Ni(HCO3)2的制备及其在安息香乙醚合成中的催化应用
来源期刊:中国有色金属学报(英文版)2014年第5期
论文作者:吴 旭 安 霞 谢鲜梅
文章页码:1440 - 1445
关键词:Ni(HCO3)2;催化;绿色合成;安息香乙醚
Key words:Ni(HCO3)2; catalysis; green synthesis; benzoin ethyl ether
摘 要:应用尿素热解法合成晶相单一、结晶度较好的Ni(HCO3)2多孔材料,借助多种表征手段对其物化性能进行表征。N2吸附-脱附结果表明Ni(HCO3)2多孔材料有99.03 m3/g的比表面积、7.8 nm左右的孔径;扫描电镜 (SEM)和粒度分布结果说明Ni(HCO3)2微球的粒径均匀;热重分析结果表明500 °C是热解Ni(HCO3)2制备NiO的适宜温度;CO2-TPD和 NH3-TPD结果表明Ni(HCO3)2具有明显的酸碱双功能活性中心;透射电镜(TEM)结果表明Ni(HCO3)2颗粒是由六边形纳米片聚集而成的,且颗粒具有孔隙结构。将Ni(HCO3)2催化剂应用于苯甲醛与乙醇一步法合成安息香乙醚反应中,Ni(HCO3)2显示出优异的催化活性,反应平衡转化率高达57.5%,安息香乙醚的选择性近100%。
Abstract: Ni(HCO3)2 with unique phase and high crystallinity was synthesized with urea hydrolysis. The as-prepared samples were well characterized in detail. N2 adsorption and desorption result manifests a high surface area of 99.03 m2/g with a pore size of 7.8 nm. Scanning electron microscopy (SEM) and particle size distribution reveal that the diameters of the formed pellets are uniform. Thermogravimetry (TG) analysis result shows that 500 °C could be the appropriate temperature for converting Ni(HCO3)2 precursors into NiO via a thermal decomposition process. CO2 and NH3 temperature-programmed desorption results show that Ni(HCO3)2 has explicit acid-base sites. Transmission electron microscopy (TEM) results vividly indicate that the pellets are aggregated by hexagonal platelets and possess porous structures. Ni(HCO3)2 can efficiently catalyze the one-step synthesis of benzoin ethyl ether from benzaldehyde and ethanol, with the conversion of benzaldehyde up to 57.5% and nearly 100% selectivity of benzoin ethyl ether.
Trans. Nonferrous Met. Soc. China 24(2014) 1440-1445
Xu WU, Xia AN, Xian-mei XIE
College of Chemistry and Chemical Engineering, Taiyuan University of Technology, Taiyuan 030024, China
Received 20 May 2013; accepted 15 January 2014
Abstract: Ni(HCO3)2with unique phase and high crystallinity was synthesized with urea hydrolysis. The as-prepared samples were well characterized in detail. N2 adsorption and desorption result manifests a high surface area of 99.03 m2/g with a pore size of 7.8 nm. Scanning electron microscopy (SEM) and particle size distribution reveal that the diameters of the formed pellets are uniform. Thermogravimetry (TG) analysis result shows that 500 °C could be the appropriate temperature for converting Ni(HCO3)2 precursors into NiO via a thermal decomposition process. CO2 and NH3 temperature-programmed desorption results show that Ni(HCO3)2has explicit acid-base sites. Transmission electron microscopy (TEM) results vividly indicate that the pellets are aggregated by hexagonal platelets and possess porous structures. Ni(HCO3)2can efficiently catalyze the one-step synthesis of benzoin ethyl ether from benzaldehyde and ethanol, with the conversion of benzaldehyde up to 57.5% and nearly 100% selectivity of benzoin ethyl ether.
