DOI: 10.11817/j.issn.1672-7207.2017.01.006
合成体系中NO3-与HMT的物质的量比对AlOOH结构及催化性能的影响
吴旭1,阴青青1,黄艳丽1,杜亚丽1, 2,谢鲜梅1
(1. 太原理工大学 化学化工学院,山西 太原,030024;
2. 太原理工大学 煤科学与技术教育部和山西省重点实验室,山西 太原,030024)
摘要:采用HMT水热分解法,通过调控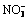 与HMT的物质的量比(n(
与HMT的物质的量比(n(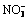 )/n(HMT))在不同合成体系下制备AlOOH催化剂,并就其在苯甲醛和乙醇一步合成安息香乙醚中的催化性能进行评价。通过XRD,FT-IR,TG-DTG,BET,TEM及NH3-TPD-MS对催化剂进行表征。研究结果表明:调控合成体系中n(
)/n(HMT))在不同合成体系下制备AlOOH催化剂,并就其在苯甲醛和乙醇一步合成安息香乙醚中的催化性能进行评价。通过XRD,FT-IR,TG-DTG,BET,TEM及NH3-TPD-MS对催化剂进行表征。研究结果表明:调控合成体系中n(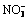 )/n(HMT)可以制备出结构和形貌不同的勃姆石。合成环境在构建AlOOH组成结构的基础上进而影响着其催化性能。取苯甲醛3 mL、乙醇40 mL、不同n(
)/n(HMT)可以制备出结构和形貌不同的勃姆石。合成环境在构建AlOOH组成结构的基础上进而影响着其催化性能。取苯甲醛3 mL、乙醇40 mL、不同n(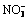 )/n(HMT)制备的AlOOH催化剂0.2 g,当反应温度为70 ℃,时间为40 min时,安息香乙醚的选择性均可达100%。高n(
)/n(HMT)制备的AlOOH催化剂0.2 g,当反应温度为70 ℃,时间为40 min时,安息香乙醚的选择性均可达100%。高n(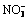 )/n(HMT)下制备的AlOOH能使苯甲醛的转化率达68.9%,低n(
)/n(HMT)下制备的AlOOH能使苯甲醛的转化率达68.9%,低n(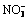 )/n(HMT)下制备的AlOOH仅使苯甲醛的转化率为32.3%,而调控n(
)/n(HMT)下制备的AlOOH仅使苯甲醛的转化率为32.3%,而调控n(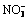 )/n(HMT)使最终合成体系为中性条件下制备的AlOOH几乎没有催化活性(苯甲醛的转化率仅为3.08%)。不同的催化性能与制备所得AlOOH结构和形貌密切相关,而AlOOH催化剂酸量的不同构建是催化差异的本质原因。
)/n(HMT)使最终合成体系为中性条件下制备的AlOOH几乎没有催化活性(苯甲醛的转化率仅为3.08%)。不同的催化性能与制备所得AlOOH结构和形貌密切相关,而AlOOH催化剂酸量的不同构建是催化差异的本质原因。
关键词:HMT水热分解;AlOOH;催化性能;苯甲醛;安息香乙醚
中图分类号:O643.3 文献标志码:A 文章编号:1672-7207(2017)01-0039-08
Effects of molar ratio of NO3- to HMT on structure and catalytic performances of AlOOH
WU Xu1, YIN Qingqing1, HUANG Yanli1, DU Yali1, 2, XIE Xianmei1
(1. College of chemistry and Chemical Engineering, Taiyuan University of Technology, Taiyuan 030024, China;
2. Key Laboratory of Coal Science and Technology of Education Ministry and Shanxi Province,
Taiyuan University of Technology, Taiyuan 030024, China)
Abstract: AlOOH were fabricated by hexamine (HMT) hydrolysis method through modulating the molar ratio of NO3- to HMT (n(NO3-)/n(HMT) and their catalytic performances toward synthesis of benzoin ethyl ether from benzaldehyde and ethanol were evaluated. Samples obtained at various molar ratio of 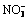 to HMT were characterized by XRD, FT-IR, TG-DTG, BET, TEM and NH3-TPD-MS. The results show that AlOOH obtained at different molar ratios of NO3- to HMT has different structures and morphologies. The further evaluation of catalytic activity demonstrates that ambient atmosphere can alter the structure of AlOOH, which has a significant effect on the catalytic behavior. All synthesized catalysts present 100% selectivity of benzoin ethyl ether under following reaction condition: temperature 70 ℃, benzaldehyde 3 mL, ethanol 40 mL, catalyst 0.2 g, reaction time 40 min, at higher molar ratio of
to HMT were characterized by XRD, FT-IR, TG-DTG, BET, TEM and NH3-TPD-MS. The results show that AlOOH obtained at different molar ratios of NO3- to HMT has different structures and morphologies. The further evaluation of catalytic activity demonstrates that ambient atmosphere can alter the structure of AlOOH, which has a significant effect on the catalytic behavior. All synthesized catalysts present 100% selectivity of benzoin ethyl ether under following reaction condition: temperature 70 ℃, benzaldehyde 3 mL, ethanol 40 mL, catalyst 0.2 g, reaction time 40 min, at higher molar ratio of 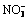 to HMT, AlOOH displays fine catalytic activity (68.9% conversion of benzaldehyde), while at lower molar ratio of NO3- to HMT a poor activity (32.3% conversion of benzaldehyde) is obtained and at neutral ambient atmosphere there is almost no catalytic activity (3.08% conversion of benzaldehyde). The totally different catalytic activity has close relation to the structures and morphologies of AlOOH synthesized under different pH values, and the amount of acid is responsible for the different catalytic performances.
