV和30 mA, 扫描速度为0.1 s/步,以铜靶为辐射源。
应用CS350型电化学工作站,采用标准三电极体系进行阳极电化学性能测试。以制备的PbO2/Ti阳极为工作电极,其测试尺寸(长×宽)为2 cm×2 cm和4 cm×4 cm的铂片作为对电极,参比电极为饱和甘汞电极(saturated calomel electrode, SCE)。CV、极化和EIS测定均在25 ℃的0.211 mol/L Na2SO4溶液和含有600 mg/L苯酚的Na2SO4溶液2种条件下完成;其中CV和极化曲线扫描速度分别为20 mV/s和10 mV/s,扫描电位范围分别为0~2.0 V和0~2.5 V;EIS工作条件为:测试电位1.80 V,交流振幅10 mV,频率范围为100 kHz~0.01 Hz。
1.3 苯酚阳极氧化实验
阳极氧化液相采用去离子水加入质量浓度为600 mg/L的苯酚(99.5%纯度)配制而成;以Na2SO4作为支持电解质,浓度为0.211 mol/L。处理单元主体长×宽×高为10 cm×5 cm×15 cm,反应区有效容积为0.5 L;以制备的PbO2/Ti作为阳极,Ti板作为阴极,电极尺寸(长×宽)为10 cm×10 cm,极板间距为5 cm;反应流程由8个相同的处理单元组成,每一单元均配置型号为BT100-12数显恒流泵。直流电源型号为KXN-1540D,可提供0~15 V的电压和0~40 A的电流输出值。采用恒温水浴保持反应温度为25 ℃;在进水流量q为0.6 L/h,改变电流密度J为50 A/m2和100 A/m2以及J为80 A/m2,在流速为1.0 L/h和2.0 L/h的条件下分别进行苯酚阳极氧化实验。在每一处理单元出水口处取样,过滤后进行数据分析。
1.4 分析方法
采用Scherrer公式计算制备的PbO2/Ti阳极表面晶体粒径d[8]:
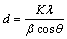 (1)
(1)
式中:K为Scherrer常数,取0.89;λ为X线的波长;β为衍射峰半高峰宽;θ为衍射角度。
扩散系数D的获取方法参照文献[9],PbO2/Ti阳极氧化苯酚传质系数km的计算式为[10-11]
 (2)
(2)
式中:Re和Sc分别为Reynolds数与Schmidt数;F为Faraday常数;c0为原水有机物浓度;de和y0分别为反应器当量直径和有效反应区高度。
以COD(chemical oxygen demand, 化学需氧量)指标描述苯酚阳极氧化效果,采用标准方法进行测试[12]。为验证理论模型与实际处理的吻合程度,对预测数据和实验结果进行回归分析和F检验;相关系数R2和FD分别采用式(3)和(4)进行计算[13-14]:
 (3)
(3)
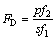 (4)
(4)
式中:xexpe和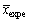 分别为实验数据和实验值的平均值;xtheo和
分别为实验数据和实验值的平均值;xtheo和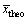 分别为理论数值及其平均值;p为回归平方和;s为残余平方和;f1为自变量个数;f2为s的自由度,其值为实验数据个数减去(f1+1)。若由式(4)计算所得的FD大于10
分别为理论数值及其平均值;p为回归平方和;s为残余平方和;f1为自变量个数;f2为s的自由度,其值为实验数据个数减去(f1+1)。若由式(4)计算所得的FD大于10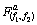 (a为置信水平),则可说明理论模型与实验数据之间具有高度相关性。
