硫酸锌和二甲基二硫代氨基甲酸钠在铅锌浮选体系中对闪锌矿的协同抑制机理
来源期刊:中国有色金属学报(英文版)2020年第9期
论文作者:崔艳芳 焦芬 覃文庆 董留洋 王旭
文章页码:2547 - 2555
关键词:吸附行为;接触角;络合;方铅矿;闪锌矿;组合抑制剂
Key words:adsorption behavior; contact angle; complexation; galena; sphalerite; combined depressant
摘 要:通过浮选试验、离子络合测试、接触角测试和XPS分析,研究组合抑制剂硫酸锌(ZnSO4)和二甲基二硫代氨基甲酸钠(DMDC)对闪锌矿的抑制机理。浮选试验表明,与单一抑制剂ZnSO4和DMDC相比,组合抑制剂ZnSO4+DMDC对闪锌矿有更好的选择性抑制效果。离子络合试验表明,DMDC对铅离子或其羟基络合物有很强的络合能力。接触角测试证明,与单一抑制剂ZnSO4和DMDC相比,组合抑制剂ZnSO4+DMDC使闪锌矿表面更加亲水。XPS分析表明,组合抑制剂通过竞争吸附阻止捕收剂在铅离子活化后的闪锌矿表面吸附,而在方铅矿表面,组合抑制剂与捕收剂产生共吸附。
Abstract: The depression mechanism of zinc sulfate (ZnSO4) and sodium dimethyl dithiocarbamate (DMDC) as the combined depressant on sphalerite was investigated by micro-flotation experiments, ion complexing tests, contact angle tests and X-ray photoelectron spectroscopy (XPS) analysis. The micro-flotation tests revealed that ZnSO4+DMDC had a better selective depression effect on sphalerite than using single ZnSO4 or DMDC. Ion complexing tests confirmed that DMDC had a strong complexing capacity with lead ions or hydroxy complexes. Contact angle tests illustrated that ZnSO4+DMDC makes the sphalerite surface more hydrophilic than ZnSO4 or DMDC. XPS analysis indicated that the combined depressant could prevent collector adsorbing on the Pb-activated sphalerite surface by a competitive adsorption method, while the combined depressant and collector were co-adsorbed on galena surface.
Trans. Nonferrous Met. Soc. China 30(2020) 2547-2555
Yan-fang CUI, Fen JIAO, Wen-qing QIN, Liu-yang DONG, Xu WANG
School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
Received 29 November 2019; accepted 18 June 2020
Abstract: The depression mechanism of zinc sulfate (ZnSO4) and sodium dimethyl dithiocarbamate (DMDC) as the combined depressant on sphalerite was investigated by micro-flotation experiments, ion complexing tests, contact angle tests and X-ray photoelectron spectroscopy (XPS) analysis. The micro-flotation tests revealed that ZnSO4+DMDC had a better selective depression effect on sphalerite than using single ZnSO4 or DMDC. Ion complexing tests confirmed that DMDC had a strong complexing capacity with lead ions or hydroxy complexes. Contact angle tests illustrated that ZnSO4+DMDC makes the sphalerite surface more hydrophilic than ZnSO4 or DMDC. XPS analysis indicated that the combined depressant could prevent collector adsorbing on the Pb-activated sphalerite surface by a competitive adsorption method, while the combined depressant and collector were co-adsorbed on galena surface.
Key words: adsorption behavior; contact angle; complexation; galena; sphalerite; combined depressant
1 Introduction
Sphalerite is the most important zinc ore, which almost always coexists with galena [1,2]. It is the primary mineral raw material for extracting zinc. Sphalerite does not respond well to short-chain thiol collectors because of the relative instability of zinc-xanthate [3]. However, inadvertent activation of sphalerite by Pb2+ could significantly improve the floatability of sphalerite, resulting in low separation efficiency of lead and zinc [4-6]. The inefficient flotation separation will increase the cost of smelting and decrease the quality of concentrate products. Therefore, it is indispensable to select effective flotation reagents to achieve flotation separation of sphalerite from galena.
In the flotation of Pb-Zn system, galena is often preferentially floated due to the good floatability of galena [7,8]. Usually, inorganic and organic depressants are used as a depressant in order to increase the difference between hydrophilicity and hydrophobicity of useful minerals and gangue minerals. Inorganic depressants mainly include cyanide, zinc sulfate, sodium sulfide and sulfur-oxy, etc [9-12]. Most of these depressants have a low price and excellent chemical properties. However, the use of these depressants often has many practical problems, such as a large loss of precious metals in mineral concentrates, high doses of zinc sulfate, and the negative environmental impact of cyanide toxicity.
