偏氟乙烯/三氟氯乙烯共聚物的合成及其微观结构
李衡峰1, 2,岳春波1,边 宏1
(1. 中南大学 材料科学与工程学院,湖南 长沙,410083;
2. 中南大学 粉末冶金国家重点实验室,湖南 长沙,410083)
摘 要:
摘 要:利用1, 1, 2-三氟三氯乙烷(F113)作溶剂,通过自由基聚合得到不同配比偏氟乙烯与三氟氯乙烯的共聚物,利用NMR,FTIR,GPC和元素分析对共聚物进行表征和讨论。对共聚物的19F NMR 结构进行分析、对比。研究结果表明:—CH2CF2CF2CFClCH2CF2—结构和—CF2CFClCF2CFClCF2—结构为随反应物三氟氯乙烯(CTFE)的增加主要的结构增长成分,增速是其他结构增长速度的2倍; F113的使用提高了共聚物中的CTFE的含量;该种反应中少量的CTFE-CTFE结构中存在明显的晶区。
关键词:
中图分类号:TQ050.4+25 文献标识码:A 文章编号:1672-7207(2009)02-0362-05
Preparation and microstructure of poly(vinylidenefluoride-co-chlorotrifluoroethylene)
LI Heng-feng1, 2, YUE Chun-bo1, BIAN Hong1
(1. School of Materials Science and Engineering, Central South University, Changsha 410083, China;
2. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China)
Abstract: A series of copolymers of different CTFE/VDF ratio were synthesized through radical polymerization using 1, 1, 2-Trichloro-1, 2, 2-Trifluoroethane as the solvent, the microstructures were studied through NMR, GPC, FTIR and element analysis to confirm the effect of the VDF/CTFE ratio on the structure. The analysis of 19F NMR to the different copolymers indicates that the structures of —CH2CF2CF2CFClCH2CF2— and —CF2CFClCF2CFClCF2— are the main increasing structure with the increase of reagent of CTFE. The using F113 as solvent of the copolymerization increases the content of CTFE in the copolymer, and the amplitude is two times of the amount of the theoretic. There are CTFE-CTFE crystal regions in the copolymer.
Key words: P(VDF-CTFE); microstructure; copolymer; polymerization
聚偏氟乙烯三氟氯乙烯(P(VDF-CTFE))是第2代氟聚合物的一种。氟原子具有较低的极化率、较强的电负性、较小的范德华半径(0.132 nm)和很强的C—F键能(485 kJ/mol),并具有优良的耐候性、优异的表面特性、光学性能和电学性能[1],与第1代PVDF相比,单轴拉伸的P(VDF-CTFE)具有少量的α相晶体,具有更低的残余极化区域[2]。楚宝林等[3]指出,低CTFE含量的P(VDF-CTFE)具有较高的偶极密度和能量密度,其形成的膜具有非常快的放电速度和较低的损耗,这使得P(VDF-CTFE)的应用更具有现实意义。高CTFE含量的P(VDF-CTFE)主要用作聚合物粘结炸药的粘结剂和电池的集电体金属的粘结剂[4]。
P(VDF-CTFE)经过部分氢化得到P(VDF-TtrFE- CTFE),是一种优异的铁电高分子聚合物。利用此方法可以根据氢化程度来调整结晶度、居里温度和介电常数,从而有效降低居里温度,提高铁电性能[5-8]。
对于P(VDF-CTFE)的微观结构,Benedetti等[9]对P(VDF-CTFE)共聚物中微观结构中形成的晶体进行了分析,得出840 cm-1处的峰值减少主要是CTFE含量增加而影响共聚物中片晶的形成;付海涛等[10]对P(VDF-CTFE)的结晶性能进行了研究,得到了其形成片晶的条件,制备出厚度为4.68 nm的片晶。但目前还没有关于P(VDF-CTFE)共聚微观结构的报道,本文作者对此进行讨论。
1 实 验
1.1 原料试剂
原料试剂为:偏氟乙烯(工业品,上海有机化学研究所);三氟氯乙烯(工业品,上海有机化学研究所);过氧化苯甲酰(BPO,分析纯,常熟金城化工)使用前用氯仿和甲醇进行重结晶、干燥得到;1, 1, 2-三氟三氯乙烷(F113分析纯,上海五联化工厂)。
1.2 共聚物的合成
合成反应式为:

称取1.21 g BPO加入到盛有F113(400 mL)的 1 L高压反应釜中,封闭反应釜,用干冰浴将其冷却后抽真空,通入25 g三氟氯乙烯后,再通入420 g偏氟乙烯,升温至室温,加热到80 ℃反应7.5 h后,降温到室温,放掉未反应气体,打开反应釜,取出溶液中的无色沉淀析出物,在真空干燥箱内于60 ℃干燥4 h,最终得到40.8 g白色橡胶状产物(产率为9.2%)。采用GPC测其相对分子质量为![]() =123 927;
=123 927;![]() = 211 741;
= 211 741;![]() = 1.71。
= 1.71。
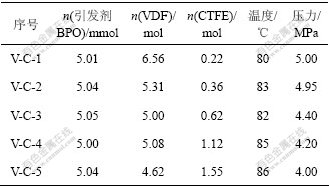
对于低配比的共聚物的共聚[11],初步选定表1中不同原料配比和条件进行聚合反应,其他反应条件与V-C-1反应条件相同。得到一系列配比的VDF-CTFE共聚物,经分子量测定其相对分子质量都为5万~20万,相对分子质量分布系数为1.5~1.8。
表1 不同配比原料的共聚反应
Table 1 Different inverse proportion of reagent to the copolymerization
1.3 共聚物结构表征
将所得共聚物溶解于丙酮中,涂于KBr盐片,用红外灯照射除去溶剂,用BIO-RAD FT-185红外光谱仪进行测试;利用HERAEUS 1106元素分析,通过单柱离子色谱方法[12]对共聚物中的F和Cl元素含量进行分析;利用氘代丙酮作溶剂在BRUKER DPX-300核磁共振仪上进行19F NMR测试;以DMF为溶剂在PERKINELMER 200Q GPC色谱仪上测定其相对分子质量及其分布。
2 结果与讨论
2.1 共聚物的组成
在VDF和CTFE自由基聚合体系中,由于CTFE分子结构更容易被引发,在聚合反应初期,BPO分解成为自由基首先引发CTFE后进行链增长反应,在高CTFE含量的反应体系中,反应更容易进行。其竞 聚率为rVDF=0.70;rCTFE=0.72[13],2种单体更趋于生 成共聚物,由于rVDF?rCTFE=0.504,存在恒比点,恒比点在fCTFE=0.483,共聚物主要为无规共聚结构。共
聚物中单体的配比根据自由基共聚体系![]() 进行计算。
进行计算。
2.2 共聚物微观结构的表征
由19F NMR对共聚物的微观结构进行分析, 19F NMR更能够说明其结构,对应的19F NMR如图1和图2所示。其中,VDF-VDF结构主要出现在-92.70×10-6,-93.80×10-6~-98×10-6,-115.14×10-6和-117.46×10-6[14];CTFE-CTFE结构主要出现在-106.19×10-6~-109.16×10-6和-109.76×10-6~ -113.00×10-6[15];在表2中,可以明显看到共聚物V-C-5的主要结构是CTFE-VDF的尾尾结构和CTFE-CTFE-VDF的头尾尾结构,表明CTFE与VDF反应中更易于形成CTFE-VDF尾尾结构。

图1 VDF-CTFE共聚物V-C-5的19F NMR谱
Fig.1 19F NMR spectra of VDF-CTFE copolymer V-C-5
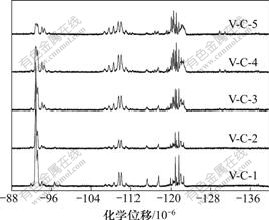
图2 不同配比VDF-CTFE共聚物的19 F NMR谱
Fig.2 19 F NMR spectra of synthesized VDF-CTFE copolymer series
图2所示为VDF-CTFE不同配比产物的19F NMR对比结果。由不同比例反应物生成共聚物的微观结构
表2 不同VDF-CTFE共聚物的19F NMR峰位结构及其比例
Table 2 Chemical shifts and assignments of 19F NMR peaks of P(VDF-CTFE) copolymer

可以得到,随着反应物中CTFE含量的增长,共聚物中VDF-CTFE头尾相连和VDF-CTFE-VDF尾尾头相连的结构由无到有,在-116.18×10-6和-116.67×10-6出现峰,积分面积随CTFE增加而增加,而-121.63× 10-6~-123.10×10-6之间的峰随反应物CTFE的增加,其积分面积增大;相反,-92.81×10-6主峰高度降低,积分面积明显减少,此为VDF-VDF头尾结构中的氟。其中,随着反应物CTFE含量的增加,VDF-VDF头尾、CTFE-VDF尾尾和VDF- VDF尾尾结构成分明显降低;相反,增大的结构部分为CTFE-CTFE和VDF-CTFE的各种结构,其中成分增幅较大的为CTFE-CTFE头尾、CTFE-CTFE-VDF头尾尾、VDF-CTFE尾尾和CTFE-VDF尾尾。可见,在低反应物CTFE含量的反应中,随着CTFE含量的增加,更容易形成—CH2CF2CF2CFClCH2CF2—结构和CTFE-CTFE头尾结构,CTFE-CTFE结构增幅不大。在反应物中,当CTFE含量为10%左右时,共聚物中CTFE-CTFE结构就开始了较大的增幅。
2.3 F113的使用对微观结构的影响
根据元素分析得到各样品的Cl含量,分别为11.7%,10%,13.28%,13.9%和16.2%,由于在溶液自由基反应中,rVDF=0.70,rCTFE=0.72,存在恒比 点,恒比点在fCTFE=0.483,反应体系中CTFE的含量低于恒比组分,产物中的反应单体的含量主要是根据
![]() 进行计算,得到共聚物中
进行计算,得到共聚物中
CTFE的含量低于fCTFE,理论上,共聚物CTFE含量为:4.37%,8.36%,14.08%,22.26%和28.64%,对应的共聚物的理论含氯量为:2.34%,4.34%,7%,10.44%和12.87%,产物中的氯含量均高于理论值,是理论值的2倍。其主要原因是:F113是制备CTFE的原材料,对CTFE具有更大的溶解度,促使实际参加反应的CTFE单体浓度比理论值大,在CTFE含量较小时,此影响更为明显,其增加幅度更大。
2.4 共聚物的红外光谱研究
选P(VDF-CTFE)-2进行FTIR测试,所得结果如图3所示。其中:在3 024,1 427和1 399 cm-1处的峰为分子结构中不同形态—CH2—的吸收峰;在 1 168 cm-1处的峰宽度大,为—CF2CH2CFCH2CF2—伸缩振动和—CF2CFCl-CF2CFCl—反对称及对称的吸收峰,由于峰位相近,且后者结构成分少,合并为一宽峰,主要为—CF2CF2CFCH2CF2—的伸缩振动特征吸收峰;在884 cm-1处的峰为—CF2CH2CF2CH2CF2—的无定形特征吸收峰;在840和503 cm-1处的峰为 —CF2CFClCF2CFCl—结晶吸收峰,说明微量的CTFE-CTFE结构存在明显的晶区,此溶剂反应更容易使共聚体在CTFE含量较低时生成结晶区。

图3 VDF-CTFE共聚物2的FTIR
Fig.3 FTIR of P(VDF-CTFE)-2
3 结 论
a. 对低CTFE配比的VDF-CTFE自由基共聚微观结构进行表征和研究,通过19F NMR对共聚物结构进行分析对比发现,随着反应物中CTFE含量的增加,分子更趋近于生成—CH2CF2CF2CFClCH2CF2—结构和—CF2CFClCF2CFClCF2—结构,此2种结构为主要的结构增长成分,其增量速度是其他结构的2倍。
b. 选择CTFE的制备原料F113作为反应体系的溶剂,提高了其共聚物中CTFE的含量(为理论值的2倍),此溶剂效应在低CTFE含量的共聚反应中更为明显,增幅更大。
c. 该种溶剂反应使共聚物中微量的CTFE-CTFE结构存在明显的晶区。
参考文献:
[1] Boutevin B, Cersosimo F, Youssef B. Studies of the alternating copolymerization of vinyl ethers with chlorotrifluoroethylene[J]. Macromolecules, 1992, 25: 2842-2846.
[2] Souzy R, Ameduri B, Boutevin B. Synthesis and (co)polymerization of monofluoro, difluoro, trifluorostyrene and ((trifluorovinyl)oxy) benzene[J]. Prog Polym Sci, 2004, 29: 75-106.
[3] CHU Bao-jin, ZHOU Xin, REN Kai-liang, et al. A dielectric polymer with high electric energy density and fast discharge speed[J]. Science, 2006, 313: 334-336.
[4] Suzuki T, Maruyama S, Oe K. Electrode, lithium secondary battery using this electrode, electric, double, layer capacitor and element: JP 10064517[P]. 1998-03-06.
[5] LU Ying-ying, Claude J, ZHANG Qi-ming, et al. Microstructures and dielectric properties of the ferroelectric fluoropolymers synthesized via reductive dechlorination of poly (vinylidene fluoride-co-chlorotrifluoroethylene)s[J]. Macro- molecules, 2006, 39: 6962-6968.
[6] Abe Y, Tashiro K. Computer simulation of structure and ferroelectric phase transition of vinylidene fluoride copolymers. (5): Influence of orientational disorder of dipole moments and domain walls on phase transitional behavior[J]. Polymer, 2001, 42: 9671-9678.
[7] WEN Jian-xun. Piezoelectric properties in uniaxially drawn copolymers of vinylidene fluoride and trifluoroethylene[J]. Japanese Journal of Applied Physics, 1984, 23: 1434-1439.
[8] LU Ying-ying, Claude J, Neese B, et al. A modular approach to ferroelectric polymers with chemically tunable curie temperatures and dielectric constants[J]. American Chemical Society, 2006, 128: 8120-8121.
[9] Benedetti E, D'Alessio A, Vergamini P, et al. Vibrational spectra of copolymers of vinylidene fluoride with some fluoro olefins[J]. Macromolcule, 1983, 6: 125-127.
[10] 付海涛, 陈 玮, 于 瀛, 等. 偏氟乙烯/三氟氯乙烯无规共聚物的结晶[J]. 高等学校化学学报, 2004, 25: 1958-1961.
FU Hai-tao, CHEN Wei, YU Ying, et al. Crystallization behavior of the copoly (vinylidene fluoride- chlorotrifluoroethylene)[J]. Chemical Journal of Chinese University, 2004, 25: 1958-1961.
[11] Otazaghine B, Sauguet L, Boucher M. Radical copolymerization of vinylidene fluoride with perfluoroalkylvinyl ethers[J]. European Polymer Journal, 2005, 41: 1747-1756.
[12] Ikeda S, Yuki T. A theoretically reproduced phase diagram for vinylidene fluoride and trifluoroethylene copolymers[J]. Polymer, 2005, 43: 4783-4789.
[13] Gee R H, Fried L E, Cook R C. Structure of chlorotrifluoroethylene/vinylidene fluoride Random copolymers and homopolymers by molecular dynamics simulations[J]. Macromolecules, 2001, 34: 3050-3059.
[14] WANG Zhi-yin, SU Ke-he, FAN Hui-qing. Structure, phase transition and electric properties of poly (vinylidenefluoride- tetrafluoroethylene) copolymer studied with density functional theory[J]. Polymer, 2007, 48: 3226-3236.
[15] Bernard B, Yves F, Yves H. Study of vinylidene fluoride (VF,) telomerization and cotelomerization. Part Ⅱ: VF2 and chlorotrifluoroethylene; (CTFE) cotelomerization with 2-hydroxyethylmercaptan by radical initiation[J]. Journal of Fluorine Chemwy, 1995, 74: 37-42.
收稿日期:2008-06-10;修回日期:2008-10-27
基金项目:国家青年自然科学基金资助项目(50703048)
通信作者:李衡峰(1972-),男,湖南茶陵人,教授,从事功能高分子材料的研究;电话:0731-8877873;E-mail: lihf@mail.csu.edu.cn