(a为置信水平),则可说明理论模型与实验数据之间具有高度相关性。
2 结果与讨论
2.1 物相表征
图1(a)所示为PbO2/Ti阳极XRD图谱。由图1(a)可看出:当2θ为25.4°,32.01°和40.68°时出现3个强峰,分别对应于(110),(101)和(211) 3个晶面,根据JCPDS 65-2826标准卡数据对比得知这是β-PbO2的典型特征峰;β-PbO2的存在能够大幅度提高阳极电化学活性,快速氧化有机污染物[15-16]。基于3个晶面衍射峰的半高峰宽并采用式(1)计算得晶粒分别为55.4,53.9和41.4 nm,平均粒径为50.23 nm。由图1(a)还可得2θ为36.37°和77.08°时的PbO衍射峰,说明在电极表面存在β-PbO2和PbO这2种物质,但还原态Pb2+会增加无效电流,降低电流效率,进而在较大程度上影响阳极的氧化能力。图1(b)所示为PbO2/Ti阳极表面的SEM图。由图1(b)可知:电极表层由排列紧密、具棱角的团聚体构成,颗粒与基体之间在扫面区域内未出现明显的空洞,这种结构有利于阻止活性氧的渗入,进而起到保护基底、防止TiO2生成的作用。团聚体大小分布基本均匀,颗粒直径为5~10 μm,可有效增加阳极比表面积;这也表明较小粒径的晶粒易于在钛板酸刻蚀麻面层有序沉积生长。
2.2 电化学性能分析
2.2.1 CV曲线与极化测试
图2(a)所示为制备的PbO2/Ti阳极在浓度为0.211 mol/L的Na2SO4溶液中的CV曲线。由图2(a)可知:当液相中无苯酚时,存在明显的氧化/还原峰,峰电位分别为1.54 V和0.53 V,这由Pb2+/Pb4+电对之间的相互转化引起[17-19];电位大于1.78 V后析氧反应开始发生,馈电流急剧增加。当液相中加入苯酚后,并未出现明显的苯酚氧化峰,表明中性溶液中PbO2/Ti阳极表面不能发生苯酚直接氧化反应,这可能由于苯酚易于在电极表面吸附,进而阻碍了电荷向电极本体的渗透,致使Pb2+/Pb4+电对在反应区电势范围内馈电流有所下降;同时,电位超过1.78 V后馈电流增加,且增加量远大于无苯酚时的情况,这可能由析氧反应和羟基自由基(·OH)的大幅度产生引起。CV曲线测试结果说明,中性溶液中苯酚发生间接氧化,苯酚氧化和水分解副反应同时发生,阳极析氧电位越高,析氧反应抑制效果越明显,电氧化效率将会越高。
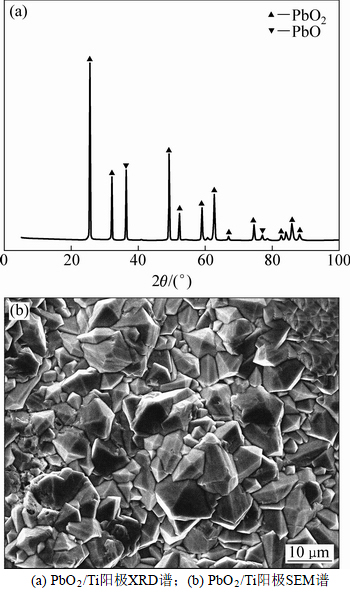
图1 PbO2/Ti阳极XRD图谱和SEM图
Fig. 1 XRD pattern and SEM micrograph of PbO2/Ti anode
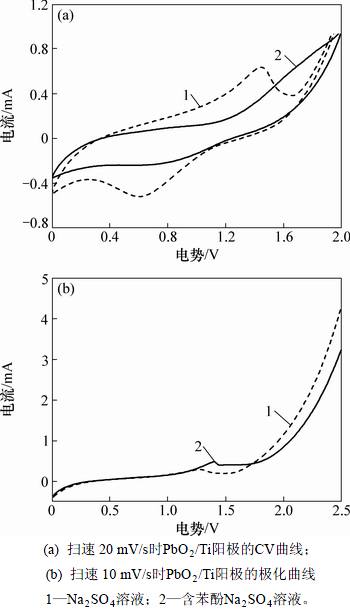
图2 扫速分别为20 mV/s和10 mV/s时PbO2/Ti阳极的CV曲线和极化曲线
Fig. 2 CV curves and polarization curves at sweeping rates of 20 mV/s and 10 mV/s
图2(b)所示为极化曲线。由图2(b)可知:在中性溶液条件下PbO2/Ti阳极的析氧电位约为1.78 V,与其他研究者的结论基本一致[20-21]。但由于实际运转条件下,为获得较大的电流密度,操作电压远大于析氧电位,造成有机物氧化和析氧2种反应并存。因此,选用高析氧电位阳极是提高电催化氧化效率的关键 因素。
