DOI��10.19476/j.ysxb.1004.0609.2019.03.13
Au-Pt-Sn��Ԫ�Ͻ���ͼ��700 ����½���
������1, 2��л ��1, 2���� ��1, 2������̩1, 2���� ��2��������1, 2
(1. ����������ѧ ���Ͽ�ѧ�빤��ѧԺ������ 650093��
2. ������������������ 650106)
ժ Ҫ��
���� X ����������(XRD)�͵���̽����������(EPMA)�ȷ����ⶨ��Au-Pt-Sn ��Ԫϵ 700 ����½��档���������Au-Pt-Sn ��ϵ 700 ����½�����3����������7����������6����������ɡ�6���������ֱ�ΪPt3Sn+FCC-A1+PtSn��PtSn+FCC-A1+Au5Sn��PtSn+Pt3Sn+Au5Sn��Pt3Sn+FCC-A1+Au5Sn��PtSn+Pt2Sn3+Liquid��PtSn+FCC-A1+Liquid��Au-Pt-Sn��ϵ�д���Au-Pt�Ͻ�ĵ����ֽⷴӦ������Pt�����ļ��٣��Ͻ��е����ֽ�������ʧ����Au16Pt30Sn54��Au16Pt20Sn64�Ͻ�����ȫ��ʧ������Pt-Sn�Ͻ�����۵��Au-Sn�Ͻ����ߣ�������700 ����½��棬��ֻ����Pt-Sn�Ͻ��࣬��Au-Sn�Ͻ����ֻ������Һ���С��Ͻ�Au16Pt69Sn15��Au16Pt54Sn30��Au16Pt42Sn42���ڴ��ڵ����ֽ��࣬��֯�ֲ��Ͼ��ȣ���ǿ�����ԱȺϽ�Au16Pt30Sn54��Au16Pt20Sn64�ĸߡ�
�ؼ��ʣ�
Au-Pt-Sn ��ϵ����ͼ�����½���������֯����ƽ���������ֽ���
���±�ţ�1004-0609(2019)-03-0538-11���� ��ͼ����ţ�TB31���� ���ױ�־�룺A
�������ʹ���ص�ɹ��Ϊ���١�С�������㡢���������������٣�������Ʒ��Ԫ�������������С�����Ǽ�������Ҫ��Ӧ���ڹؼ��ͺ��IJ�λ��Ӧ������㣬ʹ�ü�ֵ������ˣ���Ч���ͳɱ����з������ܹ�����²����ǵ�ǰ����������о���������Ҫ��������⡣Ȼ�����������١��о��ɱ��ߣ�������Ͻ���ͼ���о�ʮ�ֱ���������Ϊ������Ͻ��ʵ���о����²��Ͽ����ṩӦ�е�ָ������ˣ�����������Ͻ���ͼ�����������ϵ������ѧ���ݿ����з�������²���������Ҫ��չ�Ĺ�����
Au-Pt ��ϵ�Ͻ����ھ������õ���������ѧ���������ܣ�ʹ���ڵ�������������������ҽҩ�����ӷ�װ������õ��˹㷺��Ӧ��[1-3]������1986 �꣬OKAMOTO��[4]��һ�ζ�Au-Pt��Ԫ�Ͻ���ͼ��������������������Ҫ�Dzο�20����50��60�������X �������䷨���ȷ������Լ����跨��õ���ƽ�����ݣ�����������ѧ���ݣ���Okamoto ����������д�δ�漰��������GROLIER��[5]������OKAMOTO��[4]����ʱ�����õ�ͬ����ƽ�����ݻ����ϣ�ͬʱ����������ѧ���ݣ���������������ģ�Ͷ�Һ���Լ�����������(FCC��)����������GROLIER���������õ�Au-Pt ��Ԫϵͳ������ѧ�������Գɹ������ִֵ���ƽ�������ѧʵ�����ݡ����ǣ�����GROLIER������������ѧ��������Au-Pt Ԫ��ͼ���������ʱ��ƽ������ɷֱַ�Ϊ��Pt ��~3%Pt(Ħ������)����������ѧ����������ì�ܡ���������[2]�������ʵ���õ���Au-Pt ��Ԫ��ϵ��ƽ�����ݣ����� CALPHAD ������������Au-Pt ��Ԫ��ϵ������ѧ��������������������ģ��Redlich-Kister ��ʽ����Һ��������������Gibbs �����ܡ���������ѧ�������ɵ�������������ƽ�����ݺ�������ѧ���ʣ�������Ⱥͻ���ʣ��Ż�Au-Pt ��Ԫϵͳ����ѧ�������Ż����������Au-Pt �Ͻ�ϵͳ���ܽ�ȼ�϶�߽���Au ��ƫ�ƣ��䶥��λ����1200 �棬Au-56%Pt(Ħ������)��������ϵ��ʧ�ȷֽ������Բ�����֯�����ܵ�Ӱ�첢û�еõ�ϵͳ�о���
Au-Sn �Ͻ���н����Ժá�����ǿ�ȸߺ���ʴ���ܺõ��ŵ㣬�㷺Ӧ���ڸ߿ɿ��������������������װ��ǥ�����ϣ��úϽ�ǥ��ϵ�Ķ�Ԫ�����ɷ�Ϊ80Au/20Sn���۵�Ϊ280 �棬��Ŀǰ�۵���280~360 ����Ωһ����������۵�Ǧ���Ͻ��ǥ��[6-8]��Au-Sn ��Ԫ��ϵ����4����Ԫ�Ͻ���[9]���ֱ�ΪAuSn��AuSn2��AuSn4 �� Au5Sn��AuSn�ľ���ṹ����������ϵ������NiAs�ͽṹ���ռ�ȺΪ P63/mmc��AuSn2�ľ���ṹ����������ϵ���ռ�ȺΪPBCA�������к���24��ԭ�ӣ����� 8��Au ԭ�ӣ�16��Sn ԭ�ӣ�AuSn4�ľ���ṹҲ����������ϵ������ PtSn4�ͽṹ���ռ�ȺΪABA2��Au5Sn�ľ���ṹ����������ϵ���ռ�ȺΪR3H��
������ϸߵĻ��ԣ�Pt-Sn �����㷺Ӧ���ڴ��������缫������������������[10-12]�����⣬Pt-Sn �����ڴ����ⷽ��Ҳ����Ӧ��DZ�ܡ�Pt-Sn ��Ԫ��ϵ����5����Ԫ�Ͻ���[13]���ֱ�ΪPtSn��PtSn2��PtSn4��Pt2Sn3�� Pt3Sn������Pt3Sn���γ��¶Ƚϸߣ��ɹ�����Ӧ���ɣ���898 ��ʱ�� PtSn��Һ���������Ӧ����Pt2Sn3��748 ��ʱ��Pt2Sn3��Һ���������Ӧ������PtSn2��PtSn2��Һ����540 ��ʱ����������Ӧ������PtSn4��228 ��ʱ��ʣ���Һ���������Ӧ����ȫת��ΪPtSn4�ͦ�-Sn������-Sn������ʱת��Ϊ��-Sn[14]��
ǰ���о��������ڴ�ͳPt-Sn���������ϣ�����Au�����õ�������շ��Ʊ���Au-Pt-Sn �������ڱȽ��о�Au ���춡���������ת���ʺ�ѡ���Ե�Ӱ����ּ���һ����Au ����ǿ�˴�������[10]��ALEXANDRA��[16]��ʵ��ķ�����Au-Pt-Sn ��Ԫ��ϵ��Sn����(Sn��50%��Ħ������)��400 ����½���������о���DONG��[17]������Au-Pt-Sn ��ϵ400 �桢320 ���150 ��ĵ��½��棬����Au-20%Sn-Pt ������֯����ƽ����Ϣ�������о��ͱ�����ALEXANDRA��[16]��DONG��[17]��Ҫ�Ƕ�Au-Pt-Sn��ϵ�ĵ��µ��½�������о��������Ķ�Au-Pt-Sn��Ԫϵ�Ͻ���ͼ700 ����½�������о���̽����ϵ���ܴ��ڵ����Լ����ϵ��Ϊ���ϵ��Ʊ��������о��ṩ��ѧ���ݡ�
1 ʵ��
ʵ����ԭ��Ϊ���ȷֱ�Ϊ99.99%�ĸߴ���99.95%����99.99%����ʵ������ƵĺϽ�ɷ����1 ��ʾ�����Ͻ���Ʒ������Ϊ 5 g���Ͻ�����������½�����յ绡������Ϊ��֤�Ͻ�ɷ־��ȣ�ÿ���Ͻ���Ʒ�������� 3 �Ρ���������������������¯��ȡ��������С�飬Ȼ���ٴ�������ȷ����Ʒ�ɷ־��ȡ���ɺϽ��Ʊ�����Ʒ�������ʯӢ�������н��о��Ȼ��˻𡣽���Ʒ����700 ����Ȼ��˻�20 d��Ȼ������������ˮ�п��ٴ�𡣶Ծ������Ȼ��ȴ�����Ŀ�״�Ͻ��������ô�ɰֽĥȥ����������㣬����ĥ������õ�һ����ƽ�棬����X������������͵���̽���������֯�����ɷַ����������������Empyrean��(���ɿ�)X���������ǽ��У�����ԴΪCu K����������ѹΪ40 kV����������Ϊ40 mA��ɨ���ٶ�ѡ��10 (��)/min��ɨ��Ƕ�2��Ϊ10��~100�㡣��ò���������ɷַ������õ��ǵ���̽����������(EPMA)�������ͺ���JXA8230(�ձ�����)����ɢ�������ֱ�����20 nm�����ٵ�ѹ0~30 kV��������ΧΪ1��10-5~1��10-12  �������������ȿ���ʶ��0.2%(��������)���ϵ�Ԫ�ء�
�������������ȿ���ʶ��0.2%(��������)���ϵ�Ԫ�ء�
��1 ʵ����ƺϽ������ɷ�
Table 1 Nominal compositions of designed Au-Pt-Sn alloys
2 ���������
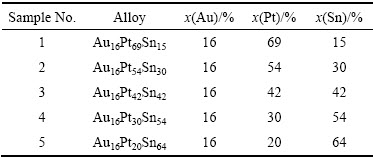
ͼ1�ͱ�2��ʾ�ֱ�Ϊ�Ͻ�1(Au16Pt69Sn15)��700 ����Ȼ��˻����Ʒ�ĵ���̽������֯��Ƭ�͵���̽�����ɷַ�����ͼ2��ʾΪƽ��Ͻ�1��XRD�ס���ͼ1(a)��(b)�п��Կ������Ͻ�1��ƽ����֯�д���3���࣬���ݱ�2�ɷַ�����֪����ɫ����Ϊ�����ֽ�Au-Pt�Ͻ���ɷ�ΪAu80.45Pt19.55(��Au������)���ṹ��Pt�ṹһ�£���ɫ֦����ΪAu4.51Pt74.28Sn21.21���ɷ���Pt3Sn�ɷֽӽ�����һ����ͼ2��ʾ��XRD���Է��֣�������嶼���Ա�Pt3Sn���궨����ɫ֦����Χ�Ļ�ɫ����ΪPt-Sn�Ͻ�������һ�ֵ����ֽ�Au-Pt�Ͻ�(��Pt������)�Ļ���ࡣ���ֵ����ֽ������ṹ��ͬ������Pt�Ľṹһ�£����ɷֲ�ͬ����ɫ���������Au�����ϸߣ��Ұ�ɫ����Pt�����ϸߡ�����ɫ�к�ɫС�����ۼ���������˲���Sn����Sn�����ϸ������ں�ɫ֦����Χ�γ���Pt-Sn�Ͻ𣬽�һ����XRD���������֪����Pt-Sn�Ͻ�ΪHCP�࣬�ɱ궨ΪPtSn���������ȷ���Ͻ�1��700 ��ʱ������3���࣬��Pt3Sn��HCP-PtSn �Լ������ֽ��γɵ����ֳɷֲ�ͬ��FCC�����塣���ʵ����˵������Au-Pt-Sn ��Ԫ�Ͻ���ϵ��Pt������700 ����½������1������Pt3Sn+FCC-A1+PtSn������������ͼ3�кϽ�1��EPMAԪ����ֲ�ͼ�����Ը�������ֱ�۵Ŀ����������ֽ⸻Pt������ĺ����ϸ�Au������ĺ����࣬��AuԪ��ֻ���зֲ��ڰ�ɫ����SnԪ�صķֲ�Ҳ�Ƚϼ����ں�ɫ����PtԪ�صķֲ���Χ�Ϲ㣬���ҽϾ��ȡ�

ͼ1 �Ͻ�1 Au16Pt69Sn15������֯
Fig. 1 Microstructure of Au16Pt69Sn15 alloy at different magnifications
��2 ͼ1(c)�����EMPA�������
Table 2 EPMA elemental analysis and possible phase of each spot marked in Fig.1(c)

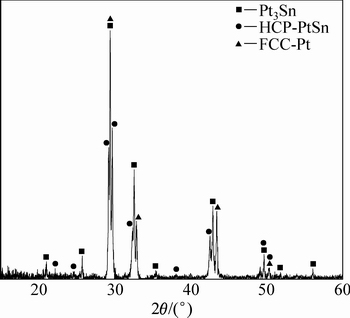
ͼ2 �Ͻ�1��XRD��
Fig. 2 XRD pattern of alloy 1 (Au16Pt69Sn15)
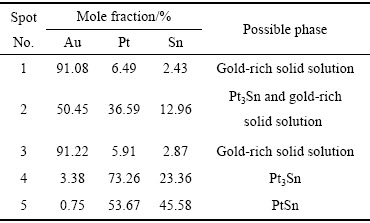
ͼ4��5��ʾ�ֱ�Ϊ�Ͻ�2(Au16Pt54Sn30)�ĵ���̽������֯��Ƭ��XRD�ס��Ͻ�2��XRD���п������궨��Pt3Sn��PtSn���ࡣ��Ͻ�1��ȣ�����Sn���������ӣ�Pt������֮���٣�PtSn����ȫ�Ӹ�Pt����������������Pt���������Ҳ����PtSn���������Pt3Sn�Ĵ������ɶ�������١���һ���ĵ���̽��ɷַ������֣��Ͻ�2Ҳ��������ɣ����3��ʾ�����ݳɷַ����Ľ��(����3)���б����ɫ��ΪPtSn��dz��ɫ����ΪPt3Sn������ΪAu-Pt�Ͻ�(��Au������)��X �������䷢�֣�Au-Pt�Ͻ����Ľṹ��Auһ�£��ɴ˿����Ʋ⣬���źϽ�ɷֵĸı䣬�����ֽ������ĽṹҲ�����仯���ɺϽ�2��XRD�����Կ�������ǿ��ij�������Ϊ�Ͻ�����Pt3Sn�������϶������о���һ����ȡ���ԡ�ͼ6��ʾΪ�Ͻ�2��EPMAԪ����ֲ�ͼ����ͼ6���Ը�������ֱ�۵ؿ�����PtԪ����SnԪ����Ҫ�ԺϽ������ʽ���ڣ�AuԪ������ֻ���зֲ��ڰ�ɫ���ֲ�����ϺϽ�1�Ĵ�
ͼ7��8��ʾ�ֱ�Ϊ�Ͻ�2(Au16Pt42Sn42)�ĵ���̽������֯��XRD�ס�������֪���Ͻ�3�����������Ͻ�2�Ļ���һ�£�ֻ�ǺϽ�3�л�ɫPt3Sn����������٣��ɸ�Pt������ȡ����֮����ɫ����ΪPtSn�࣬�����ֽ⸻Au�븻Pt������ͬʱ���ڣ��ڸ�Au����������Au5Sn���ɡ����ͼ8��ͼ9�ͱ�4������֪�������ֽ�Ͻ�������ֽṹ�������Ʋ⣬�Ͻ�3��Ʒ�γ������ֽṹ���͵ĵ����ֽ�Ͻ𣬰����˺Ͻ�1��2�ĵ����ֽ�Ͻ����͡�����Ϊ���ֽṹ�����ֽ�Ͻ�Ĺ�ͬ���ڣ�����XRD��������ż����Ͻ����Au��Pt���ֽṹ��������ڡ����ʵ����˵������ Au-Pt-Sn ��Ԫ�Ͻ���ϵ�У���700 ����½������1������PtSn+FCC-A1+ Au5Sn����������
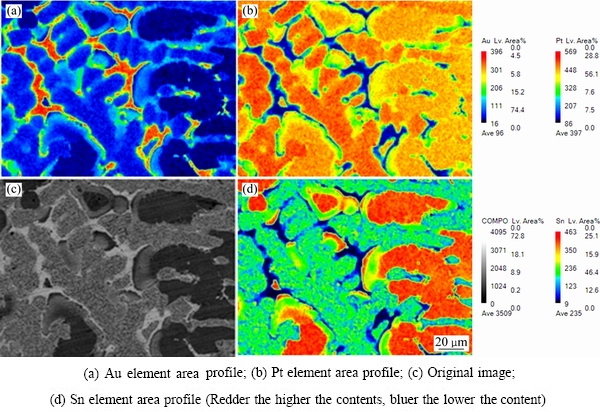
ͼ3 �Ͻ�1������֯��EPMAԪ����ֲ�����
Fig. 3 Microstructures of alloy 1 and EPMA mapping distributions of elements in alloy 1 (Au16Pt69Sn15)
ͼ4 �Ͻ�2(Au16Pt54Sn30)������֯
Fig. 4 Microstructures of alloy 2 (Au16Pt54Sn30) at different magnifications
��3 ͼ4(c)�и����EMPA�������
Table 3 EPMA elemental analysis and possible phase of each spot marked in Fig.4(c)
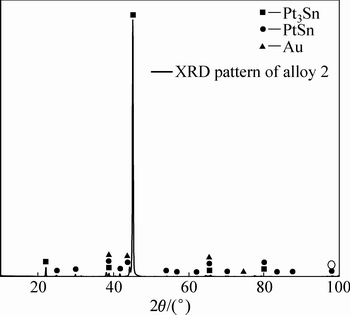
ͼ5 �Ͻ�2��XRD��
Fig. 5 XRD pattern of alloy 2 (Au16Pt54Sn30)
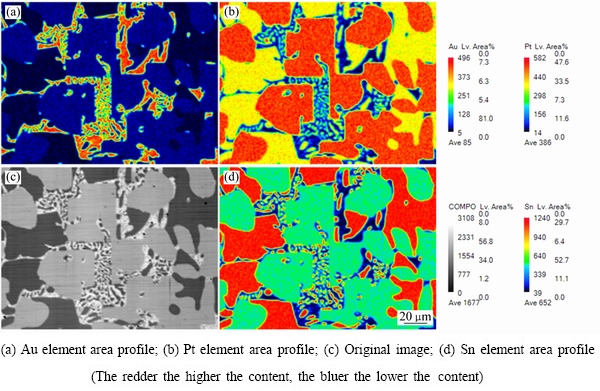
ͼ6 �Ͻ�2������֯��EPMAԪ����ֲ�����
Fig. 6 Microstructures and EPMA mapping distributions of element of alloy 2 (Au16Pt54Sn30)
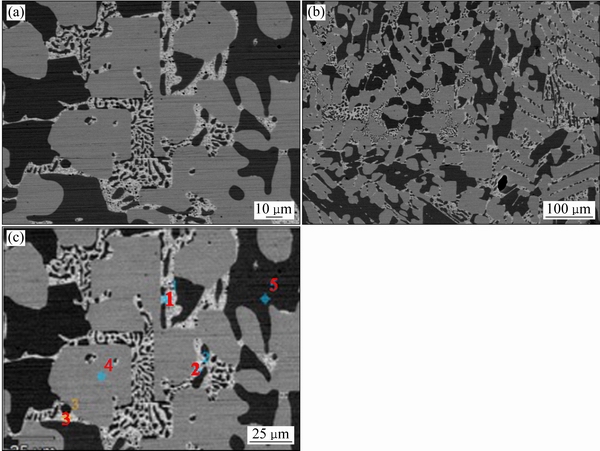
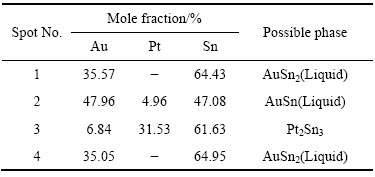
ͼ10��ʾΪ�Ͻ�4(Au16Pt30Sn54)�ĵ���̽������֯��Ƭ���Ͻ�4Ϊ�������Ͻ�����700 ��ʱ����Һ����ڣ���ɫ���ο�����Χ��֦������Ǵ�Һ�������������ࡣ���ݱ�6��7��8��EPMA�ɷַ����������ɫ������ΪPtSn�й���������Au�γɵ�Pt47.5Sn47.5Au5��Ԫ�Ͻ��࣬���ͼ11�е�XRD������֪������Ԫ�Ͻ���Ľṹ��PtSn��ͬ����������Χ��ϸС֦����ϸ��ӣ����а�ɫ֦���еĻҺ�ɫ����ΪPt2Sn3�����л���������Au���ܣ���ɫ֦����������ɫ��dz�ĻҰ�ɫ��֯����ΪҺ�������ɷ���Au47.5Sn47.5Pt5����XRD������֪��ṹ��AuSn��ͬ������ɫ֦����Χ�ڻ�ɫ����Ҳ��Һ�������ɷ���Au35.05Sn64.95����XRD������֪��ṹ��AuSn2��ͬ������Ʒ4��XRD�����Կ�������ǿ��ij�������Ϊ�Ͻ�Pt47.5Sn47.5Au5�����ο����ϴ������϶������о���һ����ȡ���ԣ����Բ�����ǿ��ij��֡���һ����ͼ12�Ͻ�Ԫ�ص���ֲ�ͼ���������֪����ɫ����������Χ��Ҫ��AuԪ�غ�SnԪ�صĸ���������ΪҺ�����������˻��¶Ƚϸߣ��˻�ʱ��ϳ�������Һ�������������ֲ�ͬ��Au-Sn�Ͻ��ࡣ�ɴ˿��Եó����ۣ��� Au-Pt-Sn ��Ԫ�Ͻ���ϵ�У���700 ����½������1������PtSn+ Pt2Sn3+Һ�����������
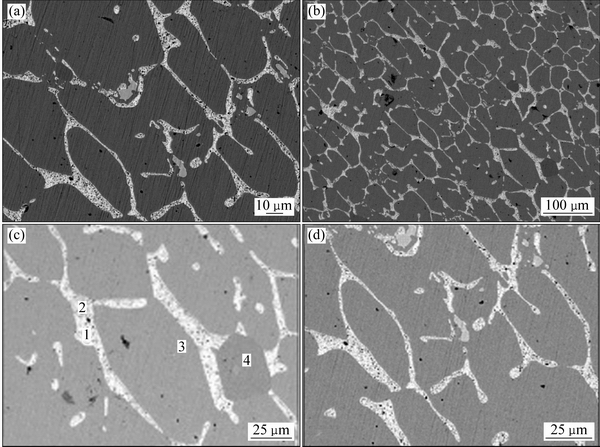
ͼ7 �Ͻ�3(Au16Pt42Sn42)������֯
Fig. 7 Microstructures of alloy 3 (Au16Pt42Sn42) alloy at different magnifications
��4 ͼ7(c)�����EMPA�������
Table 4 EPMA elemental analysis and possible phase of each spot marked in Fig.7(c)

��5 ͼ7(d)��1��EMPA�������
Table 5 EPMA elemental analysis and possible phase of spot 1 marked in Fig.7(d)

ͼ13��14��ʾΪ�Ͻ�5(Au16Pt20Sn64)�ĵ���̽������֯��Ƭ����XRD�ס�ͬ�����Ͻ�5��700 ��ʱҲ��Һ����ڡ�ͼ13�ͱ�9����10������֪���Ͻ�������AuSn��Pt2Sn3��AuSn2��AuSn4������ɣ�ͼ 15(a)�ĵ�����Ƭ�У���ɫ��������ΪPt2Sn3����ɫ�������״����ΪAuSn2����ɫ����ɷ�ΪAu24.5Sn75.5����ͼ14��һ��ȷ��Ϊ����ΪAuSn4����ɫС����ΪAuSn����һ����ͼ15�Ͻ�Ԫ�ص���ֲ�ͼ���������֪����ɫ����������Χ��Ҫ��AuԪ�غ�SnԪ�صĸ���������ΪҺ�����������˻��¶Ƚϸߣ��˻�ʱ��ϳ�������Һ����������3�ֲ�ͬ��Au-Sn�Ͻ��ࡣ���ʵ����˵������ Au-Pt-Sn ��Ԫ�Ͻ���ϵ��Sn����700 ����½������1������Pt2Sn3+Һ�����������
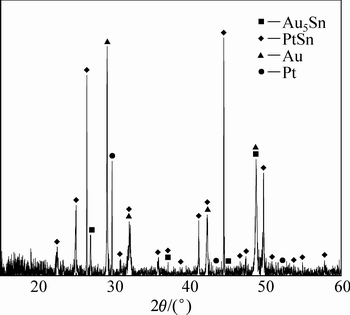
ͼ8 �Ͻ�3��XRD��
Fig. 8 XRD pattern of alloy 3 (Au16Pt42Sn42)
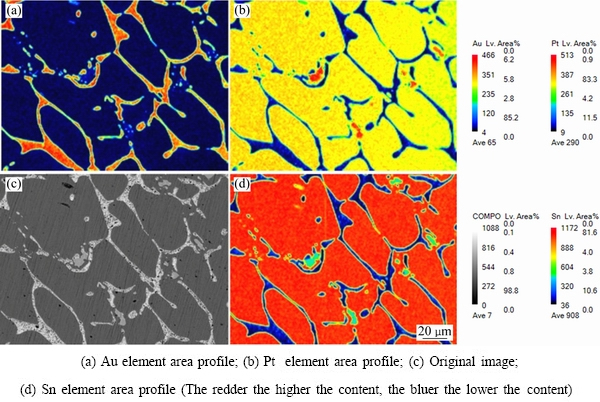
ͼ9 �Ͻ�3������֯��EPMAԪ�طֲ������
Fig. 9 Microstructures and EPMA mapping distributions of element of alloy 3 (Au16Pt42Sn42)
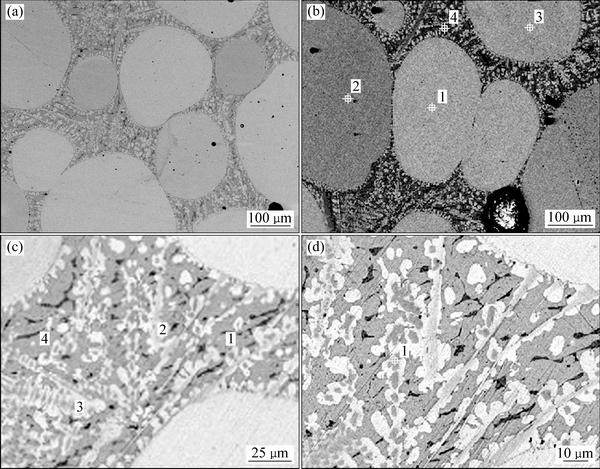
ͼ10 �Ͻ�4������֯
Fig. 10 Microstructures of alloy 4 at different magnifications
��6 ͼ10(b)�и����EMPA�������
Table 6 EPMA elemental analysis and possible phase of each spot marked in Fig.10(b)
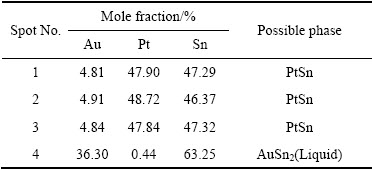
��7 ͼ10(c)�и����EMPA�������
Table 7 EPMA elemental analysis and possible phase of each spot marked in Fig.10(c)
ͨ������5���Ͻ���Ʒ����֯������֪����Ʒ�Ͻ�1~3����֯�ֲ��Ͼ��ȣ���3���Ͻ���Ʒ�Ļ������ǿ�Ƚϸߵ�Au-Pt�Ͻ�����ֽ��࣬���ԺϽ�1~3��ǿ�Ⱥ�Ӳ�Ƚϸߡ����Ͻ�4��5����֯�ֲ������ȣ��һ�����֯����ǿ�Ⱥ�Ӳ�Ƚϵ͵�Au-Sn�Ͻ��࣬���ԺϽ�4��5��ǿ�Ⱥ�Ӳ�Ⱦ��ϵ͡���������ʵ������ݺ����е�Au-Pt-Sn��ϵ����ѧ����[17-18]�������Ի��Ƴ� Au-Pt-Sn��Ԫϵ 700 ����½�����ͼ����ͼ16��ʾ��ͼ16�о���(��)��ʾ��������
��8 ͼ10(d)��1��EMPA�������
Table 8 EPMA elemental analysis and possible phase of spot 1 marked in Fig.10(d)
