文章编号:1004-0609(2013)S1-s0343-05
基于模量调控的医用多孔钛的设计与制备
徐广胜1, 2,李若琳1,寇宏超1,刘向宏2,李金山1,周 廉1
(1. 西北工业大学 国家重点凝固实验室,西安 710072;
2. 西部超导材料科技股份有限公司 超导材料国家工程实验室,西安 710018)
摘 要:
摘 要:采用多层钛网层叠烧结的方法制备多孔钛,通过控制冷压制力控制多孔钛孔隙率,进而控制多孔钛的弹性模量,使多孔钛的弹性模量和骨组织相适配。采用场发射扫描电镜观测多孔钛的微观孔形貌,采用Instron力学试验机测试压缩应力—应变曲线。通过截取应力—应变曲线上的线弹性测得弹性模量,并取σ0.2作为多孔钛的屈服强度。结果表明:采用控制冷压制力在112~225 MPa之间,可以制备出孔隙率为41.02%~58.87%的多孔钛,弹性模量为1.6~5.6 GPa。这种多孔钛在微观结构上显示出纵横向不同的孔形状和孔尺寸,力学性能也因微观结构的差异而显示出各向异性。
关键词:
中图分类号:TG146.2 3 文献标志码:A
Design and fabrication of porous medical titanium based on control of elastic modulus
XU Guang-sheng1, 2, LI Ruo-lin1, KOU Hong-chao1, LIU Xiang-hong2, LI Jin-shan1, ZHOU Lian1
(1. State Key Laboratory of Solidification Processing, Northwest Polytechnical University, Xi’an 710072, China;
2. National Engineering Laboratory for Superconducting Material, Western Superconducting Technology Co. Ltd., Xi’an 710018, China)
Abstract: The porous titanium with bone-like structure were designed and fabricated by titanium mesh-stacking-sintering based on the control of elastic modulus. The elastic modulus was controlled by the porosity which was influenced by the cold pressure during the fabrication of porous titanium. The microstructure and mechanical properties of the porous titanium specimen were investigated by scanning electron microscope (SEM) and electrical universal testing machine, respectively. The elastic modulus and yield stress (σ0.2) were calculated from the stress—strain curve. The results show that the elastic modulus changes from 1.6 GPa to 5.6 GPa when the porosity is 41.02%-58.87% by controlling the cold pressure from 112 MPa to 225 MPa. The microstructure shows that the pore has different shapes and sizes at longitudinal section and cross section, which is determined by the porous titanium’s anisotropy of the mechanical properties.