2.2.2 EIS测试
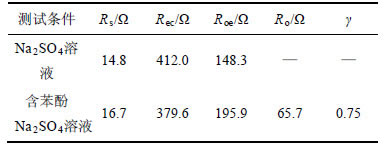
测得EIS谱图并采用ZSimpWin软件对Nyquist等效电路进行拟合可得各电路元件参数,能有效地表征电化学反应状况[22-23]。图3所示为在1.80 V时PbO2/Ti的EIS图谱。图中,以阻抗的实部为横坐标,以阻抗的虚部为纵坐标。由图3可知:在液相无苯酚条件下,电极表面主要发生析氧反应;而当含有苯酚时,析氧和有机物氧化共存,此时,若析氧阻力加大,将会增加有机物反应有效电流,反之亦然。故本研究分别采用Rs(QecRec)(Qdl(RoeW))和Rs(QecRec)(QdlRoe(RoW))电路拟合2种测试结果,其中Rs为溶液电阻,其数值与液相性质和工作电极与对电极之间的距离有关;Rec为PbO2/Ti阳极的涂层与Ti基体之间的电阻;Roe和Ro分别为析氧电阻和苯酚氧化电阻;W为Warburg 阻抗;Q为常相位角,为表征电容参数发生偏离时的物理量。电路各元件拟合结果见表1。拟合结果表明:无苯酚时,Roe较小,为148.3 Ω;有苯酚时,析氧进程被大幅度抑制,Roe增加为195.9 Ω,增加比率达到32%,这能提高苯酚氧化的有效电流。由表1还可看出:在2种情况下,溶液电阻分别为14.8 Ω和16.7 Ω,相差不大;电极电阻分别为379.6 Ω和412.0 Ω,也在误差允许范围之内,表明拟合电路较为适合。
鉴于析氧、有机物氧化的电路并联性质,在宏观操作条件下,当电流密度为J时,用于苯酚氧化的有效电流密度Jef可表示为
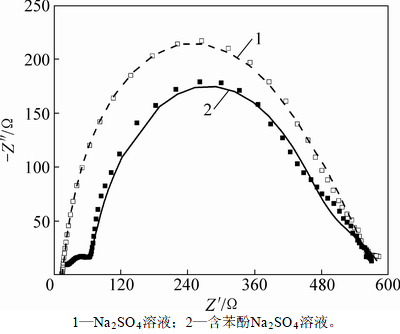
图3 1.80 V时PbO2/Ti阳极的EIS图谱
Fig. 3 EIS images of PbO2/Ti anode at potential of 1.80 V
表1 1.80 V时PbO2/Ti阳极EIS图谱拟合电路元件数值
Table 1 Obtained values for elements of simulation circuit to EIS measurements of PbO2/Ti anode at potential of 1.80 V
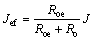 (5)
(5)
令氧化电流占比系数γ=Roe/(Roe+Ro)。由式(5)可知:增加析氧电阻,即采用高析氧电位阳极,或减小有机物氧化电阻,即采取相应手段如投加催化剂等均能增加氧化反应有效电流,有利于提高反应速率。采用表1拟合数据计算得到,制备的PbO2/Ti阳极在中性条件下,当氧化苯酚输入电流为I时,用于有效反应的电流为0.75I。
2.3 苯酚阳极氧化
2.3.1 阶段反应动力学模型
上述分析表明,用于有机物氧化的有效电流仅为输入电流的一部分,与阳极的析氧能力相关联;因此,由法拉第定律并代入氧化电流占比系数γ可得有机物氧化速率r表达式为
 (6)
(6)
式中:n为阳极氧化反应电荷转移数量。根据电化学反应本征动力学,有机物的阳极氧化可分为反应控制和扩散控制2个阶段,可由γJ与极限电流密度Jlim之间的关系界定,2种情况对应的判据分别为γJ≤Jlim和γJ>Jlim;而在扩散控制时,用于电氧化反应的有效电流为极限电流密度。据此,式(6)转化为
 (7)
(7)
式中:c(t)表示反应时间为t时的出水有机物浓度;α为操作电流密度与起始极限电流密度(nFkmc0)的比值,
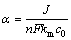 (8)