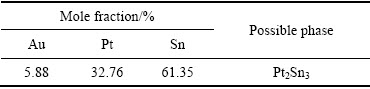
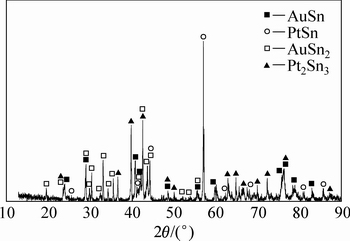
ͼ11 �Ͻ�4��XRD��
Fig. 11 XRD pattern of alloy 4 (Au16Pt30Sn54)
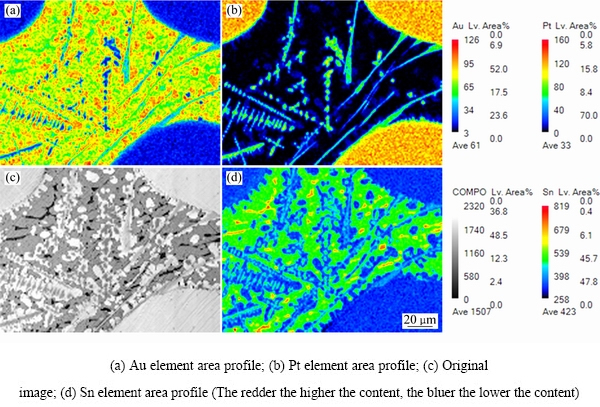
ͼ12 �Ͻ�4��EPMAԪ�طֲ������
Fig. 12 EPMA mapping of elements of alloy 4 (Au16Pt30Sn54)
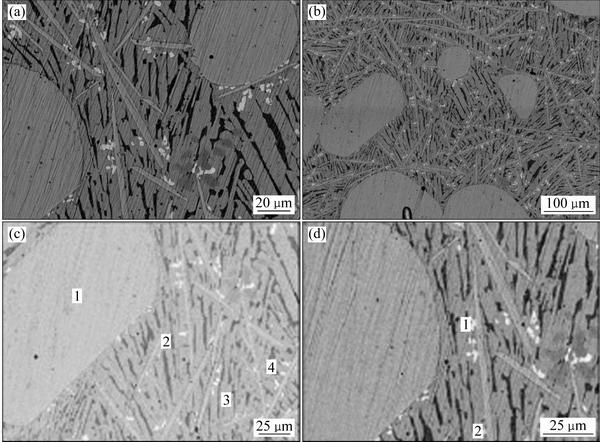
ͼ13 �Ͻ�5������֯
Fig. 13 Microstructures of alloys at different magnifications
��9 ͼ13(c)�и����EMPA�������
Table 9 EPMA elemental analysis and possible phase of each spot marked in Fig.13(c)
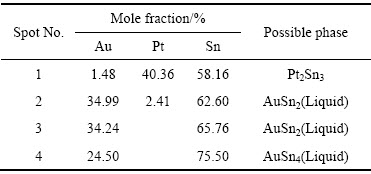
��10 ͼ13(d)�и����EMPA�������
Table 10 EPMA elemental analysis and possible phase of each spot marked in Fig.13(d)
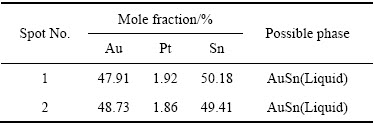
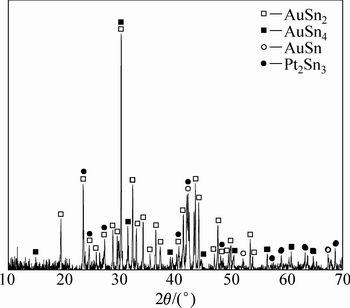
ͼ14 �Ͻ�5��XRD��
Fig. 14 XRD pattern of alloy 5 (Au16Pt20Sn64)
3 ����
1) ��700 �棬Au-Pt-Sn��Ԫϵ��3����������7���������� 6����������ɡ�6���������ֱ�ΪPt3Sn+FCC-A1+PtSn��PtSn+FCC-A1+Au5Sn��PtSn+ Pt3Sn+Au5Sn��Pt3Sn+FCC-A1+Au5Sn��PtSn+Pt2Sn3+Һ�࣬PtSn+FCC-A1+Һ�ࡣ
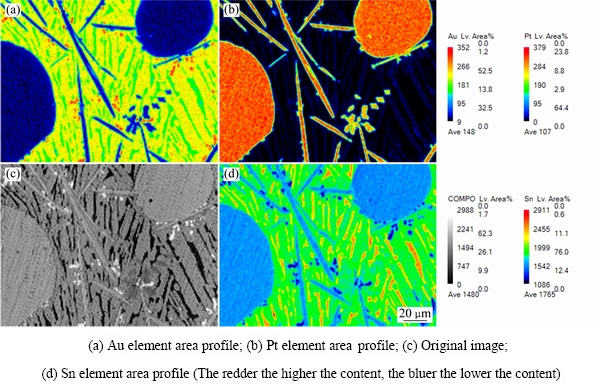
ͼ15 �Ͻ�5������֯��EPMAԪ�طֲ������
Fig. 15 Microstructures and EPMA mapping of elements of alloy 5 (Au16Pt20Sn64)
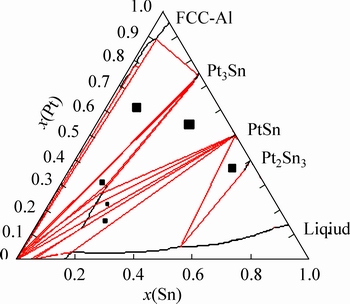
ͼ16 Au-Pt-Sn ��Ԫϵ 700 ����½���
Fig. 16 Isothermal section of Au-Pt-Sn system at 700 ��
2) ����Pt-Sn�Ͻ�����۵��Au-Sn�Ͻ���ߣ�������700 ����½��棬��ֻ����Pt-Sn�Ͻ��࣬������Sn���������ӣ������ֽ�Au-Pt�Ͻ����������ʧ���ںϽ�4 ��5����ȫ��ʧ���Ͻ�1~3���ڴ��ڵ����ֽ��࣬��֯�ֲ��Ͼ��ȣ��Ͻ�ǿ�Ⱥ�Ӳ�����ԱȺϽ�4��5�ĸߡ�
REFERENCES
[1] SHEN Yi-hong, LI Yu-ming, LIU Hai-chao. Base-free aerobic oxidation of glycerol on TiO2-supported bimetallic Au-Pt catalysts[J]. Journal of Energy Chemistry, 2015, 24(5): 669-673.
[2] XU Xiao-ning, REN Yu-ping, LI Chang-fa, LI Song, QIN Gao-wu. Thermodynamic assessment of Au-Pt system[J]. Transactions of Nonferrous Metals Society of China, 2012, 22(6): 1432-1436.
[3] LIU Ya-jun, WANG Jiang, DU Yong, SHENG Guang, LONG Zhao-hui, ZHANG Li-jun. Phase boundary migration, Kirkendall marker shift and atomic mobilities in fcc Au-Pt alloys[J]. CALPHAD, 2012, 36: 94-99.