Key words: porous titanium; mechanical adaptation; elastic modulus; yield stress; titanium mesh-stacking-sintering
多孔钛合金材料结合了钛合金的优势与多孔材料的优势,不仅实现了表面的多孔化,而且在整个植入物器件里面都是多孔化结构。这种结构既有利于骨组织从各个方向长入植入物内部,提高了植入物器件和骨组织的结合强度,又有利于因骨组织的长入多孔钛合金植入物内部,使其成为一体,而明显减少因应力集中而带来的对植入物的破坏。因此,研究如何调控多孔钛的弹性模量,并同时提高多孔钛屈服强度,使之与自然骨有更好的力学适配性具有十分重要的意 义[1]。
根据经典多孔材料弹性模量与相对密度之间的经验公式E*=C(ρ*/ρs)可知[2],多孔钛的弹性模量不仅与孔隙率密切相关,而且与多孔钛的孔型、孔径尺寸和孔的分布有关。目前,制备多孔钛的方法有钛粉烧结法[3]、造孔剂法[4]、钛丝烧结法[5]、SLM法[6]等,这些方法都可使用控制孔隙率的方法来调控弹性模量,但是,这种方法掩盖了孔隙率不均匀、大量微小裂纹的存在、氧氮含量的增高等对多孔材料的影响[7],所以,使得实验测得的力学性能数据离散型比较大,规律不易发现。可见,弹性模量的控制就比较困难。
在本文作者的前期研究中,通过对多孔钛力学行为模拟研究发现,控制孔径和孔隙率都会对多孔钛的弹性模量具有影响[8],而且方形孔的多孔钛兼具较低的弹性模量和较高的屈服强度[9]。因此,在方形孔模型基础上,采用具有方形孔的钛网,以网孔的规则方孔形通过层叠冷压的方法,制备多孔钛预制体,然后,采用真空烧结的方法,制备多孔钛。通过观察冷压力、孔隙率、弹性模量等指标,以探讨对多孔钛弹性模量的控制方法和手段。
1 实验
采用钛网层叠烧结制备多孔钛所用钛网为0.18 mm,孔径为180 μm,钛丝直径为100 μm,化学成分如表1所示。采用本课题组发明专利[10]制备不同压力下得到的等径多孔钛。使用精密线切割机,加工成d 6 mm×9 mm圆柱体样品用于力学测试。采用SEM观察多孔钛横向和纵向围观结构。
表1 多孔钛化学元素
Table 1 Chemical elements of porous titanium (mass fraction, %)

采用Instron力学试验机测试样品压缩应力应变曲线。在压缩实验中,以规定非比例压缩强度σ0.2为多孔材料的强度指标,按照GB/T 7314—2005《金属材料室温压缩实验方法》进行实验和数据处理。根据胡克定律,在应力—应变曲线的初始部分(即弹性阶段),材料的弹性模量E是该曲线上的斜率,所以,可通过压缩应力—应变曲线上弹性阶段中的曲线斜率求得其弹性模量。由于本试验压缩过程中采用西北有色金属研究院Instron电子万能力学试验机的双差动位移传感器采集变形,测量精度高,弹性阶段的曲线斜率由设备自动拟合。
2 结果与讨论
2.1 多孔钛的微观结构
图1所示为采用0.18 mm钛网在180 MPa冷压力作用下形成的多孔钛的微观结构照片。从图1可以看出:该种多孔体具有纵横向不同的微观结构。横向网孔为规则方形孔隙,尺寸为钛网尺寸180 μm,纵向孔为类方孔孔隙,尺寸范围在几十微米到几百微米之间。
采用纤维烧结法制备多孔钛是一个常用生产多孔材料的方法。研究表明[5, 11-12]:该方法制备的多孔钛具有良好生物相容性,为骨组织的长入提供一个良好的环境。但是,采用该方法制备的多孔钛均不能有效地控制孔径,且孔径分布范围比较广,因此,作者采用钛网作为原料,使用钛网层叠的方法制备多孔材料。多孔钛横向的孔径可由钛网网孔尺寸控制,纵向孔径可由冷压力大控制。从图1可以看到:孔隙尺寸和形状在纵向剖面和横截面有很大的不同。在横截面孔隙形状和分布非常规则,孔隙尺寸几乎相同,约为200mm,这是适合骨长入沿纵向的[13]。在作者先前研究中发现[14],成骨细胞不仅可以在多孔钛表面生长,而且可以沿纵向往孔里面长入。在纵向剖面,孔隙形状是类方形孔,孔隙大小比较分散,大多数低于100 μm。它不能适合骨长入,可以提供水和营养物质输送通道来维持骨长入和新陈代谢,但这需要进一步研究。
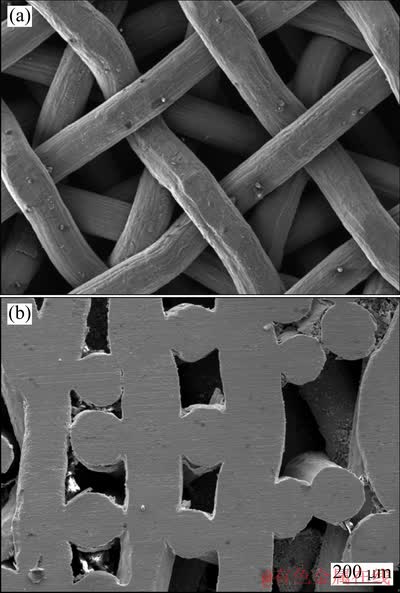
图1 多孔钛的微观结构SEM照片
Fig. 1 SEM micrographs of porous titanium with longitudinal section (a) and cross section (b)
2.2 多孔钛的力学性能
采用钛网层叠烧结方法制备的多孔钛,因其结构在纵横方向上有些差异,进而会影响到力学性能的变化。图2所示为在同一多孔体上沿纵向(轴线方向)和横向(直径方向)的应力—应变曲线。从图2可以看出:两者的应力响应过程都有明显的弹性区、平台区和密实区,二者的弹性模量和σ0.2屈服强度都相差不大,但经过屈服点后,横向上应力有明显下降,对应着多孔体结构的崩坍,而在纵向方向上则没有明显变化。
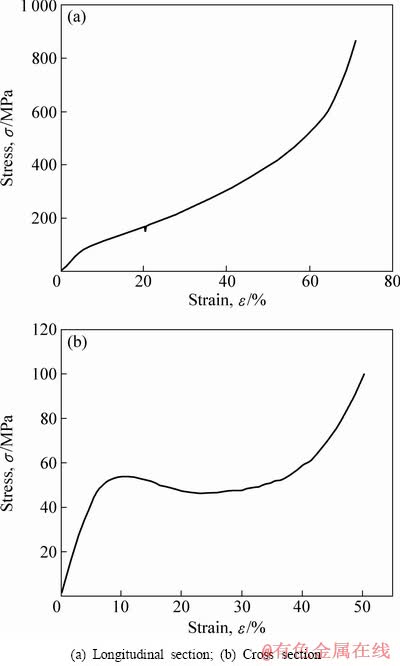
图2 多孔钛纵向、横向的应力—应变曲线
Fig. 2 Compressive stress—strain curves of porous titanium with different directions
从图2可以看出多孔钛应力—应变过程有明显的3个阶段[2]:第一个阶段为弹性变形阶段,该阶段材料变形以线弹性为主,而这个阶段很短,应变量很小,线弹性阶段主要归结为孔壁发生了弹性变形;第二个阶段为塑性屈服阶段(平台阶段),该阶段跨度很大,随着压缩过程的进行,在应力缓慢变化同时应变大幅度增加而形成一个或高或低的平台,对于弹性体材料,坍塌是由较弱区域孔壁的弹性屈曲所造成的,可恢复。对于具有塑性屈服点的材料,坍塌由弯曲边的最大力矩截面处形成塑性铰所造成;而对于脆性材料,坍塌则是由脆性断裂所造成,显然,后两者是不可恢复的;第三个阶段为密实化阶段,该阶段中孔体完全坍塌、孔壁接触并被压实,可视为对基体材料的压缩,因此,应力迅速增加,应力—应变曲线斜率急剧增大,然而相关文献中这一阶段对于多孔钛合金并不明显,但本研究中密实化阶段的现象十分明显,如图2所示。
2.3 多孔钛的弹性模量调控
图3所示为0.18 mm钛网在不同冷压力下形成的多孔钛孔隙率随冷压力变化的关系。从图3可以看出:随着冷压力的增加,多孔钛的孔隙率呈线性下降。因此,在采用钛网层叠烧结法制备多孔钛时,可以通过控制冷压力,制备不同孔隙率的多孔钛。而多孔钛的孔隙率是影响多孔钛的力学行为和力学性能的主要因素。
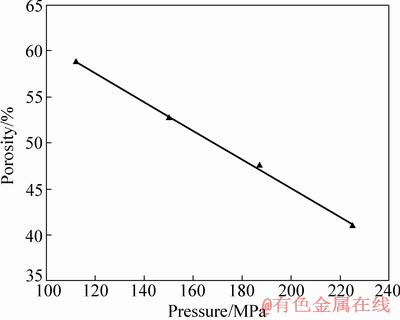
图3 多孔钛孔隙率随冷压力的变化曲线
Fig. 3 Porosity curves of porous titanium with cold pressure
与其他的多孔材料一样,采用钛网层叠烧结法制备的多孔钛的力学性能强烈依赖于孔隙度。多孔钛力学行为及力学性能随孔隙率的变化趋势如图4所示。从图4可以看出:在孔隙率为40%~60%的范围内,弹性模量和屈服强度随孔隙率的增加呈近线性降低;当孔隙率从41.02%增加到58.87%时,弹性模量从5.6 GPa下降到1.6 GPa,下降了70%;而压缩屈服强度则减少,从199 MPa下降到96 MPa,下降了52%。由此可以看出:由孔隙率变化引起的多孔钛力学性能变化中,对弹性模量的影响要大于对屈服强度(σ0.2)的影响。
从其他研究[15-16]和钛网层叠烧结发制备的多孔钛力学响应来看,多孔钛的静态力学性能不仅与构成材料、孔隙率等宏观因素有关,而且与孔形态因素有关,如孔隙定向排列、孔隙分布、孔隙、孔隙形状和大小等。但是,这些结构参数如何影响多孔钛的力学行为和力学性能,在多大程度上能够体现出来,这它需要进一步研究。
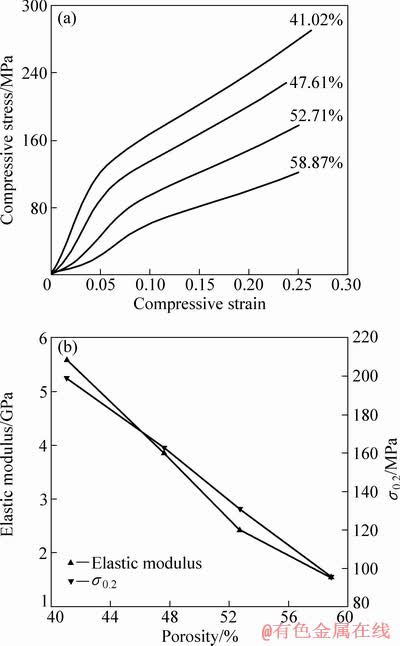
图4 多孔钛力学性能随孔隙率变化趋势图
Fig. 4 Compressive mechanical properties tendency with different porosity
3 结论
采用钛网层叠烧结法制备出弹性模量可控的多孔钛;控制网孔及冷压制力112~225 MPa,可以制备出孔隙率范围在41%~59%左右的多孔钛,进而制备出具备1.6~6.5 GPa弹性模量的多孔钛;钛网层叠烧结法制备的多孔钛在微观结构上显示出纵、横向不同的孔形状和孔尺寸,微观结构的差异使得多孔钛力学性能显示出各向异性。
REFERENCES
[1] LONG M, RACK H J. Titanium alloys in total joint replacement: A materials science perspective[J]. Biomaterials, 1998, 19(18): 1621-1639.