(8)
式(7)的边界条件为γJ=Jlim,故两反应阶段的边界浓度c(t)Cr为
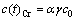 (9)
(9)
对于流经式反应器,在连续流状态下取与电场方向平行的微元进行物料衡算得
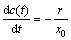 (10)
(10)
式中:x0为反应器的极板间距。将式(7)代入式(10),应用边界条件式(9)并积分得到
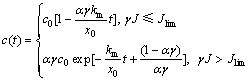 (11)
(11)
将式(9)代入式(11),计算得到到达两反应阶段的边界时间tCr为
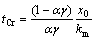 (12)
(12)
在上述理论分析中,存在两反应阶段的前提条件为γJ≤nFkmc0,即α≤1/γ;当α>1/γ时,电氧化过程始终处于扩散控制状态,则采用如上所示的方法进行分析可得出水浓度理论计算式为
 (13)
(13)
式(13)表明只有当操作电流密度足够大且超出起始极限电流密度时,有机物的阳极氧化进程才能表现为一级反应;尤其是采用析氧电位较低的阳极如IrO2,RuO2和碳电极时,可能由于氧化电流占比系数γ较小,即使在较大的操作电流密度下,电化学反应仍能有序地呈现反应控制和扩散控制2个阶段。
2.3.2 苯酚的电氧化
在不同电流密度与进水流量条件下进行了苯酚阳极氧化实验。由图4(a)可知:较大的操作电流密度可提高反应速率、降低出水COD质量浓度。这是由于增加电场强度可加快有机物自液相向电极表面的扩散速率而强化传质进程,所得实验结果与文献[24-25]所反映的趋势一致;而图4(b)反映出在相同的电流密度下,加大进水流速也可减小同一反应时间的出水有机物浓度,进而达到提高阳极氧化效率的效果。
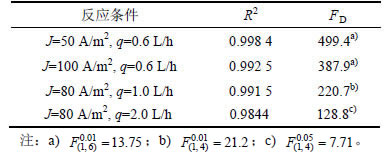
上述实验结果均可采用阶段反应动力学进行定量阐释。由式(2)可得:当电流密度为50 A/m2时,km=2.55×10-6 m/s;增加电流密度至100 A/m2时,km=3.92×10-6 m/s,后者仅为前者的1.54倍,传质系数的变化与电流密度不呈线性关系。据此,计算得到50 A/m2时的α为1.08 (α<1/γ=1.33),苯酚氧化进程起先处于反应控制阶段,结束终点tCr为1.27 h,而后有机物向阳极表面的扩散机制占主体;而100 A/m2时α为1.56,有效电流密度已超过起始极限电流密度 64.27 A/m2,致使苯酚氧化一直处于扩散控制状态,尽管其去除量高于50 A/m2时的情况,但其氧化效率已经远低于前者;由建立的动力学模型推断,若进一步增加电流密度,很可能出现有机物去除量下降的状况,PANIZZA等[26]采用BDD阳极氧化2-萘酚时有效地捕捉到了这种现象。式(2)还反映出流速可通过Re数作用于传质系数,增加流速会提高km;与流速1.0 L/h时的km为3.69×10-6 m/s相比,2.0 L/h时km为4.59×10-6 m/s,有机物自本体溶液向阳极表面的扩散进程得到较大幅度提高,因此,在相同的操作电流密度条件下,后者的氧化效率将远大于前者的氧化效率。上述理论分析与图4中实验数据反映的趋势完全相同。表2所示为实验数值和模型预测值之间的回归分析和F检验结果。由表2可知:在所有反应条件下,R2在0.984 4和0.998 4之间浮动,吻合程度相当高;同时,只有2.0 L/h时进行苯酚氧化时置信水平为95%,其余均高达99%,证明了理论与实验数值之间的高度相关性。实验分析表明,本研究所建立的阶段反应动力学能够准确预测多种反应条件下苯酚氧化进程的COD质量浓度变化趋势,也证明了模型中引入氧化电流占比系数γ的必要性和准确性。
