肼还原法制备镍纳米粒子及其机理
胡爱平,唐元洪,彭 坤,罗小兰,朱文海
(湖南大学 材料科学与工程学院,湖南 长沙,410082)
摘 要:在乙二醇中用肼作还原剂还原Ni2+制备镍纳米粒子。用X射线衍射仪测定出产物的相结构,用扫描电子显微镜观察产物的微观结构。根据反应现象和产物的相结构,研究温度、pH值调节剂种类和pH值对镍纳米粒子形成的影响,并推断其形成机理。研究结果表明,用肼作还原剂还原Ni2+制备纳米Ni的最佳反应温度为60 ℃,用NaOH作pH值调节剂调pH值到10~11得到的镍纳米粒子为体心立方结构,平均粒径为25 nm。在Ni纳米粒子的形成过程中,NaOH不仅起到调节pH值的作用,还起催化作用。
关键词:Ni纳米粒子;肼;合成;形成机理
中图分类号:O781;TN304.3 文献标识码:A 文章编号:1672-7207(2007)06-1063-04
Synthesis and formation mechanism of nickel nanoparticles by hydrazine reduction
HU Ai-ping, TANG Yuan-hong, PENG Kun, LUO Xiao-lan, ZHU Wen-hai
(College of Materials Science and Engineering, Hunan University, Changsha 410082, China)
Abstract: Nickel nanoparticles were obtained by hydrazine reduction of nickel chloride in ethylene glycol in an air atmosphere. The resultant particles were characterized by X-ray diffractometer and scanning electron microscope. The effects of temperature and alkalinity on the reaction were studied. The results show that the optimal conditions are as follows: temperature of 60 ℃, and NaOH as pH value adjusting reagents, pH value of 10-11. Under the optimal conditions, the resultant particles is pure crystalline nickel with an face centered cubic (fcc) structure and mean diameter of about 25 nm. According to the experiment phenomena in the process of reaction and the phase structure of resultant particles, it can be concluded that the reaction can be carried out due to NaOH as catalyst and pH adjusting reagent.
Key words: nickel nanoparticles; hydrazine; synthesis; formation mechanism