[2] GIBSON L J. Biomechanics of cellular solids[J]. Journal of Biomechanics, 2005, 38(3): 377-399.
[3] WAN Xiao-jun. Processing and mechanical properties of porous Ti-7.5Mo alloy[J]. Transactions of Nonferrous Metals Society of China, 2011, 21(6): 1335-1339.
[4] WANG Yue-qin, TAO Jie, ZHANG Jin-long, WANG Tao. Effects of addition of NH4HCO3 on pore characteristics and compressive properties of porous Ti-10% Mg composites[J]. Transactions of Nonferrous Metals Society of China, 2011, 21(5): 1074-1079.
[5] HE Guo, LIU Ping, TAN Qing-biao. Porous titanium materials with entangled wire structure for load-bearing biomedical applications[J]. Journal of the Mechanical Behavior of Biomedical Materials, 2012, 5(1): 16-31.
[6] van BAEL S, KERCKHOF G, MOESEN M, PYKA G, SCHROOTEN J, KRUTH J P. Micro-CT-based improvement of geometrical and mechanical controllability of selective laser melted Ti6Al4V porous structures[J]. Materials Science and Engineering A, 2011, 528(24): 7423-7431.
[7] SALLICA-LEVA E, JARDINI A L, FOGAGNOLO J B. Microstructure and mechanical behavior of porous Ti6Al4V parts obtained by selective laser melting[J]. Journal of the Mechanical Behavior of Biomedical Materials, 2013, 26:98-108.
[8] GONG Mi, KOU Hong-chao, YANG Yu-song, XU Guang-sheng, LI Jin-shan, ZHOU Lian. Investigation of pore structures on mechanical properties of porous Ti by 3D finite element models [J]. Advanced Materials Research, 2013, 647: 683-687.
[9] 宫 谜. 医用多孔钛力学性质的计算机模拟与实验研究[D]. 西安: 西北工业大学, 2013: 33-34.
GONG Mi. Numerical and experimental study on mechanical properties of porous bio-titanium[D]. Xi’an: Northwest Polytechnical University, 2013: 33-34.
[10] 寇宏超, 李金山, 徐广胜, 唐 斌, 王 军, 周 廉. 一种梯度多孔钛合金的制备方法[P]. 中国专利: 201310005574.3. 2013-01-08.
KOU Hong-chao, LI Jin-shan, XU Guang-sheng, TANG Bin, WANG Jun, ZHOU Lian. A fabricated method of gradient porous titanium and titanium alloys[P]. China Patent: 201310005574.3. 2013-01-08.
[11] ZOU Chun-ming, ZHANG Er-lin, ZENG Song-yan. Preparation, microstructure and mechanical properties of porous titanium sintered by Ti fibers[J]. Journal of Material Science: Materials in Medicine, 2008, 19(1): 401-405.
[12] KITAOKA K, YAMAMOTO H, TANI T, HOSHIJIMA K, NAKAUCH M. Mechanical strength and bone bonding of a titanium fiber mesh block for intervertebral fusion[J]. Journal of Orthopacdic Science, 1997, 2(2): 106-113
[13] YAMADA H, YOSHIHARA Y, HENMI O. Cementless total hip replacement: Past, present and future[J]. Journal of Orthopacdic Science, 2009, 14(2): 228-241.
[14] XU Guang-sheng, KOU Hong-chao, LI Ruo-lin, LIU Xiang-hong, LU Ting-li, LI Qi, ZHOU Lian. Investigation on osteoblast growth on the modified surface of porous titanium[J]. Advanced Materials Research, 2013, 647: 98-103.
[15] JUNG H D, YOOK S W, JANG T S, LI Y L, KIM H E. Dynamic freeze casting for the production of porous titanium (Ti) scaffolds[J]. Materials Science and Engineering C, 2013, 33(1): 59-63.
[16] SHEN H, BRINSON L C. A numerical investigation of porous titanium as orthopedic implant material[J]. Mechanics of Materials, 2011, 43(8): 420-30.
(编辑 陈灿华)
基金项目:国家重点基础研究发展计划资助项目(2012CB619101)
收稿日期:2013-07-28;修订日期:2013-10-10
通信作者:寇宏超,副教授,博士;电话:029-88460568;E-mail:hchkou@nwpu.edu.cn