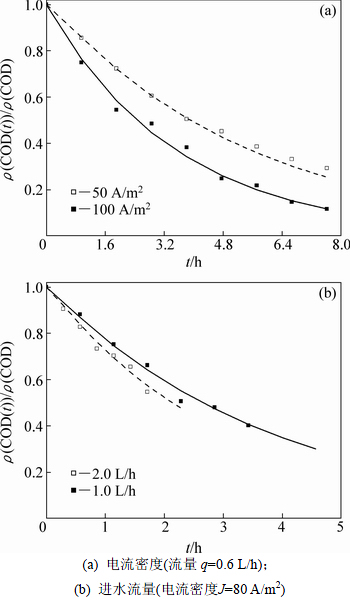
图4 电流密度和进水流量对PbO2/Ti阳极氧化苯酚的影响
Fig. 4 Effect of applied current densities and flow rates on COD removal during electro-oxidation of phenol on PbO2/Ti anode
表2 PbO2/Ti阳极氧化苯酚的实验、预测值之间的回归分析和F检验结果
Table 2 Regressive analysis and F-test results during electro-oxidation of phenol on PbO2/Ti anode
3 结论
1) 加入NaF采用电镀法所制备的PbO2/Ti阳极,表面主要晶型为β-PbO2,间杂少量PbO;计算得到晶粒平均粒径为50.23 nm,晶粒可形成直径为5~10 μm的团聚体,可在钛板酸刻蚀麻面层有序沉积、固着。
2) 所制备的PbO2/Ti阳极具有较高的电氧化活性,析氧电位达到1.78 V,但中性条件下苯酚不能在阳极表面发生直接氧化;析氧过程与苯酚氧化过程共存,氧气的析出减小了苯酚氧化的有效电流。
3) 当液相中含有苯酚时,在电位为1.80 V时EIS图谱可采用等效电路图Rs(QecRec)(QdlRoe(RoW))描述,通过拟合可得各电路元件参数。鉴于析氧、有机物氧化反应可同时发生,引入氧化电流占比系数γ表征苯酚氧化的有效电流。
4) 基于法拉第定律和电化学反应本征动力学,引入系数γ建立了通用条件下的有机物阳极氧化阶段反应动力学;该模型可对各种实验条件下的出水COD质量浓度进行精确预测。
参考文献:
[1]  T, PALO P, et al. Anodic oxidation of ketoprofen on boron-doped diamond (BDD) electrodes: Role of operative parameters[J]. Chem Eng J, 2010, 162(3): 1012-1018.
T, PALO P, et al. Anodic oxidation of ketoprofen on boron-doped diamond (BDD) electrodes: Role of operative parameters[J]. Chem Eng J, 2010, 162(3): 1012-1018.
[2] PANIZZA M, KAPALKA A, COMNINELLIS C H. Oxidation of organic pollutants on BDD anodes using modulated current electrolysis[J]. Electrochim Acta, 2008, 53(5): 2289-2295.
[3] RODRIGO M A, MICHAUD P A, DUO I, et al. Oxidation of 4-Chlorophenol at Boron-Doped Diamond electrode for wastewater treatment[J]. J Electrochem Society, 2001, 148(5): D60-D64.