[4] OKAMOTO H, MASSALSKI T. The Au-Pt system[J]. Journal of Phase Equilibria, 1985, 6(1): 46-56.
[5] GROLIER V, SCHMID F R. Experimental study of Au-Pt-Sn phase equilibria and thermodynamic assessment of Au-Pt and Au-Pt-Sn system[J]. Journal of Electronic Materials, 2008, 37(3): 264-278.
[6] HU Jie-qiong, XIE Ming, ZHANG Ji-ming, LIU Man-men, YANG You-cai, CHEN Yeng-tai. First principles study of Au-Sn intermellic[J]. Acta Physica Sinica, 2013, 62(24): 247102.
[7] ARABI F, THEOLIER L, MARTINEAU D, DELETAGE J Y, MEDINA M, WOIRGARD E. Power electronic assemblies: Thermo-mechanical degradations of gold-tin solder for attaching devices[J]. Microelectronics Reliability, 2016, 64: 409-414.
[8] HUANG M L, YANG Y C, CHEN Y, DONG C. Microstructure and mechanical properties of Sn-rich Au-Sn solders designed using cluster-plus-glue-atom model[J]. Materials Science and Engineering A, 2016, 664: 221-226.
[9] DEBSKI A, GASIOR W, MOSER Z, MAJOR R. Enthalpy of formation of intermetallic phases from the Au-Sn system[J]. Journal of Alloys and Compounds, 2010, 491(1/2): 173-177.
[10] PATRICIA G, CORRADINIA ERMETE ANTOLINI, JOELMA PEREA. Electro-oxidation of ethanol on ternary non-alloyed Pt�CSn�CPr/C catalysts[J]. Journal of Power Sources, 2015, 275: 377-383.
[11] LAI Yu-long, HE Song-bo, LUO Sha, BI Wen-jun, LI Xian-ru, SUN Cheng-lin, SESHAN K. Hydrogen peroxide modified Mg-Al-O oxides supported Pt-Sn catalysts for paraffin dehydrogenation[J]. Catalysis Communications, 2015, 69: 39-42.
[12] SHAN Yu-ling, WANG Ting, SUI Zhi-jun, ZHU Yi-an, ZHOU Xing-gui. Hierarchical MgAl2O4 supported Pt-Sn as a highly thermostable catalyst for propane dehydrogenation[J]. Catalysis Communications, 2016, 84: 85-88.
[13] ZHOU Wei, LIU Li-juan, LI Bao-ling, WU Ping, SONG Qing-gong. Structural, elastic and electronic properties of intermetallics in the Pt-Sn system: A density functional investigation[J]. Computational Materials Science, 2009, 46(4): 921-931.
[14] Vincent Grolier, Rainer Schmid-Fetzer. Thermodynamic analysis of the Pt-Sn system[J]. Journal of Alloys and Compounds, 2009, 46(4): 921-931.