to HMT, AlOOH displays fine catalytic activity (68.9% conversion of benzaldehyde), while at lower molar ratio of NO3- to HMT a poor activity (32.3% conversion of benzaldehyde) is obtained and at neutral ambient atmosphere there is almost no catalytic activity (3.08% conversion of benzaldehyde). The totally different catalytic activity has close relation to the structures and morphologies of AlOOH synthesized under different pH values, and the amount of acid is responsible for the different catalytic performances.
Key words: HMT hydrolysis; AlOOH; catalytic performances; benzaldehyde; benzoin ethyl ether
勃姆石(AlOOH)作为一种重要的含氧氢氧化物,已成为制备工业催化剂重要载体Al2O3的主要前驱体[1-4],因其结构性能决定了下游产品Al2O3的应用,现阶段围绕勃姆石结构和形貌的调控制备开展了大量研究。如HE等[5]以AlCl3和氨水为反应物,H2SO4为导向剂,通过调控H2SO4溶液的浓度,制备了一系列纳米棒状勃姆石。周秋生等[6]用碱式乙酸铝和氨水为前驱物,以水为反应介质,通过控制反应温度和反应时间调控制备出不同粒度和形貌的勃姆石。CAI等[7]研究了在Al(NO3)3·9H2O和尿素水溶液的反应体系下,借助不同的硫酸盐水热合成了不同形貌的勃姆石。陆光伟等[8]用十二烷基苯磺酸钠作为模板剂,以AlCl3和 NaOH为反应物,通过调变NaOH的量,合成了一维棒状和二维片状的AlOOH。YU等[9]将铝球加入到偏铝酸钠溶液中生成Al(OH)3,Al(OH)3微球经过水热处理自组装成各种花球状勃姆石。MATHIEU等[10]以 AlCl3·6H2O 和 NaOH 溶液在碱性条件下,以聚丙烯酸钠为尺寸/形貌控制剂,制备了圆球型的勃姆石粒子。在以往对勃姆石的研究中,大多提及的是关于制备条件和形貌控制方面的问题,而对勃姆石直接应用的研究则鲜有报道。为此,本文作者以Al(NO3)3·9H2O和HMT为原料,通过调控n( )/n(HMT)进而控制合成体系终点pH,在不同环境体系下制备不同形貌和结构的AlOOH;以苯甲醛与乙醇一步催化合成安息香乙醚为探针反应评价出不同环境体系下制备的AlOOH催化性能有显著差异;通过XRD,FT-IR,TG-DTG,TEM,BET,NH3-TPD-MS对催化剂进行表征分析,以揭示其催化性能差异的本质原因。
)/n(HMT)进而控制合成体系终点pH,在不同环境体系下制备不同形貌和结构的AlOOH;以苯甲醛与乙醇一步催化合成安息香乙醚为探针反应评价出不同环境体系下制备的AlOOH催化性能有显著差异;通过XRD,FT-IR,TG-DTG,TEM,BET,NH3-TPD-MS对催化剂进行表征分析,以揭示其催化性能差异的本质原因。
1 实验
1.1 材料准备
材料为:硝酸铝(Al(NO3)3·9H2O),分析纯,天津市恒兴化学试剂制造有限公司生产;六次甲基四胺(HMT),分析纯,天津市科密欧化学试剂有限公司生产;无水乙醇(CH3CH2OH),分析纯,天津市申泰化学试剂有限公司生产;苯甲醛(C7H6O),分析纯,天津市申泰化学试剂有限公司生产。
1.2 催化剂制备
调控 与HMT的物质的量比(n(
与HMT的物质的量比(n( )/n(HMT))分别为8:1,3:1和1:1;将0.1 mol/L的Al(NO3)3·9H2O和0.1 mol/L的HMT配制成混合溶液,剧烈搅拌0.5 h后置于100 mL干净不锈钢反应釜中,密封后放入烘箱,于140 ℃进行水热处理。12 h后取出并冷却至室温,抽滤、洗涤至中性,80 ℃干燥12 h,研磨待用。
)/n(HMT))分别为8:1,3:1和1:1;将0.1 mol/L的Al(NO3)3·9H2O和0.1 mol/L的HMT配制成混合溶液,剧烈搅拌0.5 h后置于100 mL干净不锈钢反应釜中,密封后放入烘箱,于140 ℃进行水热处理。12 h后取出并冷却至室温,抽滤、洗涤至中性,80 ℃干燥12 h,研磨待用。
所得催化剂的标记:n( )/n(HMT)=8:1时制备的催化剂记为C-81;n(
)/n(HMT)=8:1时制备的催化剂记为C-81;n( )/n(HMT)=3:1时制备的催化剂记为C-31;n(
)/n(HMT)=3:1时制备的催化剂记为C-31;n( )/n(HMT)=1:1时制备的催化剂记为C-11。
)/n(HMT)=1:1时制备的催化剂记为C-11。
1.3 催化剂表征
1.3.1 X线衍射分析(XRD)
用Rigaku/max-2500型X线粉末衍射仪(Cu Kα辐射源,λ=0.154 nm,40 kV,100 mA)连续扫描法测定,扫描范围2θ为5°~75°,扫描速度为8 (°)/min,步长为0.03°。
1.3.2 红外分析(FT-IR)
傅里叶变换红外光谱(FT-IR)通过KBr压片技术,利用BruckerVECTOR22光谱仪记录4 000~400 cm-1波数范围内功能基团的红外吸收谱图,扫描数为32次。
1.3.3 热重分析(TG-DTG)
利用南京大展机电研究所生产的STA-200型综合热分析仪测定催化剂的热分解行为。实验条件如下:样品用量为5~10 mg;纯度为99%的N2流量为 20 mL/min,采用程序升温加热至800 ℃,升温速率为 10 ℃/min。
1.3.4 比表面积分析(BET)
利用美国Micromeritics ASAP 2020型自动吸附仪进行测定。样品在测定前进行高真空100 ℃条件下脱气处理5 h,吸附剂为高纯氮。通过BET法分析比表面积,用BJH法分析孔径。
1.3.5 透射电镜(TEM)
利用JEOL JEM-2100F电子显微镜,在200 kV工作电压下观察产物的形貌。
1.3.6 NH3程序升温脱附(NH3-TPD-MS)
在天津先权生产的 TP-5000 型程序升温脱附仪进行,同时采用英国Hiden公司生产的QIC20质谱检测器检测尾气中质核比m/z=17的信号。
1.4 活性评价
将装有回流装置的三口烧瓶固定在集热式恒温磁力搅拌器上,加入3 mL苯甲醛、40 mL乙醇,加热至70 ℃后分别将0.2 g不同合成环境下制备的AlOOH催化剂加入其中。微量进样器抽取反应液进行气相色谱分析,每10 min取1次样。用瑞利集团色谱仪器中心气相色谱(GC)仪对产物进行定性检测,GC分析条件如下:色谱柱FFAP(30 m×0.32 mm×0.5 μm),载气为N2,流量为30 mL/min;柱温为180 ℃,气化室温度为200 ℃,FID检测,检测器温度为230 ℃,进样量为0.2 μL。
2 结果与讨论
2.1 催化剂的表征结果
2.1.1 XRD表征
图1所示为制备合成体系中不同n( )/n(HMT)下所得样品的XRD谱。由图1可知:不同n(
)/n(HMT)下所得样品的XRD谱。由图1可知:不同n( )/ n(HMT)下制备所得催化剂其衍射峰位置与AlOOH的PDF卡片JCPDS PDF No.832384相一致,谱图中未见其他杂质物相衍射峰存在,说明在上述条件下所得产物均为纯相AlOOH。差别之处在于不同合成体系n(NO3-)/n(HMT)下制备的AlOOH结晶度不同,C-81晶型比较弥散,半峰宽较大,结晶度较低,但随着 n(
)/ n(HMT)下制备所得催化剂其衍射峰位置与AlOOH的PDF卡片JCPDS PDF No.832384相一致,谱图中未见其他杂质物相衍射峰存在,说明在上述条件下所得产物均为纯相AlOOH。差别之处在于不同合成体系n(NO3-)/n(HMT)下制备的AlOOH结晶度不同,C-81晶型比较弥散,半峰宽较大,结晶度较低,但随着 n( )/n(HMT)逐渐减小,制备所得的C-11和C-31的衍射峰逐渐由宽变窄,强度有所增加,表明C-11和C-31晶型发育趋于完好,结晶度提高。
)/n(HMT)逐渐减小,制备所得的C-11和C-31的衍射峰逐渐由宽变窄,强度有所增加,表明C-11和C-31晶型发育趋于完好,结晶度提高。

图1 合成体系中不同n(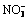 )/n(HMT)下所得AlOOH样品的XRD谱
)/n(HMT)下所得AlOOH样品的XRD谱
Fig.1 XRD patterns of samples synthesized under various molar ratios of 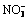 to HMT
to HMT
2.1.2 FT-IR表征
图2所示为制备合成体系中不同n(NO3-)/n(HMT)下所得AlOOH红外光谱图。由图2可知:合成体系中不同n( )/n(HMT)下产物的谱图均出现了AlOOH的典型特征吸收峰,文献[11-15]将图中的吸收峰归属于:3 109 cm-1处的肩峰对应(Al) O—H的对称伸缩振动;1 635 cm-1处的峰对应于游离水中O—H的弯曲振动;1 384 cm-1处的吸收峰很可能是胶体状的AlOOH表面吸附
)/n(HMT)下产物的谱图均出现了AlOOH的典型特征吸收峰,文献[11-15]将图中的吸收峰归属于:3 109 cm-1处的肩峰对应(Al) O—H的对称伸缩振动;1 635 cm-1处的峰对应于游离水中O—H的弯曲振动;1 384 cm-1处的吸收峰很可能是胶体状的AlOOH表面吸附 导致的;1 172 cm-1和1 069 cm-1处的峰分别归属于Al—O—H的不对称弯曲振动和 Al—OH 的对称弯曲振动峰;760,630和482 cm-1的吸收带分别对应于Al—O的扭曲振动、伸缩振动和弯曲振动,这是AlOOH晶体的骨架特征吸收峰,也可表明AlOOH晶体的形成。从图2可以看出:C-11和C-31的FT-IR谱图几乎没有差别,但C-81在1 384 cm-1处的吸收峰明显比C-11和C-31的强,这可能与C-81有更多的结构缺陷、配位不饱和等因素而导致阴离子在其表面吸附量不同有关,而通常认为较多的结构缺陷位会导致物质具有较大的酸量;同时3 279 cm-1处 (Al) O—H 不对称伸缩振动的特征吸收峰消失,而在3 400 cm-1附近出现了对应于游离水中O—H伸缩振动的新特征吸收峰。这表明催化剂C-81中含有较多游离水,而一般认为游离水的存在可使催化剂形成适当酸中心。
导致的;1 172 cm-1和1 069 cm-1处的峰分别归属于Al—O—H的不对称弯曲振动和 Al—OH 的对称弯曲振动峰;760,630和482 cm-1的吸收带分别对应于Al—O的扭曲振动、伸缩振动和弯曲振动,这是AlOOH晶体的骨架特征吸收峰,也可表明AlOOH晶体的形成。从图2可以看出:C-11和C-31的FT-IR谱图几乎没有差别,但C-81在1 384 cm-1处的吸收峰明显比C-11和C-31的强,这可能与C-81有更多的结构缺陷、配位不饱和等因素而导致阴离子在其表面吸附量不同有关,而通常认为较多的结构缺陷位会导致物质具有较大的酸量;同时3 279 cm-1处 (Al) O—H 不对称伸缩振动的特征吸收峰消失,而在3 400 cm-1附近出现了对应于游离水中O—H伸缩振动的新特征吸收峰。这表明催化剂C-81中含有较多游离水,而一般认为游离水的存在可使催化剂形成适当酸中心。

图2 合成体系中不同n(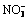 )/n(HMT)下所得AlOOH样品的FT-IR谱图
)/n(HMT)下所得AlOOH样品的FT-IR谱图
Fig. 2 FT-IR spectra of samples synthesized under various molar ratios of 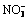 to HMT
to HMT
2.1.3 TG-DTG表征
对制备合成体系中不同n( )/n(HMT)下所得的AlOOH样品进行TG-DTG表征,结果如图3所示。从图3可以看出质量损失过程大致分为3个阶段[16-20]:第1阶段在70 ℃附近,对应于样品外表面吸附水的脱除;第2阶段质量损失温度约为200 ℃,可能是发生了孔道内外化学吸附水的脱除;随着温度继续升高,出现第3个质量损失峰,应该是样品开始失去结合水而向Al2O3转变。由图3可知:3种催化剂在低温段脱除吸附水时的温度没有表现出明显的差异,而在脱除结合水时的温度却不尽相同。C-81在 345 ℃附近失去结合水,而C-31和C-11在400 ℃附近才失去结合水。这种结果预示着C-81结构比较弥散容易失去结合水,而C-31和C-11晶型完整,层间结合水的结合力较强,所以,失去结合水所需温度较高,这与XRD表征结果相一致。
)/n(HMT)下所得的AlOOH样品进行TG-DTG表征,结果如图3所示。从图3可以看出质量损失过程大致分为3个阶段[16-20]:第1阶段在70 ℃附近,对应于样品外表面吸附水的脱除;第2阶段质量损失温度约为200 ℃,可能是发生了孔道内外化学吸附水的脱除;随着温度继续升高,出现第3个质量损失峰,应该是样品开始失去结合水而向Al2O3转变。由图3可知:3种催化剂在低温段脱除吸附水时的温度没有表现出明显的差异,而在脱除结合水时的温度却不尽相同。C-81在 345 ℃附近失去结合水,而C-31和C-11在400 ℃附近才失去结合水。这种结果预示着C-81结构比较弥散容易失去结合水,而C-31和C-11晶型完整,层间结合水的结合力较强,所以,失去结合水所需温度较高,这与XRD表征结果相一致。

图3 合成体系中不同n( )/n(HMT)下所得AlOOH样品的TG-DTG谱图
)/n(HMT)下所得AlOOH样品的TG-DTG谱图
Fig. 3 TG-DTG spectra of samples synthesized under various molar ratios of  to HMT
to HMT
2.1.4 N2物理吸附
图4所示为制备合成体系中不同n( )/n(HMT)下所得AlOOH样品的吸附-脱附等温线和孔径分布曲线(插入图)。根据IUPAC分类,不同条件下制备的AlOOH均为Ⅳ型吸附脱附等温线,并且滞后环形状都为H3型,说明3种催化剂都具有狭长裂口型的孔结构,且为宽孔,孔口窄而短。3种催化剂具体的织构性质见表1。从表1可以看出:C-81具有较大的比表面积,而C-31和C-11比表面积较小但孔径较大,这可能与二者的结晶度较高有关。
)/n(HMT)下所得AlOOH样品的吸附-脱附等温线和孔径分布曲线(插入图)。根据IUPAC分类,不同条件下制备的AlOOH均为Ⅳ型吸附脱附等温线,并且滞后环形状都为H3型,说明3种催化剂都具有狭长裂口型的孔结构,且为宽孔,孔口窄而短。3种催化剂具体的织构性质见表1。从表1可以看出:C-81具有较大的比表面积,而C-31和C-11比表面积较小但孔径较大,这可能与二者的结晶度较高有关。
表1 合成体系中不同n( )/n(HMT)下AlOOH样品的比表面积和孔容
)/n(HMT)下AlOOH样品的比表面积和孔容
Table 1 Special surface area of AlOOH samples synthesized under various molar ratios of  to HMT
to HMT
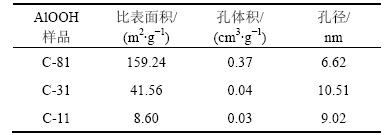
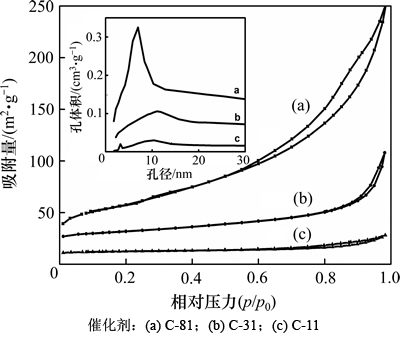
图4 合成体系中不同n( )/n(HMT)下AlOOH样品的吸附-脱附等温线和孔径分布图
)/n(HMT)下AlOOH样品的吸附-脱附等温线和孔径分布图
Fig. 4 N2 adsorption–desorption isotherms and pore size distribution (inset) of AlOOH samples synthesized under various molar ratios of  to HMT
to HMT
2.1.5 TEM表征
图5所示分别为C-81,C-31和C-11的TEM图。由图5可以看出:高n( )/n(HMT)下制备的AlOOH形貌呈现为纳米纤维状和条状;低n(
)/n(HMT)下制备的AlOOH形貌呈现为纳米纤维状和条状;低n( )/n(HMT)下制备的AlOOH形貌已经表现为纳米片状结构。据文献报道[21-24]:层片状AlOOH是由溶液中初始形成的Al(OH)3经过水解反应脱水形成,其层与层之间通过羟基上的氢键结合,因为合成溶液pH对层间氢键的结合有一定的影响,故pH会显著影响AlOOH的形貌。在酸性溶液中,由于H+能结合羟基和氧未共用电子对,破坏了AlOOH层间的氢键,从而促进了一维定向生长;而在碱性溶液中H+浓度不足以破坏层间氢键,所以导致产物形貌呈片状。
)/n(HMT)下制备的AlOOH形貌已经表现为纳米片状结构。据文献报道[21-24]:层片状AlOOH是由溶液中初始形成的Al(OH)3经过水解反应脱水形成,其层与层之间通过羟基上的氢键结合,因为合成溶液pH对层间氢键的结合有一定的影响,故pH会显著影响AlOOH的形貌。在酸性溶液中,由于H+能结合羟基和氧未共用电子对,破坏了AlOOH层间的氢键,从而促进了一维定向生长;而在碱性溶液中H+浓度不足以破坏层间氢键,所以导致产物形貌呈片状。

图5 不同n( )/n(HMT)下AlOOH样品的TEM图
)/n(HMT)下AlOOH样品的TEM图
Fig.5 TEM of samples synthesized under various molar ratios of  to HMT
to HMT
2.1.6 NH3-TPD-MS表征
对制备合成体系中不同n( )/n(HMT)下得到的AlOOH进行NH3-TPD-MS分析,见图6。这3种催化剂吸附峰面积积分计算所得酸量如表2所示。由图6和表2可以看出:不同合成体系n(
)/n(HMT)下得到的AlOOH进行NH3-TPD-MS分析,见图6。这3种催化剂吸附峰面积积分计算所得酸量如表2所示。由图6和表2可以看出:不同合成体系n( )/n(HMT)下制备的AlOOH均在150℃附近出现NH3的脱附峰,说明3种催化剂均存在弱酸位,且酸量表现出明显的差异,弱酸酸量由大到小顺序为C-81,C-11,C-31。这可能是由于高n(
)/n(HMT)下制备的AlOOH均在150℃附近出现NH3的脱附峰,说明3种催化剂均存在弱酸位,且酸量表现出明显的差异,弱酸酸量由大到小顺序为C-81,C-11,C-31。这可能是由于高n( )/n(HMT)下制备的AlOOH存在较多的缺陷位,从而导致其具有较多的酸量。
)/n(HMT)下制备的AlOOH存在较多的缺陷位,从而导致其具有较多的酸量。

图6 合成体系中不同n( )/n(HMT)下AlOOH样品的NH3-TPD-MS图
)/n(HMT)下AlOOH样品的NH3-TPD-MS图
Fig. 6 NH3-TPD-MS spectra of samples synthesized under various molar ratios of  to HMT
to HMT
表2 不同样品的NH3-TPD积分计算结果
Table 2 NH3-TPD integral calculations of different catalysts

2.2 催化剂活性评价结果
将所制备的C-81,C-31和C-11催化剂用于苯甲醛和乙醇生成安息香乙醚的一步催化反应中进行活性评价,70 ℃下回流40 min,苯甲醛的转化率和安息香乙醚的选择性结果如表3所示。由表3可知:在适宜反应条件下,尽管合成体系中不同n( )/n(HMT)下制备的催化剂对催化苯甲醛与乙醇合成安息香乙醚的反应均表现出一定的活性,但合成的C-81的催化活性要明显高于C-31和C-11的催化活性。出现这样的结果与催化剂拥有的不同酸量有直接关系,因C-81有更多的酸量,故具有更强的催化活性。而其酸量的不同与催化剂的结构息息相关,说明合成体系中不同n(
)/n(HMT)下制备的催化剂对催化苯甲醛与乙醇合成安息香乙醚的反应均表现出一定的活性,但合成的C-81的催化活性要明显高于C-31和C-11的催化活性。出现这样的结果与催化剂拥有的不同酸量有直接关系,因C-81有更多的酸量,故具有更强的催化活性。而其酸量的不同与催化剂的结构息息相关,说明合成体系中不同n( )/n(HMT)所营造的合成环境对催化剂结构有显著影响,而催化剂结构不同会导致其具有不同的酸量。基于优势催化剂的评选,下面考察C-81在不同反应条件下的催化性能。
)/n(HMT)所营造的合成环境对催化剂结构有显著影响,而催化剂结构不同会导致其具有不同的酸量。基于优势催化剂的评选,下面考察C-81在不同反应条件下的催化性能。
表3 不同n( )/n(HMT)下AlOOH样品对催化活性的影响
)/n(HMT)下AlOOH样品对催化活性的影响
Table 3 Catalytic activity of AlOOH samples synthesized under various molar ratios of  to HMT
to HMT

2.2.1 催化剂用量对反应的影响
取苯甲醛3 mL、乙醇40 mL,改变AlOOH(C-81)的用量,在70 ℃下反应40 min,考察该反应催化剂的适宜用量,结果见图7。由图7可知:该催化剂能有效促使苯甲醛的转化,不同用量催化剂下苯甲醛的转化率也有所差别,当添加0.2 g AlOOH催化剂时,苯甲醛转化率可达68.9%;继续增加催化剂投入量,苯甲醛转化率基本保持不变。故较适宜的催化剂用量为0.2 g。
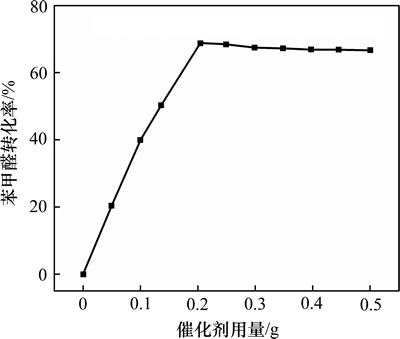
图7 催化剂用量对苯甲醛转化率的影响
Fig. 7 Effect of catalyst quality on reaction conversion
2.2.2 反应温度对反应的影响
取苯甲醛3 mL、乙醇40 mL、催化剂AlOOH(C-81) 0.2 g,设定反应时间为40 min来考察该反应的适宜温度,结果见图8。从图8可以看出:随着反应温度的升高,苯甲醛的转化率逐渐增加至趋于平衡。这应该与反应属于吸热反应、升高温度有利于反应向正方向移动有关。但当反应温度为80 ℃时,高于乙醇沸点后,反应平衡转化率的降低可能和乙醇沸腾挥发导致反应物减少有关。故本实验适宜的反应温度为70 ℃。
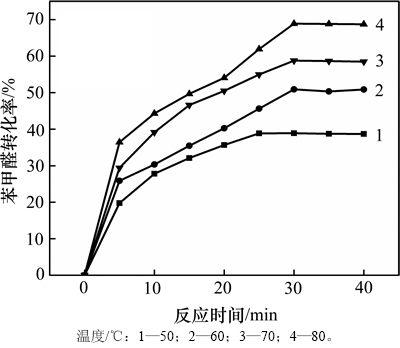
图8 反应温度对苯甲醛转化率的影响
Fig. 8 Effects of temperature on reaction conversion
2.2.3 反应时间对反应的影响
取苯甲醛3 mL、乙醇40 mL、催化剂AlOOH(C-81) 0.2 g,于70 ℃反应不同时间苯甲醛转化率的变化曲线如图9所示。由图9可知:反应开始后的30 min,苯甲醛的转化率迅速提高,而随后延长反应时间对苯甲醛转化率影响不大。估计这与反应 30 min左右即可达到平衡有关。所以,优选的反应时间为30~50 min,故本实验适宜的反应时间为40 min。

图9 反应时间对苯甲醛转化率的影响
Fig. 9 Effect of time on reaction conversion
2.3 催化剂稳定性评价结果
为了进一步了解AlOOH(C-81)的稳定性,对回收后重复使用5次的催化剂进行表征,结果如图10所示。从图10可以看出:重复使用5次后AlOOH催化剂依然能使苯甲醛高效的转化,具体为,前3次重复使用均能使苯甲醛转化率大于65%,第4次使用时转化率稍微下降至63.4%,重复使用第5次时苯甲醛的转化率才下降至62.5%,同时,在重复使用过程中安息香乙醚的选择性都近100%,这说明该催化剂有很强的稳定性。图11所示为该AlOOH催化剂重复使用后的XRD谱。从图11可以看出:催化剂除了结晶度的微弱变化外,都很好地保持了AlOOH的层状结构,催化剂反应前后结构基本不变是使其具备很优良重复利用性的保证。

图10 重复使用次数对苯甲醛转化率和选择性的影响
Fig. 10 Effects of reusability on reaction conversion and selectivity
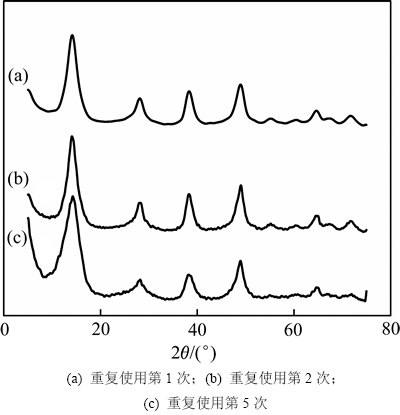
图11 重复次数对催化剂结构的影响
Fig. 11 Effects of reusability on catalyst structure
3 结论
1) 采用HMT水热分解法,通过调控 与HMT的物质的量比(n(
与HMT的物质的量比(n( )/n(HMT))制备出晶相单一,结构形貌各异的AlOOH样品。
)/n(HMT))制备出晶相单一,结构形貌各异的AlOOH样品。
2) 制备合成体系中不同n( )/n(HMT)所得的AlOOH在苯甲醛与乙醇一步合成安息香乙醚反应中的催化性能有显著差异。在适宜反应条件下,高n(
)/n(HMT)所得的AlOOH在苯甲醛与乙醇一步合成安息香乙醚反应中的催化性能有显著差异。在适宜反应条件下,高n( )/n(HMT)下制备的AlOOH样品可以很好地催化苯甲醛和乙醇反应制备安息香乙醚;在70 ℃,苯甲醛体积为3 mL,乙醇体积为40 mL,AlOOH用量为0.2 g,30 min后达到平衡后苯甲醛的转化率可达68.9%,且其拥有良好的重复利用性能,可循环多次使用。
)/n(HMT)下制备的AlOOH样品可以很好地催化苯甲醛和乙醇反应制备安息香乙醚;在70 ℃,苯甲醛体积为3 mL,乙醇体积为40 mL,AlOOH用量为0.2 g,30 min后达到平衡后苯甲醛的转化率可达68.9%,且其拥有良好的重复利用性能,可循环多次使用。
3) AlOOH结构形貌差异构建的酸量是影响催化活性差异的关键因素。高n( )/n(HMT)体系制备的AlOOH样品其晶型弥散,比表面积大,游离水含量高,呈纤维状;不同结构构建的酸量是催化活性差异的本质原因。
)/n(HMT)体系制备的AlOOH样品其晶型弥散,比表面积大,游离水含量高,呈纤维状;不同结构构建的酸量是催化活性差异的本质原因。
参考文献:
[1] CHEN Xiangying, ZHANG Zhongjie, LI Xueliang, et al. Controlled hydrothermal synthesis of colloidal boehmite (γ-AlOOH) nanorods and nanoflakes and their conversion into γ-Al2O3 nanocrystals[J]. Solid State Communications, 2008, 145(7/8): 368-373.
[2] RAYBAUD P, DIGNE M, IFTIMIE R, et al. Morphology and surface properties of boehmite (γ-AlOOH): a density functional theory study[J]. Journal of Catalysis, 2001, 201(2): 236-246.
[3] 杨燕敏.镁铝碳酸根类水滑石的制备及其形成机理研究[D]. 北京: 北京化工大学理学院, 2012: 1-63
YANG Yanmin. The synthesis and formation mechanism study of magnesium and aluminum carbonate layered double hydroxide[D]. Beijing: Beijing University of Chemical Technology. College of Science, 2012: 1-63.
[4] YANG Huaming, HU Yuehua, YANG Wuguo, et al. Alteration of diaspore by thermal treatment[J]. Journal of Central South University of Technology, 2004, 11(2): 173-175.
[5] HE Taobo, XIANG Lan, ZHU Shenlin. Hydrothermal preparation of boehmite nanorods by selective adsorption of sulfate[J]. Langmuir, 2008, 24(15): 8284-8289.
[6] 周秋生, 李小斌, 熊翔, 等. 一水软铝石纳米粉体的水热合成及表征[J]. 中国有色金属学报, 2001, 11(增刊): 206-209.
ZHOU Qiusheng, LI Xiaobin, XIONG Xiang, et al. Hydrothermal synthesis and characterizations of boehmite powder[J]. The Chinese Journal of Nonferrous Metals, 2001, 11(Suppl): 206-209.
[7] CAI Weiquan, YU Jiaguo, CHENG Bei, et al. Synthesis of boehmite hollow core/shell and hollow microspheres via sodium tartrate-mediated phase transformation and their enhanced adsorption performance in water treatment[J]. The Journal of Physical Chemistry C, 2009, 113: 14739-14746.
[8] 陆光伟, 杨琪, 邓意达, 等. 水热法制备一维纳米 γ-AlOOH的形态结构[J]. 无机材料学报, 2009, 24(3): 463-468.
LU Guangwei, YANG Qi, DENG Yida, et al. Fabrication of morphology of one-dimensional nano-γ-AlOOH via hydrothermal route[J]. Journal of Inorganic Materials, 2009, 24(3): 463-468.
[9] YU Xiaoxiao, YU Jiaguo, CHENG Bei, et al. Synthesis of hierarchical flower-like AlOOH and TiO2/AlOOH superstructures and their enhanced photocatalytic properties[J]. The Journal of Physical Chemistry C, 2009, 113(40): 17527-17535.
[10] MATHIEU Y, LEBEAU B, VALTCHEV V. Control of the morphology and particle size of boehmite nanoparticles synthesized under hydrothermal conditions[J]. Langmuir, 2007, 23(18): 9435-9442.
[11] KRISHNA PRIYA G, PADMAJA P, WARRIER K G K, et al. Dehydroxylation and high temperature phase formation in sol-gel boehmite characterized by Fourier transform infrared spectroscopy[J]. Journal of Materials Science Letters, 1997, 16(19): 1584-1587.
[12]  S. Hydrothermal crystallization of boehmite from freshly precipitated aluminium hydroxide[J]. Materials Letters, 1999, 40(6): 269-274.
S. Hydrothermal crystallization of boehmite from freshly precipitated aluminium hydroxide[J]. Materials Letters, 1999, 40(6): 269-274.
[13] TANG Zhe, LIANG Jilei, LI Xuehui, et al. Synthesis of flower-like Boehmite (γ-AlOOH) via a one-step ionic liquid-assisted hydrothermal route[J]. Journal of Solid State Chemistry, 2013, 202: 305-314.
[14] 熊飞, 王达健, 蒙延双, 等. 拟薄水铝石胶溶过程参数及胶团结构[J]. 材料与冶金学报, 2004, 3(3): 236-240.
XIONG Fei, WANG Dajian, MENG Yanshuang, et al. Peptizing process parameters and colloidal particle structure of pseudo-boehmite[J]. Journal of Materials and Metallurgy, 2004, 3(3): 236-240.
[15] 卫荣荣, 高志华, 郝树宏, 等. 不同制备方法对AlOOH结构和甲醇脱水性能的影响[J]. 化工学报, 2015, 66(6): 2112-2117
WEI Rongrong, GAO Zhihua, HAO Shuhong, et al. Effects of different preparation methods on structures and catalytic performances of AlOOH for methanol dehydration[J]. Journal of Chemical Industry and Engineering, 2015, 66(6): 2112-2117.
[16] 王永在, 范有莲. γ-AlOOH纳米晶的微波水热法制备及表征[J].人工晶体学报, 2009, 38(4): 930-933.
WANG Yongzai, FAN Youlian. Peparation and characterization of nanocrystals γ-ALOOH via microwave hydrothermal method[J]. Journal of Synthetic Crystals, 2009, 38(4): 930-933.
[17] SHAHEEN W M. Thermal solid–solid interactions and catalytic properties of V2O5/Al2O3 system treated with Li2O and Ag2O[J]. Materials Science and Engineering: B, 2006, 135(1): 30-37.
[18] HONG T L, LIU H T, YEH C T, et al. Electron microscopic studies on pore structure of alumina[J]. Applied Catalysis A: General, 1997, 158(1/2): 257-271.
[19] MEN Y, GNASER H, ZIEGLER C. Adsorption/desorption studies on nanocrystalline alumina surfaces[J]. Analytical and Bioanalytical Chemistry, 2003, 375(7): 912-916.
[20] 吴建锋, 徐晓虹, 张欣. 以硝酸铝为原料制备铝溶胶的研究[J].陶瓷学报, 2007, 28(3): 155-159.
WU Jianfeng, XU Xiaohong, ZHANG Xin. Study and preparation of aluminum sol from inorganic salt Al(NO3)3[J]. Journal of Ceramics, 2007, 28(3): 155-159.
[21] CAI Weiquan, YU Jiaguo, GU Shihai, et al. Facile hydrothermal synthesis of hierarchical boehmite: sulfate-mediated transformation from nanoflakes to hollow microspheres[J]. Crystal Growth & Design, 2010, 10(9): 3977-3982.
[22] HAO Baohong, FANG Keming, XIANG Lan, et al. Synthesization and crystallization mechanism of nano-scale γ-AlOOH with various morphologies[J]. International Journal of Minerals, Metallurgy, and Materials, 2010, 17(3): 376-379.
[23] HE Taobao, XIANG Lan, ZHU Shenlin. Different nanostructures of boehmite fabricated by hydrothermal process: effects of pH and anions[J]. Cryst Eng Comm, 2009, 11(7): 1338-1342.
[24] YANG Qi. Synthesis of γ-Al2O3 nanowires through a boehmite precursor route[J]. Bulletin of Materials Science, 2011, 34(2): 239-244.
(编辑 杨幼平)
收稿日期:2016-01-06;修回日期:2016-03-02
基金项目(Foundation item):化工资源有效利用国家重点实验室开放基金资助项目(CRE-2015-C-106);山西省自然科学基金资助项目(201601D102007) (Project(CRE-2015-C-106) supported by State Key Laboratory of Chemical Resource Engineering; Project(201601D102007) supported by the Natural Science Foundation of Shanxi Province, China)
通信作者:吴旭,博士,副教授,从事无机材料的调控制备及其催化应用研究;E-mail: wuxu@tyut.edu.cn