[4] 王雅琼, 顾彬, 许文林. 不同金属氧化物膜电极上苯酚的电催化氧化[J]. 化工学报, 2007, 58(8): 2087-2093.
WANG Yaqiong, GU Bin, XU Wenlin. Electrocatalytic oxidation of phenol on several metal oxide film electrodes[J]. J Chem Industry Eng, 2007, 58(8): 2087-2093.
[5] EL-GHENYMY A, CENTELLAS F, GARRIDO J A, et al. Decolorization and mineralization of Orange G azo dye solutions by anodic oxidation with a boron-doped diamond anode in divided and undivided tank reactors[J]. Electrochim Acta, 2014, 130: 568-576.
[6] QUIROZ M A,  J L, REYNA S, et al. Degradation of 1-hydroxy-2,4-dinitrobenzene from aqueous solutions by electrochemical oxidation: role of anodic material[J]. J Hazard Mater, 2014, 268: 6-13.
J L, REYNA S, et al. Degradation of 1-hydroxy-2,4-dinitrobenzene from aqueous solutions by electrochemical oxidation: role of anodic material[J]. J Hazard Mater, 2014, 268: 6-13.
[7] ZHAO G H, CUI X, LIU M C, et al. Electrochemical degradation of refractory pollutant using a novel microstructured TiO2 nanotubes/Sb-doped SnO2 electrode[J]. Environ Sci Technol, 2009, 43: 1480-1486.
[8] FUKUDA K, KIKUYA K, ISONO K, et al. Foliated natural graphite as the anode material for rechargeable lithium-ion cells[J]. J Power Sources, 1997, 69(1/2): 165-168.
[9] 王晓娟, 邬蓓蕾, 马淳安. O, O-二丁基二硫代磷酸锌在玻碳电极上的电氧化特性[J]. 化工学报, 2013, 64(7): 2550-2555.
WANG Xiaojun, WU Beilei, MA Chunan. Electrochemical oxidation characteristics of zinc O, O, O/, O/-tetrabutyl bis (phosphorodithioate) on glassy carbon electrode[J]. J Chem Industry Eng, 2013, 64(7): 2550-2555.
[10] WANG L Z, HU Y L, ZHANG Y L, et al. A novel cost-saving strategy for electrochemical oxidation of organic matters by multi-current controlled operation[J]. Sep Purif Technol, 2013, 109: 18-22.
[11] 杨绮琴, 方北龙, 童叶翔. 应用电化学[M]. 广州: 中山大学出版社, 2000: 64-78.
YANG Qiqin, FANG Beilong, TONG Yexiang. Applied electrochemistry[M]. Guangzhou: Sun Yat-sen Univ Press, 2000: 64-78.
[12] 国家环保总局. 水和废水检测分析方法[M]. 4版. 北京: 中国环境科学出版社, 2002: 211-213.
State Environmental Protection Administration. Monitoring and analysis method of water and waste water[M]. 4th ed. Beijing: China Environment Science Press, 2002: 211-213.
[13] 王立章, 赵跃民. 填充床电极反应器基础理论[M]. 徐州: 中国矿业大学出版社, 2013: 118-130.
WANG Lizhang, ZHAO Yuemin. Fundamental theory of packed-bed electrode reactor in wastewater treatment[M]. Xuzhou: China Univ Mining Technol Press, 2013: 118-130.
[14] 侯影飞, 孔瑛, 郭宁, 等. 施氏假单胞菌UP-1降解二苯并噻吩的动力学模型[J]. 石油学报(石油加工), 2009, 25(5): 702-706.
HOU Yinfei, KONG Ying, GUO Ning, et al. Kinetic model of dibenzothiophene biodegradation by Pseudomonas stutzeri Up-1[J]. Acta Petrolei Sin (Petroleum Process Sect) 2009, 25(5): 702-706.
[15] 洪萍, 王凤武, 徐迈, 等. β-PbO2/TiO2纳米管电极的制备及其电催化降解苯酚[J]. 应用化学, 2014, 31(9): 1096-1100.
HONG Ping, WANG Fengwu, XU Mai, et al. Preparation of β-PbO2/TiO2 nanotube arrays electrode and electro-catalytic degradation of phenol[J]. Chin J Appl Chem, 2014, 31(9): 1096-1100.
[16] 曹江林, 吴祖成, 李红霞, 等. PbO2阳极在硫酸溶液中的析氧失活行为[J]. 物理化学学报, 2007, 23(10): 1515-1519.
CAO Jianglin, WU Zhucheng, LI Hongxia, et al. Inactivation of PbO2 Anodes during oxygen evolution in sulfuric acid solution[J]. Acta Phys Chim Sin, 2007, 23(10): 1515-1519.
[17] LIU Y, LIU H. Comparative studies on the electrocatalytic properties of modified PbO2 anodes[J]. Electrochim. Acta, 2008, 53(16): 5077-5083.
[18] BLOOD P J, BROWN I J, SOTIROPOULOS S. Electrodeposition of lead dioxide on carbon substrates from a high internal phase emulsion (HIPE)[J]. J Appl Electrochem, 2004, 34(1): 1-7.
[19] MUNICHANDRAIAH N. Potentiodynamic behaviour of β-lead dioxide in neutral media at positive potentials[J]. J Electroanal Chem Interfacial Electrochem, 1991, 309(1/2): 199-211.
[20] SOUZA F L, AQUINO J M, IRIKURA K, et al. Electrochemical degradation of the dimethyl phthalate ester on a fluoride-doped Ti/β-PbO2 anode[J]. Chemosphere, 2014, 109: 187-194.
[21] LI H Y, CHEN Y, ZHANG Y H, et al. Preparation of Ti/PbO2-Sn anodes for electrochemical degradation of phenol[J]. J Electroanal Chem, 2013, 689: 193-200.
[22] 马忠, 原鲜霞, 夏小芸, 等. 反应pH值对微波法合成的Mo修饰的Pt/C催化剂结构和乙醇电氧化催化性能的影响[J]. 物理化学学报, 2014, 30(5): 973-979.
MA Zhong, YUAN Xianxia, XIA Xiayun, et al. Effects of pH on the properties of Mo-modified Pt/C synthesized by microwave-assisted method as catalyst for electro-oxidation of ethanol[J]. Acta Phys Chim Sin, 2014, 30(5): 973-979.
[23] 梁镇海, 丁永波, 孙颜发, 等.渗氮钛基PbO2耐酸阳极的电化学性能[J]. 稀有金属材料与工程, 2010, 39 (增刊1): 56-59.
LIANG Zhenhai, DING Yongbo, SUN Yanfa, et al. Study on electrochemical properties of TiN0.26/SnO2-Sb2Ox/PbO2 electrode[J]. Rare Metal Mater Eng, 2010, 39(Suppl. 1): 56-59.
[24] DUAN X Y, MA F, YUAN Z X, et al. Electrochemical degradation of phenol in aqueous solution using PbO2 anode[J]. J Taiwan Inst Chem E, 2013, 44(1): 95-102.
[25] WEI J J, ZHU X P, NI J R. Electrochemical oxidation of phenol at boron-doped diamond electrode in pulse current mode[J]. Electrochim Acta, 2011, 56(15): 5310-5315.
[26] PANIZZA M, MICHAUD P A, CERISOLA G, et al. Anodic oxidation of 2-naphthol at boron-doped diamond electrodes[J]. J Electroanal Chem, 2001, 507: 206-214.
(编辑 杨幼平)
收稿日期:2016-10-22;修回日期:2017-02-09
基金项目(Foundation item):江苏省重点研发计划项目(BE2017640);徐州市科技计划项目(KC16SS090)(Project (BE2017640) supported by the Key Research & Development Plan of Jiangsu Province; Project (KC16SS090) supported by the Xuzhou Science and Technology Plan Project)
通信作者:王立章,博士,副教授,从事环境功能材料和电化学水处理技术研究;E-mail:wlzh0731@126.com