[15] WANG Jian-she, XI Jing-yu, ZHANG Lei, ZHANG Jiu-jun, GUO Xun, ZHAO Jian-hong, SONG Cheng-ying, WANG Liu-cheng. Synthesis of highly active SnO2-CNTs supported Pt-on-Au composite catalysts through site-selective electrodeposition for HCOOH electrooxidation[J]. Electrochimica Acta, 2013, 112: 480-485.
[16] ALEXANDRA N T, LAILA O, KJEKSHUS A, KJEKSHUS A OLSEN. The tin-rich part of the Au-Pt-Sn system[J]. Journal of alloys and compounds, 2001, 314(1/2): 92-95.
[17] DONG Hong-qun, VUORINEN V, BROAS M, PAULASTO-KR�OCKEL M. Thermodynamic reassessment of the Au-Pt-Sn system and microstructural evolution of the (AuSn)eut-Pt interconnection[J]. Journal of Alloys and Compounds, 2016, 688: 388-398.
Isothermal section of Au-Pt-Sn ternary system at 700 ��
HU Jie-qiong1, 2, XIE Ming1, 2, CHEN Song1, 2, CHEN Yong-tai1, 2, WANG Song2, WANG Sai-bei1, 2
(1. School of Materials Science and Engineering, Kunming University of Science and Technology, Kunming 650093, China;
2. Kunming Institute of Precious Metals, Kunming 650106, China)
Abstract: The isothermal section of the Au-Pt-Sn ternary system at 700 �� was investigated by X-ray diffractometer and electron probe microanalyzer. The results show that the isothermal section of the Au-Pt-Sn ternary system at 700 �� is composed of three single-phase regions, seven two-phase regions and six three-phase regions. The six three-phase regions including Pt3Sn+FCC-A1+PtSn, PtSn+FCC-A1+Au5Sn, PtSn+Pt3Sn+Au5Sn, Pt3Sn+FCC-A1+Au5Sn, PtSn+Pt2Sn3+Liquid, PtSn+FCC-A1+Liquid. The spinodal decomposition reaction of Au-Pt alloy exists in the Au-Pt-Sn system. With decreasing Pt content, the spinodal decomposition phase in the alloy gradually disappears, and it completely disappears in Au16Pt30Sn54 and Au16Pt20Sn64 samples. Because the melting point of Pt-Sn alloy phase is higher than that of Au-Sn alloy, most of phases in 700 �� isothermal section are Pt-Sn alloy, while Au-Sn alloy phase mainly exists in liquid phase. The microstructures distribution of Au16Pt69Sn15, Au16Pt54Sn30 and Au16Pt42Sn42 alloy are more uniform and the strengths of the alloy are higher than that of Au16Pt30Sn54 and Au16Pt20Sn64 alloy because of the existence of spinodal decomposition phase.
Key words: Au-Pt-Sn system; phase diagram; isothermal section; microstructure; phase equilibrium; spinodal decomposition
Foundation item: Projects (2017YFB0305700) supported by the National Key R&D Program of China; Projects (U1602275, U1602271) supported by the National Natural Science Foundation of China; Projects (2018ZE011, 2018ZE012, 2018ZE022, 2018ZE026) supported by the Major Science and Technology Projects of Yunnan Province, China; Projects (2018FB088, 2017FB144) supported by the Applied Basic Research Foundation of Yunnan Province, China
Received date: 2018-01-16; Accepted date: 2018-05-10
Corresponding author: XIE Ming; Tel: +86-871-68328841; E-mail: powder@ipm.com.cn
(�༭ ������)
������Ŀ�������ص��з��ƻ�������Ŀ(2017YFB0305700)��������Ȼ��ѧ����������Ŀ(U1602275��U1602271)������ʡ�ش�Ƽ�ר��(2018ZE011��2018ZE012��2018ZE022, 2018ZE026)������ʡӦ�û����о��ƻ�������Ŀ(2018FB088��2017FB144)
�ո����ڣ�2018-01-16�������ڣ�2018-05-10
ͨ�����ߣ�л �������ڣ���ʿ���绰��0871-68328841��E-mail��powder@ipm.com.cn
ժ Ҫ������ X ����������(XRD)�͵���̽����������(EPMA)�ȷ����ⶨ��Au-Pt-Sn ��Ԫϵ 700 ����½��档���������Au-Pt-Sn ��ϵ 700 ����½�����3����������7����������6����������ɡ�6���������ֱ�ΪPt3Sn+FCC-A1+PtSn��PtSn+FCC-A1+Au5Sn��PtSn+Pt3Sn+Au5Sn��Pt3Sn+FCC-A1+Au5Sn��PtSn+Pt2Sn3+Liquid��PtSn+FCC-A1+Liquid��Au-Pt-Sn��ϵ�д���Au-Pt�Ͻ�ĵ����ֽⷴӦ������Pt�����ļ��٣��Ͻ��е����ֽ�������ʧ����Au16Pt30Sn54��Au16Pt20Sn64�Ͻ�����ȫ��ʧ������Pt-Sn�Ͻ�����۵��Au-Sn�Ͻ����ߣ�������700 ����½��棬��ֻ����Pt-Sn�Ͻ��࣬��Au-Sn�Ͻ����ֻ������Һ���С��Ͻ�Au16Pt69Sn15��Au16Pt54Sn30��Au16Pt42Sn42���ڴ��ڵ����ֽ��࣬��֯�ֲ��Ͼ��ȣ���ǿ�����ԱȺϽ�Au16Pt30Sn54��Au16Pt20Sn64�ĸߡ�


