文章编号:1004-0609(2007)08-1391-05
微波条件下杂原子MCM-41介孔分子筛的合成
姜廷顺1,殷广明2,赵 谦1,殷恒波1,唐雅静1
(1. 江苏大学 化学化工学院,镇江 212013;
2. 齐齐哈尔大学 化学化工学院,齐齐哈尔 161006)
摘 要:
以十六烷基三甲基溴化铵(CTAB)为模板剂,硅酸钠、氯化镍、氯化钴和氯化铜为原料,通过微波辐射法合成纯硅MCM-41和Ni(Co、Cu)MCM-41介孔分子筛。采用X射线衍射、透射电子显微镜、Fourier变换红外光谱和比表面积孔径分析等测试手段对所合成的介孔分子筛进行表征。考察了分别将杂原子Ni、Co和Cu引入MCM-41介孔分子筛对其介孔有序性、比表面积及孔径大小的影响。结果表明:在微波条件下可以合成出纯硅MCM-41和掺杂Ni(Co、Cu)MCM-41介孔分子筛,样品经550 ℃焙烧后模板剂被有效去除,合成样品的介孔结构没有被破坏;杂原子Ni(或Co、Cu)引入纯硅MCM-41介孔分子筛后,其比表面积和平均孔径均增大,且介孔有序性变好。
关键词:
中图分类号:O 643 文献标识码:A
Synthesis of hetero-atom MCM-41 mesoporous
molecular sieve under microwave condition
JIANG Ting-shun1, YIN Guang-ming2, ZHAO Qian1, YIN Heng-bo1, TANG Ya-jing1
(1. School of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang 212013, China;
2. School of Chemistry and Chemical Engineering, Qiqihaer University, Qiqihaer 161006, China)
Abstract: Silica MCM-41 and Ni (Co or Cu) MCM-41 mesoporous molecular sieve were synthesized by microwave irradiation method using cetyltrimethyl ammonium bromide as template and sodium silicate, nickel chloride, cobalt chloride and cooper chloride as raw materials, respectively. The synthesized samples were characterized by X-ray diffractometry, transmission electron microscopy, specific surface area analysis, Fourier transform infrared spectroscopy and N2 physical adsorption. After hetero-atom (Ni, Co or Cu) was introduced into silica MCM-41 mesoporous molecular sieve, the effects of hetero-atom (Ni, Co or Cu) on its specific surface area, mesoporous ordering and pore size were also investigated. The results show that silica MCM-41 and Ni (Co or Cu) MCM-41 mesoporous molecular sieves are synthesized. The template is effectively removed from the samples after calcinations at 550 ℃, and the pore structure of the synthesized samples is not damaged. After hetero-atom Ni (Co or Cu) is introduced into silica MCM-41 mesoporous molecular sieve, its specific surface area and average pore size increase and mesoporous order becomes good.
Key words: mesoporous molecular sieve; microwave synthesis; characterization; specific surface area
自1992年美国Mobil公司的研究人员[1]首次报道水热法合成出规则的具有六方排列MCM-41介孔分子筛以来,由于其具有的独特孔结构和大比表面积等特点,介孔分子筛一直是催化材料研究的热门课题之一。
近年来,研究人员采用水热法合成出多种不同类型的介孔分子筛[2?10]。 然而,水热法合成介孔分子筛需要较长的晶化时间和一定的晶化温度。与水热合成法相比,微波辐射合成在许多方面表现出其优越的特性,微波是一种频率在300 MHz~300 GHz 之间的电磁波,微波条件下分子筛的合成是将分子筛的合成体系置于微波辐射范围内,在微波加热条件下,在瞬间使整体物系温度达到结晶化温度,大幅度缩短晶化时间,获得均匀细小的晶粒[11]。微波辐射技术在MCMs系列介孔分子筛的合成方面得到广泛应用[11?13],但是多数工作都限于纯硅MCM-41的微波合成。由于纯硅MCM-41介孔分子筛表面几乎呈电中性、且酸中心少等特点,限制了其在许多催化反应中的应用。若在纯硅MCM-41介孔分子筛的骨架中引入过渡金属改变其比表面积和平均孔径,便可改变其催化性能,进而用作各种不同功能的催化剂。因此各种杂原子介孔分子筛的合成引起人们的兴趣[14?16],然而微波辐射合成杂原子介孔分子筛的研究工作还未见系统报道。为此,本文作者采用微波辐射合成掺杂Ni(或Co、Cu)MCM-41介孔分子筛,同时考察不同杂原子的引入对纯硅MCM-41介孔分子筛结构的影响。
1 实验
1.1 纯硅MCM-41的合成
按照摩尔比n(CTAB)?n(SiO2)=0.2?1,首先将6.4 g CTAB溶解于20 mL蒸馏水中,25 g硅酸钠溶解于30 mL蒸馏水中,然后将硅酸钠水溶液慢慢滴入CTAB的水溶液中搅拌10 min,用硫酸溶液(5 mol/L)调节混合物的pH值至11,继续搅拌80 min,使溶液变为粘稠的透明凝胶状。再将混合物料移入250 mL圆底烧瓶中,置于带回流装置的NN?S570MFS型微波炉内,在微波功率为220 W下回流加热2.5 h。取出后冷却过滤,洗涤至中性,然后在130 ℃下干燥过夜,烘干得未焙烧样品s-MCM-41,将s-MCM-41放入马弗炉内,以2 ℃/min的速率升温至550 ℃,于空气流中焙烧10 h,得纯硅介孔分子筛c-MCM-41。
1.2 掺杂金属原子的介孔分子筛的合成
按摩尔比n(M)(M=Co,Ni,Cu)?n(CTAB)?n(Si) =0.05?0.2?1,分别将金属盐CoCl2?6H2O、NiCl2?6H2O或CuCl2?6H2O充分溶解在10 mL蒸馏水中,6.4 g CTAB溶解在10 mL蒸馏水,25 g硅酸钠溶解于30 mL蒸馏水中,然后将硅酸钠溶液和金属盐溶液在不断搅拌下加入CTAB溶液中,搅拌10 min,用硫酸溶液(5 mol/L)调节混合物的pH值至11,将混合物继续搅拌80 min后,移入250 mL圆底烧瓶中,置于带有回流装置的微波炉内,在微波功率为220 W下回流加热2.5h,将产物冷却、抽滤、洗涤至中性,分别烘干得未焙烧样品s-CoMCM-41、s-NiMCM-41和s-CuMCM-41。将未焙烧样品放入马弗炉内,以2 ℃/min的速率升温至550 ℃,于空气流中焙烧10 h,得到含杂原子的介孔分子筛c-CoMCM-41、c-NiMCM-41和c-CuMCM-41。
1.3 样品的表征
样品的XRD测试采用Rigaku D/MAX 2500PC型X射线粉末衍射仪测定,Cu Kα(λ=0.154 18 nm)、扫描速度1(?)/min、扫描范围1?~10?。样品的骨架红外测定采用Nicolet公司(美国)的Nexus FT?IR470型红外光谱仪进行测定,KBr压片,测量范围为400~4 000 cm?1。样品的比表面和孔径分布测定采用Quntachrome公司(美国)的NOVA2000型表面积和孔径分析仪进行测定,比表面积采用BET法计算,孔径分布和孔体积采用BJH法[17]计算。采用Phillips TENCNAI?12型透射电子显微镜(加速电压100~120 kV)分析介孔分子筛的微观形貌。
2 结果与讨论
2.1 样品的比表面积和孔径分布
图1所示为样品的N2吸附?脱附等温线,图2所示为样品的孔径分布曲线,由BET法计算的比表面积和由BJH法计算的平均孔径值列于表1。由图1可见,样品的吸附等温线为Ⅳ型,与介孔材料相符合,说明合成了介孔分子筛。相对压力为0.3~0.4范围内,3种掺杂金属原子样品的吸附等温线均出现明显突跃,说明掺杂金属原子样品的比表面积和孔体积大,表1的数值也说明这一点。从图2和表1的数据也可得出,掺杂金属原子后介孔分子筛的介孔孔径略有增加,这可能是由于半径比硅大的金属离子进入孔壁所造成的。而且从计算的数据可以看出,引入金属杂原子Co后介孔分子筛的比表面积增加最大,引入金属杂原子Ni后介孔分子筛的平均孔径增加最多。
表1 几种介孔分子筛的XRD和N2吸附?脱附分析结果
Table 1 XRD and N2 adsorption-desorption results of hetero-atom MCM-41 mesoporous molecular sieve
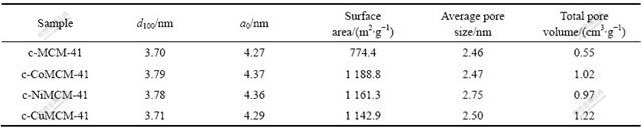

图1 样品的N2吸附?脱附等温线
Fig.1 Nitrogen adsorption-desorption isotherms of samples
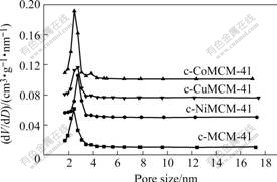
图2 几种介孔分子筛的孔径分布曲线
Fig.2 Pore size distribution curves of hetero-atom MCM-41 mesoporous molecular sieve
2.2 样品的小角度XRD分析
样品的(100)晶面间距d100(nm)的计算公式为
![]()
样品的单位晶胞参数a0(nm)的计算公式为
![]()
图3所示为样品的小角度XRD谱。根据衍射角度,计算得到d100和a0的值列于表1。由图3可见,在衍射角约为2.3?附近几种介孔材料均出现一个衍射峰,在较高衍射角处有强度较弱的衍射峰,这与文献[1]中报道的MCM-41介孔分子筛的XRD谱相似,说明所合成的样品具有规整的六方孔道结构,合成出有序性很好的杂原子MCM-41介孔分子筛。此外,由XRD谱可见,与纯硅MCM-41分子筛相比,含杂原子介孔分子筛的(100)衍射峰明显向小角度移动,说明含杂原子介孔分子筛的孔径要比纯硅的大,这与表1所列的结果一致。另外,由图3还可以看出,掺杂金属原子样品的(100)衍射峰强度比纯硅样品的高,说明在微波辐射的作用下Cu、Co和Ni等的存在有利于合成有序性高的介孔分子筛。

图3 几种介孔分子筛的XRD谱
Fig.3 XRD patterns of hetero-atom MCM-41 mesoporous molecular sieve
2.3 FT-IR分析
图4所示为c-MCM-41焙烧前后的FT-IR谱,其它介孔分子筛的FT-IR谱与图4相似。在FT-IR谱上2 922、2 852和1 467 cm?1处的衍射峰为模板剂的特征峰;1 620~1 640 cm?1处的衍射峰是由吸附水分子的变形振动引起的;1 050和1 221 cm?1处的衍射峰是由Si—O—Si不对称伸展振动引起的。样品经550 ℃焙烧后,2 922、2 852和1 467 cm?1处的衍射峰消失,而其它峰没有变化,说明经550 ℃焙烧后样品中的模板剂已被有效去除。
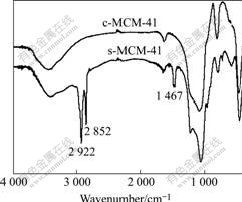
图4 c-MCM-41焙烧前后的FT-IR谱
Fig.4 FT-IR spectra of c-MCM-41 before and after calcinations
2.4 TEM分析
图5所示为几种介孔分子筛的TEM像。由图5可见,对于微波法合成的纯硅和含杂原子Ni或Cu的介孔分子筛样品c-MCM-41、c-NiMCM-41和c-CuMCM-41,在平行于孔道方向可以明显观察到六方形孔道,在垂直于孔道方向也可明显观察到互不相通的直通孔道;而含Co的介孔分子筛c-CoMCM-41具有虫蛀状的介孔结构,与水热法所合成样品的介孔结构类似[6]。由图还可以看出,所合成介孔分子筛的平均孔径在2~3 nm之间,这与比表面积孔径测定法和XRD所得的结果相吻合。
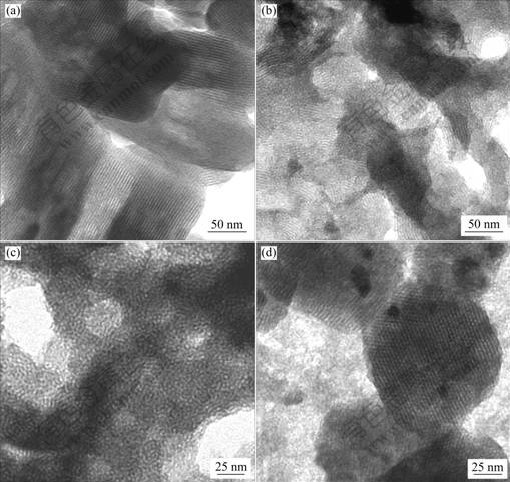
图5 介孔分子筛的TEM像
Fig.5 TEM images of hetero-atom MCM-41 mesoporous molecular siece:
(a) c-MCM-41; (b) c-NiMCM-41; (c) c-CoMCM-41; (d) c-CuMCM-41
3 结论
1) 在微波辐射条件下,成功合成了纯硅MCM-41和掺杂Ni(Co、Cu)MCM-41介孔分子筛。经550 ℃焙烧后模板剂被有效去除,介孔结构没有被破坏。
2) 在微波辐射合成条件下,杂原子掺入纯硅MCM-41介孔分子筛使其比表面积增大,平均孔径亦增大,且Cu、Co、Ni的掺入提高了介孔分子筛的有序性。
[1] Beck J S, Vartuli J C, Roth W J, Leonowicz M E, Kresge C T, Schmitt K D, Chu C T W, Olson D H, Sheppard E W, McCullen S B, Higgins J B, Schlenkert J L. A new family of mesoporous molecular sieves prepared with liquid crystal templates[J]. J Am Chem Soc, 1992, 114(27): 10834?10843.
[2] Sastre G, Catlow C R, Corma A. Influence of the intermolecular interactions on the mobility of heptane in the supercages of MCM-22 zeolite. A molecular dynamics study[J]. Phys Chem B, 2002, 106(3): 956~962.
[3] 姜廷顺, 陈宇飞, 赵 谦, 陆路德, 杨绪杰. 合成皂石为前驱物制备高稳定性含镍介孔分子筛[J]. 硅酸盐学报, 2006, 34(7): 805?809.
JIANG Ting-shun, CHEN Yu-fei, ZHAO Qian, LU Lu-de, YANG Xu-jie. Preparation of Ni-mesoporous molecular sieves of high stability by using saponite as a precursor[J]. Journal of the Chinese Ceramic Society, 2006, 34(7): 805?809.
[4] Dorset D L, Kennedy G J. Crystal structure of MCM?70: A microporous material with high framework density[J]. J Phys Chem B, 2005, 109(29): 13891?13898.
[5] Banerjee S, Santhanam A, Dhathareyan A, Rao P M. Synthesis of ordered mesostructured nickel oxide[J]. Langmuir, 2003, 19(13): 5522?5525.
[6] 姜廷顺, 赵 谦, 殷恒波. 含镍和钴介孔分子筛的稳定性和孔结构[J]. 中国有色金属学报, 2006, 16(9): 1621?1627.
JIANG Ting-shun, ZHAO Qian, YIN Heng-bo. Pore structure and stability of Ni (or Co)-containing mesoporous molecular sieves[J]. The Chinese Journal of Nonferrous Metals, 2006, 16 (9): 1621?1627.
[7] Zhao D Y, Feng J L, Huo Q S, Melosh N, Fredrickson G H, Chmelka B F, Studcky G D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores[J]. Science, 1998, 279(5350): 548?552.
[8] Selvaraj M, Panduragan A, Seshadri K S, Sinha P K, Lai K B. Synthesis, characterization and catalytic application of MCM-41 mesoporous molecular sieves containing Zn and Al[J]. Appl Catal A: Generral, 2003, 242(2): 347?365.
[9] Song C S, Reddy K M. Mesoporous molecular sieve MCM-41 supported Co-Mo catalyst for hydrodesulfurization of dibenzothiophene in distillate fuels[J]. Appl Catal A, 1999, 176(1): 1?10.
[10] Kapoor M P, Ichihashi Y, Kuraoka K J, Shen W J, Matsurnura Y. Catalytic methanol decomposition over palladium deposited on mesoporous cerium oxide[J]. Catal Lett, 2003, 88(1/2): 83?87.
[11] 许 磊, 王公慰, 魏迎旭, 齐 越, 刘宪春, 包信和. MCM-41介孔分子筛合成研究(Ⅱ)——微波辐射合成法[J]. 催化学报, 1999, 20(3): 251?255.
XU Lei, WANG Gong-wei, WEI Ying-xu, QI Yue, LIU Xian-chun, BAO Xin-he. Study on synthesis of mesoporous molecular sieve(Ⅱ)—Microwave synthesis method[J]. Chinese Journal of Catalysis, 1999, 20(3): 251?255.
[12] 银董红, 秦亮生, 刘建福, 尹笃林. 微波固相法制备ZnCl2/MCM-41催化剂及其催化性能[J]. 物理化学学报, 2004, 20(9): 1150?1154.
YIN Dong-hong, QIN Liang-sheng, LIU Jian-fu, YIN Du-lin. Preparation of ZnCl2/MCM-41 catalysts by the solid-state interaction under microwave heating and their catalytic performances[J]. Acta Phys Chim Sin, 2004, 20(9): 1150?1154.
[13] 颜学武, 韩小伟, 曹 毅, 魏一伦, 朱建华. 微波法研制碱土金属氧化物负载型MCM?48碱性介孔材料[J]. 无机化学学报, 2002, 18(11): 1101?1106.
YAN Xue-wu, HAN Xiao-wei, CAO Yi, WEI Yi-lun, ZHU Jian-hua. Preparation of basic MCM?48 mesoporous materials loaded alkaline earth oxides through microwave radiation[J]. Chinese Journal of Inorganic Chemistry, 2002, 18(11): 1101?1106.
[14] Lim S Y, Ciuparu D, Pak C, Dobek F, Chen Y, Harding D, Pfefferle L, Haller G. Synthesis and characterization of highly ordered Co-MCM-41 for production of aligned single walled carbon nanotubes(SWNT)[J]. J Phys Chem B, 2003, 107(39): 11048?11056.
[15] Gao X T, Wachs I E, Wong M S, Ying J Y. Structural and reactivity properties of Nb-MCM-41: Comparison with that of highly dispersed Nb2O5/SiO2 catalysts[J]. J Catal, 2001, 203(1): 18?24.
[16] Okumura K, Nishigaki K, Niwa M. Prominent catalytic activity of Ga-containing MCM-41 in the Friedel-Crafts alkylation[]. Micropor Mesopor Mater, 2001, 44/45: 509?516.
[17] Barrett E P, Joyner L G, Halenda P P. The determination of pore volume and area distributions in porous substances(Ⅰ). Computation isotherms [J]. J Am Chem Soc, 1951, 73(1): 373?380.
基金项目:江苏省高校自然科学基金资助项目(04KJD130040);江苏大学高级人才基金资助项目(06JDG074)
收稿日期:2006-12-25;修订日期:2007-04-17
通讯作者:姜廷顺,博士,副教授;电话:0511-5883589;E-mail: tshjiang@ujs.edu.cn
摘 要:以十六烷基三甲基溴化铵(CTAB)为模板剂,硅酸钠、氯化镍、氯化钴和氯化铜为原料,通过微波辐射法合成纯硅MCM-41和Ni(Co、Cu)MCM-41介孔分子筛。采用X射线衍射、透射电子显微镜、Fourier变换红外光谱和比表面积孔径分析等测试手段对所合成的介孔分子筛进行表征。考察了分别将杂原子Ni、Co和Cu引入MCM-41介孔分子筛对其介孔有序性、比表面积及孔径大小的影响。结果表明:在微波条件下可以合成出纯硅MCM-41和掺杂Ni(Co、Cu)MCM-41介孔分子筛,样品经550 ℃焙烧后模板剂被有效去除,合成样品的介孔结构没有被破坏;杂原子Ni(或Co、Cu)引入纯硅MCM-41介孔分子筛后,其比表面积和平均孔径均增大,且介孔有序性变好。