近年来,纳米金属材料如铁、镍纳米粒子由于在磁流体[1-2]、磁记录系统[3-4]、催化[5-6]、光电子[7]和药物传输[3, 7-8]等领域具有潜在的应用而得到广泛关注。制备纳米金属粒子的方法有很多,如气相沉积法[9-10],模板法[11-12],溶胶凝胶法[13]和电化学沉积法[14]等。气相沉积法设备昂贵,模板法可以通过调节模板剂的量来控制粒子的粒径,但是,有机模板剂在反应完之后不易除去;溶液化学还原法工艺最简单,产物粒径、形貌等容易控制,因此,受到人们关注。在溶液法中,还原剂一般采用硼氢化钠和肼。当用硼氢化钠作还原剂时,反应快且较完全,但硼氢化钠价格高,并且在产品中常会带入硼等杂质。肼是一种价格低廉的还原剂,且不会给产品带来难以剔除的杂质。所以,经常被用于还原制备不同的金属[15],目前,人们对其有关反应机理的研究较少。为此,本文作者在乙二醇中用肼做还原剂,在较低的温度下制备镍纳米粒子,并根据反应的现象及碱调节剂对反应的影响推断其反应的机理。
1 实 验
1.1 原材料
原材料为:NiCl2?6H2O, N2H4?H2O,乙二醇和NaOH,均为分析纯;实验所用水为去离子水。
1.2 合 成
在设定的温度下,先向三口烧瓶中加入乙二醇溶液,然后,将二氯化镍溶解于乙二醇溶液中,再加入适量的水合肼和1 mol/L NaOH溶液,剧烈搅拌一段时间后有黑色粒子形成。在反应的过程中,溶液的颜色由绿变紫,然后呈白紫色,最后变黑。在 60 ℃时强烈搅拌30 min,将溶液离心,黑色沉淀在80 ℃下空气中干燥2 h,待检测;往滤液中加入一定量的Na2CO3溶液,若无沉淀生成,则表明滤液中检不出Ni2+,说明反应进行完全。
1.3 表 征
利用D-5000 X射线衍射仪测定样品的相结构。扫描的条件是:Cu靶Kα,管电压为35 kV,管电流为30 mA,采用步进扫描方式进行测量,步长为0.02?,积分时间为0.2 s,扫描范围为20?~80?。
利用扫描电镜观察产物形貌,测试样本的制备方法是:首先将粉末加入到乙二醇溶剂中超声分散15 min,然后用滴管滴到铜片上,在室温下晾干后,喷上碳膜,利用JEOF-6010扫描电子显微镜进行 测试。
2 结果与讨论
2.1 结构分析
在乙二醇中用肼还原得到的样品的XRD光谱图如图1所示。3个特征峰(2θ=44.5?,51.8?和76.4?)分别对应镍的(111),(200)和(222)面的特征峰,这表明产物为面心立方结构的晶体镍。XRD图谱中没有出现NiO和Ni(OH)2的杂质峰,这说明用肼还原生成Ni不需要惰性气氛,也可能是反应本身有惰性气体放出而不再需要另外的惰性气体保护。
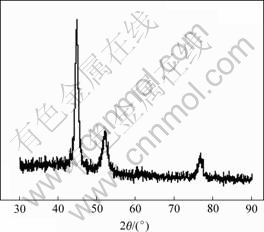
图1 制备得到的镍纳米粒子的XRD谱
Fig.1 XRD pattern of prepared Ni nanoparticles
2.2 形貌特征
分别用酒精,水,乙二醇和丙酮作溶剂分散Ni粒子,其SEM照片如图2所示。从图2可以看出,Ni粒子在乙二醇溶剂中分散最好(图2(c)),粒子粒径大约为25 nm。这说明不但可以在乙二醇中还原得到Ni纳米粒子,且Ni纳米粒子在乙二醇中分散性好。这可能是因为乙二醇含有较多的羟基,羟基与镍之间形成配位键,从而起到分散的作用,阻止粒子之间的团聚。
2.3 实验条件的确定
2.3.1 pH值调节剂的作用
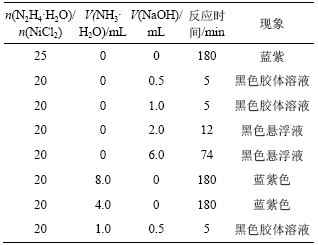
在碱性条件下,肼的电极电位小于Ni2+/Ni的电极电位,才能使Ni2+还原成Ni。因此,对pH调节剂的种类和用量的确定很重要。在50 mL乙二醇中加入0.2256 g NiCl2?H2O,分别用过量的肼、不同量的氨水和1 mol/L NaOH来调节溶液的pH值,所得的实验现象如表1所示。
从表1可知, 当增加肼的浓度或用氨水调节pH值到10以上时,得不到Ni纳米粒子。用1 mol/L NaOH调节pH值时,都可得到Ni纳米粒子。用NH3·H2O调节pH值时不能得到Ni纳米粒子的原因有2个:Ni2+与NH3形成比较稳定的[Ni(NH3)6]2+ 配合物,使反应体系的电极电势差减小,不利于反应向生成Ni的方向进行;NH3·H2O的碱性不够强,在NH3·H2O的调节下,肼不能还原Ni2+。加入不同量的NaOH,能得到不同粒径的Ni,随着NaOH加入量的增加,颗粒的粒径增大。这说明OH- 作为反应物,在粒子的成核和核生长过程中起关键作用。
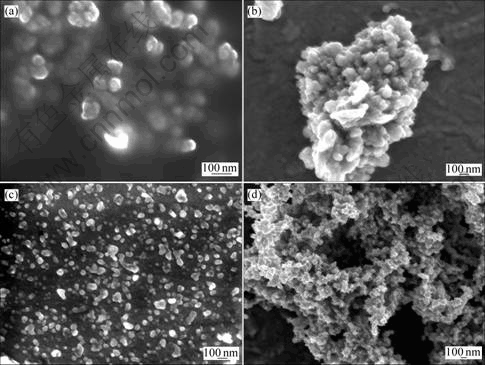
溶剂:(a) 酒精;(b) 水;(c) 乙二醇;(d) 丙酮
图2 用不同的溶剂分散得到的镍Ni纳米粒子的SEM图片
Fig.2 SEM images of Ni nanoparticles dispersed in different media
表1 采用不同的pH值调节剂时的实验现象
Table 1 Experiment phenomena of reaction by different pH adjusting reagents
根据以上2个现象,可以推测,在反应过程中,NaOH不仅起调节pH值的作用,还可能起催化作用,这与文献[16]报道的一致。
2.3.2 温度的影响
在不同的反应温度下需要的反应时间和得到的Ni粒子粒径不同,结果如图3所示。
当在室温下反应24 h时,溶液颜色保持蓝紫色,
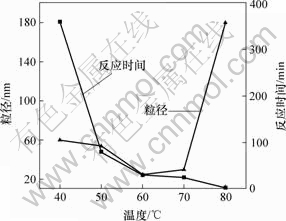
图3 温度对反应时间和产物粒径的影响
Fig.3 Effects of temperature on size of nickel particles and reaction time
得不到黑色物质;当反应温度超过40 ℃时,随着反应温度的升高,反应时间缩短,粒子变小;当温度高过60 ℃时 , 随着温度升高,反应速度加快, 粒子变大;当反应温度为80 ℃时,反应物放入之后,立即有黑色物质生成,很难控制反应的进行。这是因为随着温度的升高,反应物活性提高,成核速率加快,同时也减小了反应物的过饱和度,控制了核的生长,能快速地生成较小的粒子。随着温度的进一步升高,生成的粒子越容易团聚,导致粒径变大。根据以上分析,为了得到粒径小且分散性好的Ni纳米粒子,反应温度应控制在40~60 ℃。
2.4 Ni粒子的形成机理
实验结果表明:
a. 在60 ℃时,将二氯化镍加入到乙二醇中时,溶液呈绿色,无黑色物质形成,这说明,Ni2+ 是被肼还原的而不是被乙二醇还原的[16]。
b. 在其他条件相同的情况下,用不同的碱调节pH值到10以上,只有用NaOH时,才能形成Ni纳米粒子,这说明NaOH可能起催化作用。
c. 在空气气氛下生成的产物中没有镍的氧化物,这说明在反应进行过程中,可能有惰性气体生成,起到保护作用。
综合以上分析,还原过程可以表述如下:
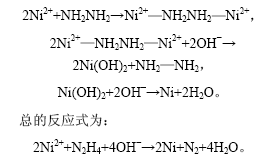
3 结 论
a. 在乙二醇溶液中,不需要惰性气体保护用肼还原可以制得Ni纳米粒子。
b. 在最佳的还原条件下:反应温度为60 ℃,用NaOH调节pH至10~11,得到的Ni粒子为体心立方结构,平均粒径为25 nm。
c. 在Ni纳米粒子的形成过程中, NaOH不仅起到调节pH值的作用, 还起催化作用。
参考文献:
[1] Ebner A D, Ritter J A, Ploehn H J. Magnetic hetero-flocculation of paramagnetic colloidal particles[J]. Journal of Colloid and Interface Science, 2000, 225: 39-46.
[2] Estournes C, Lutz T, Happich J. Nickel nanoparticles in silica gel: Preparation and magnetic properties[J]. Journal of Magnetism and Magnetic Materials, 1997, 173(1/2): 83-92.
[3] Jordan A, Scholz R, Wust P, et al. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible super paramagnetic nanoparticles[J]. Journal of Magnetism and Magnetic Materials, 1999, 201(1/3): 8-15.
[4] Wilcoxon J P, Provencio. Use of surfactant micelles to control the structural phase of nanosize iron clusters[J]. J Phys Chem B, 1999, 103(45): 91-94.
[5] Ermakova M A, Ermakov D Y. High-loaded nickel-silica catalysts for hydrogenation prepared by sol-gel route: Structure and catalytic behavior[J]. Applied Catalysis A: General, 2003, 245(2): 277-288.
[6] 李新军, 李芳柏, 古国榜, 等. 磁性纳米光催化剂的制备及其光催化性能[J]. 中国有色金属学报, 2001, 11(6): 971-975.
LI Xin-jun, LI Fang-bai, GU Guo-bang, et al. Preparation of nanometer-sized magnetic photo-catalysts and their photo-catalytic activity and properties[J]. The Chinese Journal of Nonferrous Metals, 2001, 11(6): 971-975.
[7] DUAN Ying-wen, LI Jian-gong. Structure study of nickel nanoparticles[J]. Materials Chemistry and Physics, 2004, 87: 452-454.
[8] Gruttner C, Teller J. New types of silica-fortified magnetic nanoparticles as tools for molecular biology applications[J]. Journal of Magnetism and Magnetic Materials, 1999, 194(1/3): 8-15.
[9] Battaghn G, Carnera A, Dons D R. Pulsed electron beam irradiation of nickel single crystals with silver overlayers[J]. Thin Solid Films, 1986, 145(1): 147-160.
[10] Xia B, Lenggoro I W, Okuyama K. Preparation of Ni particles by ultrasonic spray pyrolysis of NiCl2?H2O precursor containing ammonia[J]. J Mater Sci, 2001, 30: 1701-1705.
[11] TAO Xiao-jun, LI Zhi-wei, CHEN Hong-jie, et al. A new approach to synthesize nickel nanoparticles[J]. Wuli Huaxue Xuebao, 2005, 21(5): 569-572.
[12] 勾 华, 张朝平, 罗玉萍, 等. 微乳液和反相微乳液法在合成和制备纳米铁系化合物上的应用[J]. 贵州大学学报, 2001, 18(2): 143-145.
GOU Hua, ZHANG Chao-ping, LUO Yu-ping, et al. The applied of microemulsion and reverse microemulsion method in prepared for iron and iron series compounds[J]. Journal of Guizhou University, 2001, 18(2): 143-145.
[13] 沈宏芳, 陈文革, 谷臣清. 铁-镍纳米粉末的制备及表征[J]. 机械工程材料, 2005, 29(4): 31-33.
SHEN Hong-fang, CHEN Wen-ge, GU Chen-qing. Preparation and characterization of Fe-Ni nano-composite powders[J]. Materials for Mechanical Engineering, 2005, 29(4): 31-33.
[14] 王立平, 高 燕, 薛群基, 等. 电沉积镍纳米晶材料制备及性能[J]. 电镀与涂饰, 2004, 23(3): 1-2.
WANG Li-ping, GAO Yan, XUE Qun-ji, et al. Study on electrodeposition of graded Ni-Co nanocrystalline alloys[J]. Electroplating and Finishing, 2004, 23(3): 1-2.
[15] NI Xiao-min, SU Xiao-bo, ZHENG Hua-gui. Studies on the one-step preparation of iron nanoparticles in solution[J]. Journal of Crystal Growth, 2005, 275: 548-553.
[16] Wu S H, Chen D H. Synthesis and characterization of nickel nanoparticles by hydrazine reduction in ethylene hlycol[J]. Journal of Colloid and Interface Science, 2003, 259: 282-286.
收稿日期:2007-04-20;修回日期:2007-06-25
基金项目:国家自然科学基金资助项目(50501008)
作者简介:胡爱平(1973-),女,湖南宁乡人,博士研究生,从事纳米功能材料研究
通信作者:唐元洪,教授,博士生导师;电话:0731-8821778;E-mail: yhtang2000@163.com