Key words: Ni(HCO3)2; catalysis; green synthesis; benzoin ethyl ether
1 Introduction
Considering the development trend of green fine chemical industry [1,2], an ever-increasing degree of interest has been focused on the application of heterogeneous catalysts [3] as well as the requirements for “green” technologies in the synthesis of fine chemicals and pharmaceuticals intermediates. With the development of nano-science and technology, nano-structured materials with special morphology have emerged and been applied as heterogeneous catalysts. Different porous morphologies can change the physical and chemical properties of materials, so the excellent functionality of porous structural material can be used in fine chemicals and environmental engineering [4].
Benzoin ethyl ether is a kind of practically valuable photoinitiators [5,6], and is generally synthesized by a two-step process from benzaldehyde and ethanol. First, benzaldehyde is condensated into benzoin in which cyanide is used as the catalyst [7]; second, ethanol and benzoin dehydrate and etherify to yield benzoin ethyl ether on the condition of acidity. In view of the low product yield and non-environmentally benign catalyst in the conventional synthesis procedures of benzoin ethyl ethers, seeking for suitable catalysts and developing clean benzoin ethyl ether synthesis process has been recognized to be one of the most important challenges in green chemistry.
In recent years, some research groups have successfully introduced the hydrotalcite-like compounds [8] as catalysts into the synthesis reaction of benzoin ethyl ether [9-11]. Although the hydrotalcite-like compounds showed fine catalytic performance, the yield of the product was relatively low, which may be due to the low content of B acid [12]. In addition, the preparation costs of the catalysts are relatively high. Driven by the economically and environmentally benign requirements, it is urgent to find an optimal catalyst for the reaction with high activity and selectivity. In view of this, herein, we synthesized benzoin ethyl ether using an environmentally benign heterogeneous catalyst Ni(HCO3)2. Studies with Ni(HCO3)2 as catalyst for the resultant reaction have, as far as we are aware, not yet been reported. In this procedure, not only the cyanide poisoning is avoided, but also the low-cost and green synthesis method of benzoin ethyl ether can be obtained in one step instead of the traditional two steps. This synthesis method may be suitable for the commercial production.
In the present work, Ni(HCO3)2was synthesized through the urea decomposition method, and the as-prepared samples were well characterized by X-ray diffraction, Fourier-transform infrared, particle size distribution, thermogravimetry analysis, N2adsorption/ desorption, scanning electron microscopy, transmission electron microscopy, CO2 and NH3 temperature- programmed desorption.
2 Experimental
2.1 Preparation of Ni(HCO3)2
All the reagents/reactants used were of analytical grade. The urea decomposition method was used to prepare Ni(HCO3)2 with a Ni(NO3)2·6H2O to C16H33(CH3)3NBr molar ratio of 10. Ni(NO3)2·6H2O and C16H33(CH3)3NBr were dissolved in deionized water, and then urea ([urea]/[NO3]- molar ratio of 3) was dissolved in the solution. The obtained slurry was aged for 30 min under vigorous stirring and then transferred into a Teflon-lined autoclave. The Teflon-line autoclave was heated at 110-150 °C to start the hydrolysis reaction. The reaction was maintained for 12-48 h and then cooled down itself. The formed solid was collected in a beaker, and the solution delaminated. The solid was then washed with deioned water until the pH became 7-8. The pellets were separated and subsequently dried at 80 °C for 12 h.
2.2 Characterization of Ni(HCO3)2
Powder X-ray diffraction patterns (XRD) of the synthesized products were recorded on Rigaku D/max-2500 instrument (40 kV, 100 mA) using Cu Kα radiation at a scanning speed of 8 (°)/min, with the scanning territory of 5°-60°.
The Fourier-transform infrared spectroscopy (FT-IR) spectra were recorded using the KBr pellet technique on a BruckerVECTOR22 spectrometer in 4000-450 cm-1 to identify functional groups present.
The size distribution of the product particles were measured using laser scattering particle size distribution analyzer (model LA-300).
Thermogravimetric analysis (TGA) was carried out on a SDT600 apparatus. Analysis was done from 20 to 700 °C at a heating rate of 10 °C/min under nitrogen (40 mL/min) for thermal stability analysis.
Specific surface areas and pore diameters of the synthesized products were measured in an automatic Micromeritics ASAP2020 by nitrogen adsorption. Before testing, the samples were outgassed at 100 °C and 101.325 kPa vacuum for 5 h in advance. The adsorbate was high purity nitrogen. The surface areas were determined by the BET method in the nitrogen adsorption data. The pore diameter and the pore size distribution were determined by the BJH method.
NH3-TPD or CO2-TPD experiment was performed on a TP-5000 instrument with a thermal conductivity detector (TCD). The catalyst adsorbed NH3 or CO2 at 50 °C until saturation and purged with helium for 30 min to remove the physisorbed NH3or CO2. The TPD data were collected in flow helium from 50 to 805 °C at a heating rate of 10 °C/min.
The morphologies and dimensions of the synthesized products were examined with a JEOL JSM-6700F field emission scanning electron microscope (FE-SEM).
Transmission electron microscope (TEM) was taken on a JEOL JSM-100F electron microscope, operating at 100 kV to observe the morphology and measure the sizes of the particles.
2.3 Catalytic activity tests of Ni(HCO3)2
A 250 mL three-necked flask, with installed condenser and thermometer, was fixed in a DF-101S magnetic force stirrer, then the required amounts of benzaldehyde, ethanol and Ni(HCO3)2 catalyst (pretreated at 100 °C under a stream of N2for 3 h) were added. Reaction was conducted at a constant temperature and normal pressure, and the reaction mixture was analyzed using an HP (C6890A=5973MSD) gas chromatograph equipped with a FFAP column (30 m×0.32 mm ×0.5 μm) and an FID detector [9].
3 Results and discussion
3.1 Structure and morphology
XRD analysis was adopted to analyze the crystal structure and phase composition of the obtained products [13]. The XRD pattern of the products obtained by urea hydrolysis method is shown in Fig. 1. In this case, diffraction peaks marked with digit are indexed to Ni(HCO3)2, which accord well with standard powder diffraction patterns (JCPDS#: 1520782). Besides, it is found that the temperature and time of the hydrothermal treatment are very important to the crystallinity of the products [8]. The phase of Ni(HCO3)2 could not be obtained with hydrothermal treatment conditions of 110 °C for 48 h. Furthermore, it is no better for the hydrothermal treatment condition of 120 °C for 12 h under which only poor crystallinity of the products could be obtained. However, when the hydrothermal treatment conditions are set at 150 °C and 12 h, Ni(HCO3)2 with fine crystallinity can be achieved. Moreover, the relative crystallinity of the product could not be significantly improved by prolonging the time of the hydrothermal treatment at 150 °C according to the comparison of patterns c and d in Fig. 1. Thus, the optimal temperature and time of the hydrothermal treatment were set at 150 °C and 12 h. No impurities could be detected in this pattern, which suggests that Ni(HCO3)2 with high purity can be obtained and characterized under the current synthesis conditions.

Fig. 1 XRD patterns of products at different hydrothermal conditions
IR analysis is not a diagnostic tool to study the structure of product, but it can be useful to identify the presence of foreign anions in the interlayer. In addition, information about the type of bonds formed by the anions and their orientations can also be obtained. Figure 2 displays the IR spectra of the samples prepared. Broad absorption bands around 3456 cm-1 can be attributed to —OH stretching vibrations of hydroxy groups. The band at 1543 cm-1 is mainly due to the H—OH bending vibration of physically adsorbed water. The four weak bands at 824, 1061, 1391, and 1459 cm-1 are characteristics of carbonate ions [14]. The presence of the υ1 band (1061 cm-1) and υ2 band (824 cm-1), together with the splitting of the υ3 vibration mode into two bands (1391 and 1459 cm-1), indicates a low symmetry (C2υ) of the 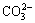 . The band at 680 cm-1 can be associated with Ni—O—H bending vibrations.
. The band at 680 cm-1 can be associated with Ni—O—H bending vibrations.

Fig. 2 FT-IR spectrum of Ni(HCO3)2
Figure 3 presents the results of particle size distribution. We can observe that the domain of the particle size distribution of the products is rather narrow and the median particle size of Ni(HCO3)2 is mainly located nearby 5 μm, which is mainly due to the influence of C16H33(CH3)3NBr on the characteristic of Ni(HCO3)2.
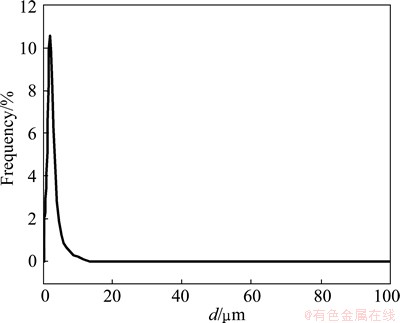
Fig. 3 Particle size distribution curve of Ni(HCO3)2
Thermogravimetry (TG) analysis was conducted towards Ni(HCO3)2 precursors in N2 atmosphere to investigate their thermal behaviors during the annealing process ranging from 20 to 700 °C [15]. As it can be seen in Fig. 4, the TG curve shows two successive decomposed stages corresponding to dehydration and decarboxylation processes, respectively. The mass losses mainly locate in two temperature regions. In the case of initial mass variation occurring in the temperature of 100-180 °C, the mass loss of 5% can be ascribed to the removal of chemically adsorbed and structural water. As for the subsequent mass change within the range of 320-480 °C, a sharp mass loss of 32.5% related to the decomposition of nickel carbonate hydroxide can be clearly noted in TG curve. When the temperature reaches 500 °C, the total mass loss is up to 37.5%, which is incompatible with the stoichiometric mass loss of 58.6% calculated from chemical reactions. It might be due to the influence of C16H33(CH3)3NBr to the characteristic of Ni(HCO3)2 and the exact cause is still under investigation. When temperature elevating over 500 °C, the TG curve becomes flat, which implies that no further mass loss occurs. So we can conclude that 500 °C could be the proper temperature for converting Ni(HCO3)2 precursors into NiO via thermal decomposition process.

Fig. 4 TG–DTG plots of Ni(HCO3)2
N2 adsorption/desorption was studied and the results are given in Fig. 5. As it is seen in Fig. 5, the specific surface area measured for Ni(HCO3)2 is 99.03 m2/g and the average pore size is 7.8 nm with pore volume (v) of 0.21 cm3/g. Based on the classification by IUPAC [16,17], the isotherm of the sample is of type IV. The plateau at high p/p0 and the low N2 adsorption at lower p/p0 value are characteristics of IV. The sample presents a H3-type hysteresis loop according to the classification of IUPCA, which represents a typical material with slit-shaped pores or aggregates of platelet-like particles. Therefore, the specific surface area and pore size make the materials attractive for catalytic application.

Fig. 5 N2adsorption–desorption isotherms and pore size distributions (inset) of Ni(HCO3)2
Figure 6 displays the CO2-TPD and NH3-TPD of Ni(HCO3)2 catalyst. There is one peak (around 400 °C) on the profiles, which suggests the existence of middle strength acid and base sites on the catalyst.
The morphology of the obtained Ni(HCO3)2was characterized using a scanning electron microscope (SEM) and transmission electron microscope (TEM) [18]. The morphology of Ni(HCO3)2is spherical and the diameters are uniform, which can be observed distinctly from SEM image in Fig. 7(a). The TEM image in Fig. 7(b) reveals that the pellets were aggregated by hexagonal platelets, but not an entire or single crystalloid. Therefore, the pellets possess pore structures. Anyway, the appearance of such a characteristic morphology should be critical for practical applications.

Fig. 6 CO2-TPD (a) and NH3-TPD (b) of Ni(HCO3)2
3.2 Structure and morphology of calcined Ni(HCO3)2pellets
With the hydroxyl and carbonate groups removed from Ni(HCO3)2 precursors, NiO crystal phase is expected to be formed [19]. It is interesting to note that the obtained samples are still loose spherical after the Ni(HCO3)2 pellets were calcined at 500-800 °C for 2 h. Under the optimal conditions, the diameters of the nickel oxide microspheres are uniform and approximate 5 μm and the specific surface area for NiO is as high as 748 m2/g. Such large surface area is expected of great interest in catalysts and catalyst supports. Details will be reported subsequently.
3.3 Catalytic performance of Ni(HCO3)2
Based on the above method, Ni(HCO3)2 was acquired on the most optimal preparation conditions and used in the synthesis reaction of benzoin ethyl ether via benzaldehyde to evaluate their catalytic performance. The conditions of the reaction were as follows: the dosage of Ni(HCO3)2 catalyst (after pretreatment with N2 blowing for 2 h) of 0.1 g, benzaldehyde of 3 mL and ethanol of 30 mL, reaction temperature of 60 °C and reaction time of 60 min. Figure 8 exhibits the chromatogram analysis of the reaction mixture when the reaction performed for 30 min. It can be seen from the chromatogram that the constituent peaks can be respectively ascribed to ethanol, benzaldehyde and benzoin ethyl ether from left to right. It can be easily confirmed that benzoin ethyl ether was synthesized efficiently from benzaldehyde and ethanol with Ni(HCO3)2 as the catalyst. In terms of catalytic performance, the equilibrium conversion of benzaldehyde was up to 57.5% and the selectivity of benzoin ethyl ether was nearly 100%. Besides, the catalyst could be recycled in the process of synthesis.

Fig. 7 SEM (a) and TEM (b) images of Ni(HCO3)2
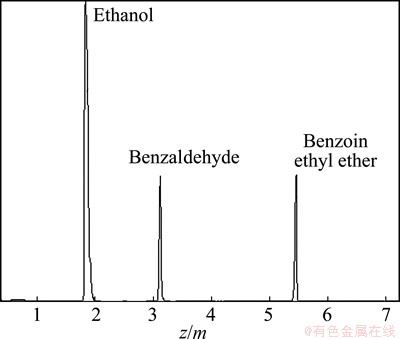
Fig. 8 Chromatogram of reaction system of ethanol and benzaldehyde
The reason why benzaldehyde and ethanol, catalyzed by Ni(HCO3)2, can generate benzoin ethyl ether may be of the porous structure and appropriate acid-base sites of the catalyst [20]. The possible reaction routine is outlined in Fig. 9. As to this sequence of steps, firstly, Ni(HCO3)2 complex is supposed to be bonded with two ethanol molecules in succession by coordinated bond, where cis-C6H14NiO8 formed; secondly, cis-C6H14NiO8 is linked with C6H5CHO by hydrogen bond and meanwhile a transition state appeared by rearrangement; thirdly, C6H5CHOC2H5OH is obtained as a second transition state; finally, C6H5CHOC2H5OH and C6H5CHO, undergoing aldol condensation invent a new complex by hydrogen bond, that is, the object product is acquired. These analyses provide a background for the subsequent discussion of proposed reaction mechanisms and kinetic models for this catalytic procedure. A more detailed investigation into the catalytic mechanism of this reaction will be covered in the subsequent study.
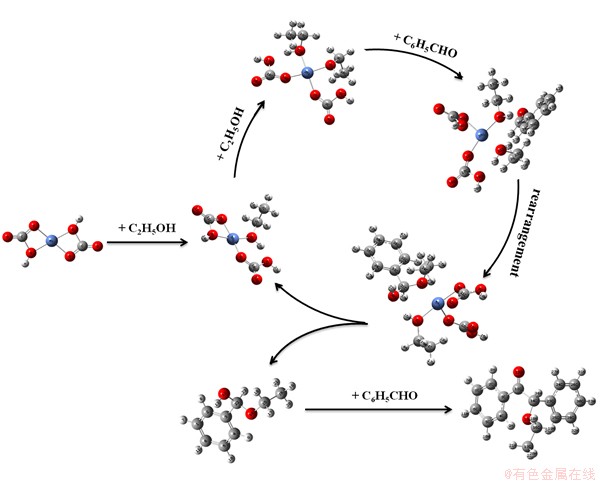
Fig. 9 Possible reaction routine
4 Conclusions
1) This work illustrated an easy and economical way for Ni(HCO3)2pellets production. The as-prepared samples were well characterized and the result vividly demonstrated that Ni(HCO3)2 possessed porous structure, explicit acid-base sites, uniform particle size distribution and high thermal stability.
2) As a green and economic catalyst, Ni(HCO3)2 was introduced into the synthesis reaction of benzoin ethyl ether where the conversion of benzaldehyde was up to 57.5% and the selectivity of benzoin ethyl ether was nearly 100%. In addition, the catalyst could be recycled.
References
[1] SHANG Dui-cai, TONG Zhong-liang. Green synthesis technology and example of fine chemicals [M]. Beijing: Chemical Industry Press, 2011. (in Chinese)
[2] MURUGAVELU M, KARTHIKEYAN B. Synthesis characterization and evaluation of green catalytic activity of nano Ag-Pt doped silicate [J]. Journal of Alloys and Compounds, 2013, 547: 68-75.
[3] CAVANI F, TRIFIRO F, VACCARI A. Hydrotalcite-type anionic clays: Preparation, properties and applications [J]. Catal Today, 1991, 11: 173-177.
[4] ZHAO Bin, KE Xiao-kang, BAO Jian-hua, WANG Chun-ling, DONG Lin, CHEN Yu-wen, CHEN Hui-lan. Synthesis of flower-like NiO and effects of morphology on its catalytic properties [J]. J Phys Chem C, 2009, 113: 14440-14447.
[5] BAI Xin-dei, ZHA Ping, YIN Ying-wu. Research status of UV curing coatings [J]. Journal of Tsinghua University: Science and Technology, 2001, 41(10): 30-32. (in Chinese)
[6] XU Bo-gang. Synthesis benzoin ethyl ether: China, 200810152003 [P]. 2010-05-26. (in Chinese)
[7] XU Li-wen, GAO Yang, YIN Jian-jun. Efficient and mild benzoin condensation reaction catalyzed by simple 1-N-alkyl- 3-methylimidazolium salts [J]. Tetrahedron Letters, 2005, 46(32): 5317-5320.
[8] PISKOVA A, BEZDICKA P. High-temperature X-ray powder diffraction as a tool for characterization of smectites, layered double hydroxides, and their intercalates with porphyrins [J]. Applied Clay Science, 2010, 49(4): 363-371.
[9] XIE Xian-mei, YAN Kai, WU Xu. Efficient synthesis of benzion methylether catalyzed by hydrotalcite containing cobalt [J]. Cataysis Communications, 2008, 6: 1128-1131.
[10] XIE Xie-mei, AN Xia, YAN Kai, WU Xu. A new way to synthesize benzoin isopropyl ether on Cu-Fe-hydrotalcite [J]. Natural Gas Chemistry, 2010, 19(1): 77-80.
[11] XIE Xian-mei, CHENG Shu-yan, WU Xu, LIAO Jia-you. Synthesis of NiAl-HTLcs/ZSM-5 composite and its application in benzoin ethyl ether [J]. Reaction Integrated Ferroelectrics, 2011, 129: 18-24.
[12] LEI Xiao-dong, ZHANG Fa-zhi, YANG Lan, TIAN Yuan-yuan, FU Shan-shan, LI Feng, EVANS D G, DUAN Xue. Highly crystalline activated layered double hydroxides as solid acid-base catalysts [J]. AIChE, 2007, 53: 932-940.
[13] YAN Kai, WU Xu, AN Xia, XIE Xian-mei. Facile synthesis and catalytic properity of spinel ferrity by a template method [J]. Alloys and Compounds, 2013, 552: 405-408.
[14] MORANDEIRA A, BOSCHLOO G, HAGFELDT A, HAMMARSTROM L. Coumarin 343-NiO films as nanostructured photocathodes in dye-sensitized solar cells: Ultrafast electron transfer, effect of the I-3(-)/I- redox couple and mechanism of photocurrent generation [J]. J Phys Chem C, 2008, 112: 9530-9535.
[15] TAN Xiao-yan, LI Gui-ying, ZHAO Ying, HU Chang-wei. Effect of preparation method on the surface properties and activity of Ni07Cu0.3Fe2O4 nanoparticles [J]. Journal of Alloys and Compounds, 2010, 493: 55-63.
[16] JIAO Qing-ze, LIU Hong-bo, ZHAO Yun, ZHANG Zhe. Preparation of Cu/Cr hydrotalcite-like compounds [J]. J Mater Sci, 2009, 44: 4422-4428.
[17] GUO Qun, ZHANG Zhi-zhi, ZHANG Xi-wen, LING Fei-xiang, SUN Wan-fu, LI Rui-feng, XIE Ke-chang. Preparation and characterization of mesoporous silica-pillared montmorillonite [J]. J Porous Mater, 2009, 16: 209-213.
[18] ZHU Qi, LI Ji-guang, LI Xiao-dong, SUN Xu-dong. Selective synthesis and shape-dependent photoluminescence properties of (Y0.95Eu0.05)2O3 submicron spheres and microplates [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 2471-2476.
[19] ZENG Wen, MIAO Bin, LIN Li-yang, XIE Jin-yue. Facile synthesis of NiO nanowires and their gas sensing performance [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(S1): s100-s104.
[20] RHODES C J. Zeolite mediated reactions: Mechanistic aspects and environmental application [J]. Progress in Reaction Kinetics and Mechanism, 2008, 33(1): 1-79.
吴 旭,安 霞,谢鲜梅
太原理工大学 化学与化工学院,太原 030024
摘 要:应用尿素热解法合成晶相单一、结晶度较好的Ni(HCO3)2多孔材料,借助多种表征手段对其物化性能进行表征。N2吸附-脱附结果表明Ni(HCO3)2多孔材料有99.03 m3/g的比表面积、7.8 nm左右的孔径;扫描电镜 (SEM)和粒度分布结果说明Ni(HCO3)2微球的粒径均匀;热重分析结果表明500 °C是热解Ni(HCO3)2制备NiO的适宜温度;CO2-TPD和 NH3-TPD结果表明Ni(HCO3)2具有明显的酸碱双功能活性中心;透射电镜(TEM)结果表明Ni(HCO3)2颗粒是由六边形纳米片聚集而成的,且颗粒具有孔隙结构。将Ni(HCO3)2催化剂应用于苯甲醛与乙醇一步法合成安息香乙醚反应中,Ni(HCO3)2显示出优异的催化活性,反应平衡转化率高达57.5%,安息香乙醚的选择性近100%。
关键词:Ni(HCO3)2;催化;绿色合成;安息香乙醚
(Edited by Hua YANG)
Foundation item: Project (50872086) supported by the National Natural Science Foundation of China; Project (2012021006-3) supported by the Natural Science Foundation of Shanxi Province, China; Project (2012L022) supported by Special/Youth Foundation of Taiyuan University of Technology, China; Project (20120321033-02) supported by Science and Technology Research of Shanxi Province, China
Corresponding author: Xian-mei XIE; Tel: +86-351-6018564; E-mail: s2003707@163.com
DOI: 10.1016/S1003-6326(14)63210-6