In view of these problems, organic depressants are receiving more and more attention from many researchers because of their good selectivity and environmental friendliness [13,14]. For example, chitosan could be used as a depressant in Cu-Pb, Zn-Pb, and Pb-Fe system and the mechanism of depressant chitosan was uncovered [15-17]. Sodium dimethyl dithiocarbamate (DMDC) is a good depressant since it has a strong complexing capacity with metal ions, which could decrease the activation of the mineral surface [18,19]. Sodium humate could depress galena in Cu-Pb separation system [20,21]. In addition, other representative reagents like starches [22,23], dextrin [24], and cellulose [25,26] also have been extensively studied and achieved good results.
Researches on inorganic depressants and organic reagents have made large achievements, but in most cases, the depression performance of these depressants is not satisfactory in practice due to the complexity of the flotation system. The combined depressants of inorganic and organic reagents have some advantages, but there are few reports about this kind of combined depressants. In this work, we investigated the depression performance of the novel combined depressant ZnSO4+DMDC in the differential flotation separation of Pb-Zn sulfides. The depression mechanism was revealed by ion complexing tests and contact angle tests, which provided a basis for the flotation separation of sphalerite from galena.
2 Experimental
2.1 Materials
The naturally pure minerals of galena and sphalerite were all purchased from Guangxi, China. The mineral samples were ground and screened to 38-75 μm for the micro-flotation tests, ICP analysis and contact angle analysis. The samples (<38 μm) continued to be ground to 2 μm for chemical analysis, X-ray photoelectron spectroscopy (XPS) analysis and X-ray diffraction (XRD) analysis. The screening process was dry screening. According to the results of X-ray diffraction (XRD) spectroscopy (Fig. 1) and chemical analysis (Table 1), the purity of the galena was 96.67 wt.%, and the purity of the sphalerite was 96.11 wt.%. Based on the result of XRD and chemical analysis, it showed that galena and sphalerite had an extremely high purity.
Depressant (CH3)2NCSSNa (DMDC) was purchased from Zhuzhou Fortune Chemical Industry Co., Ltd., China. Diethyldithiocarbamate (DDTC), ZnSO4, lead nitrate and methyl isobutyl carbinol (MIBC) from Zhuzhou Flotation Reagents Factory in Hunan, China, were all analytical grade reagents and used as collector, depressant, activator and frother, respectively. The concentrations of hydrochloric acid (HCl) and sodium hydroxide (NaOH) were 1 mol/L, and these were used to adjust pH in the tests. The deionized water (resistance over 16 MΩ·cm) was used in the micro- flotation experiment and mechanism measurements experiment.
2.2 Micro-flotation tests
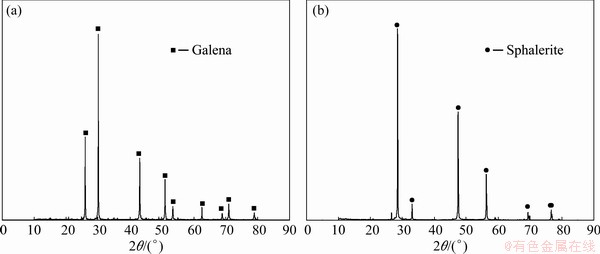
Fig. 1 XRD spectra of galena and sphalerite
Table 1 Main chemical compositions of mineral samples
Micro-flotation tests of single minerals and artificially mixed minerals were both conducted to evaluate the depression ability of combined depressant DMDC+ZnSO4 in Pb-Zn separation. The sample mass of single and mixed mineral tests (galena and sphalerite at a mass ratio of 1:1) was 2 g, respectively. The flotation tests were carried out in an XFG flotation machine (Jilin Exploration Machinery Plant, Changchun, China) with a 40 mL plexiglass cell, and the impeller speed was fixed at 1620 r/min. Each flotation test, the minerals were cleaned by ultrasonic treatment. The desired pH value was adjusted by adding HCI or NaOH. Then, activator, depressant, collector and frother were added to the slurry and the conditioning time was taken as 2 min. The concentrate and tailing products were collected after flotation, and the flotation recovery ε was calculated. The formula is as follows:
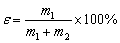 (1)
(1)
where m1 and m2 represent the mass of the concentrate and tailing, respectively.
For artificially mixed mineral experiment, the flotation procedure was the same as the single mineral flotation test. After flotation, the lead and zinc grades of the concentrates or tailings were analyzed by chemical method, and the recovery was also calculated.
2.3 Ion complexing tests
Ion complexing tests were conducted to investigate the complexation ability of DMDC by UV-vis spectroscopy (UV-9100, Japan) and inductively coupled plasma atomic emission spectrometry (SPECTRO BLUE SOP, Germany). For UV–vis spectroscopy, different metal ions (Pb2+ and Zn2+) with the concentration of 1.0×10-5 mol/L were mixed with the ligand (DMDC) of the same concentration in buffer solution (pH=6.86). Then, the solution was diluted to 50 mL. The absorbance of the solution was determined by UV–vis spectroscopy at a scanning wavelength of 200 to 700 nm. For inductively coupled plasma atomic emission spectrometry, 2.0 g of the samples were added to flotation machine with a 40 mL cell, and this was followed by the addition of lead ions, either alone or in addition to DMDC. The supernatant was used to determine the lead ions by using the ICP.
2.4 Contact angle measurement
Contact angles of mineral samples were measured using a contact angle instrument analysis system (JY-82C). The samples were prepared in accordance with single mineral flotation, and the pellet was prepared from flotation samples under the pressure of 18 MPa for 2 min. The contact angle of the pressed pellet of powdered mineral samples was measured by a sessile drop method. The contact angle of the sample was measured three times in different positions and averaged.
2.5 XPS analysis
The XPS analysis was conducted to study the chemical composition of mineral surface and chemical state of elements under the different reagents [27,28]. The samples were prepared as the flotation process. The flotation slurry pH was about 9, the concentration of depressant (DMDC and ZnSO4) was 2×10-4 mol/L, and the concentration of collector (DDTC) was 5×10-5 mol/L. Then, the samples were transferred to a vacuum drying oven to dry. The XPS tests have used the equipment of K-Alpha+ (Thermo Fisher Scientific, USA). The accuracy of the XPS measurement is 0.1 eV. The standard carbon 1s spectrum photoelectron peak was based on the binding energy of 284.8 eV.
3 Results and discussion
3.1 Micro-flotation results
3.1.1 Flotation tests of single mineral
The flotation recoveries of galena and sphalerite under different pH using depressants DMDC and ZnSO4 are shown in Fig. 2. It is shown that the flotation recovery of galena was almost stable at about 90% in the pH of 4-10. However,the flotation recovery of sphalerite decreased rapidly from 84.9% to 21.7% using depressant DMDC and decreased from 94.2% to about 32% using depressant ZnSO4 at pH 4-10. Although single depressant (DMDC or ZnSO4) had a strong depression effect on sphalerite, good separation results cannot be achieved due to the complexity of actual production. So DMDC and ZnSO4 were used as a combined depressant to separate galena from sphalerite. As shown in Fig. 2, the recovery of sphalerite decreased to 4.9%, and the recovery of galena was above 90% when DMDC and ZnSO4 were used as the combined depressant at pH 9. This indicated that the combined depressant had a better selectivity than the single depressant.
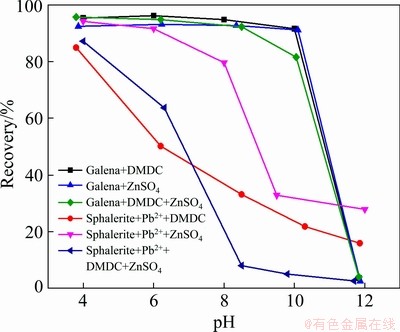
Fig. 2 Effect of pH on recovery of galena and sphalerite using depressant DMDC and ZnSO4
The flotation recoveries of galena and sphalerite as a function of the concentration of combined depressant (the mole ratio is 1:1) are depicted in Fig. 3. The results in Fig. 3 illustrated that an increase in the concentration of depressant decreased the sphalerite flotation. When the concentration of depressant reached 2×10-4 mol/L, the difference of the flotation recoveries between galena and sphalerite achieved optimum value.
The effect of the proportion of DMDC in combined depressant on flotation behavior was investigated, and the results are shown in Fig. 4. The results showed that with the increase in the proportion of DMDC, the recovery of galena remained above 90%, while the flotation recovery of sphalerite decreased first and then increased. The optimum mole ratio of DMDC to ZnSO4 was 1:1, and the flotation recovery of sphalerite was around 4%. In this case, it can achieve the flotation separation of sphalerite from galena by using the combined depressant ZnSO4 + DMDC.
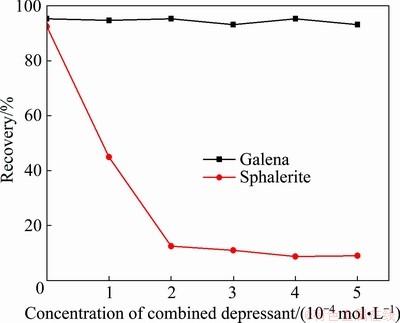
Fig. 3 Effect of concentration of combined depressant on flotation recovery of galena and sphalerite
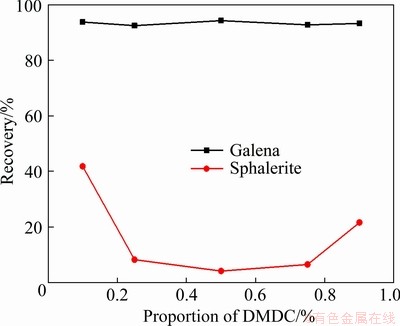
Fig. 4 Effect of proportion of DMDC in combined depressant on flotation recovery of galena and sphalerite
3.1.2 Flotation tests of artificially mixed minerals
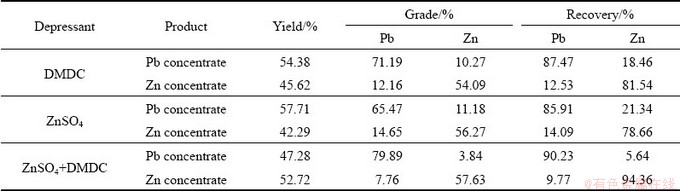
In order to study the excellent selective depression property of the combined depressant, the flotation separation of artificially mixed minerals (galena-sphalerite) was studied at pH 9, and the results are shown in Table 2. It is shown that the recovery of Zn is 18.46% and 21.34% in Pb concentrate with a single depressant of DMDC or ZnSO4. However, a concentrate with a recovery of 90.23% Pb and 5.64% Zn, a grade of 79.89% Pb and 3.84% Zn was obtained with the addition of 2×10-4 mol/L combined depressant. This result showed that the separation sphalerite from galena was unsatisfactory with a single depressant, but the lead-activated sphalerite can be effectively separated from galena with the combined depressant DMDC+ZnSO4.
Table 2 Flotation results of galena-sphalerite artificially mixed minerals
3.2 Ion complexing analysis
To study the depression mechanism of DMDC in the flotation separation of sphalerite and galena, UV-visible spectroscopy and ICP were measured, and the results are illustrated in Figs. 5 and 6. Figure 5 illustrates the complexation of DMDC with Pb2+ and Zn2+, and it was shown that there were three maximum adsorption peaks appeared in 208, 252 and 278.5 nm for DMDC and there were two maximum peaks disappeared in 252 and 278.5 nm after the addition of Pb2+. However, in terms of Zn2+, the adsorption peaks had no significant change, indicating that DMDC had a strong complex capacity with lead ions or hydroxy complexes of lead.
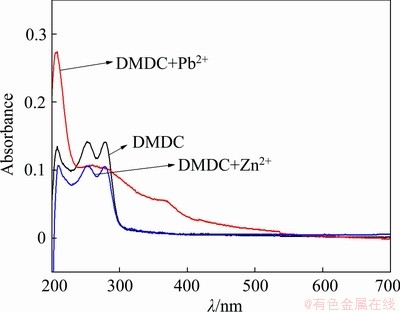
Fig. 5 Spectrophotometric adsorptions of ligand of DMDC and its complexes with Pb2+ and Zn2+
ICP was carried out to better study the complexation ability of DMDC. Figure 6 showed the concentration of Pb2+ in flotation slurry as a function of pH. It is shown that with increasing pH, the concentration of Pb2+ decreased rapidly. This could be attributed to the formation of hydroxyl compounds. In the previous study [29-31], we have illustrated that Pb(OH)+ became the dominant species in solution at pH>7. In addition, the concentration of Pb2+ decreased rapidly at a wide pH range compared with no DMDC addition, indicating the formation of the complex between DMDC and Pb2+. This could greatly reduce the concentration of Pb2+ in the slurry, thereby reducing the activation of sphalerite. These results are consistent with previous literature [32].
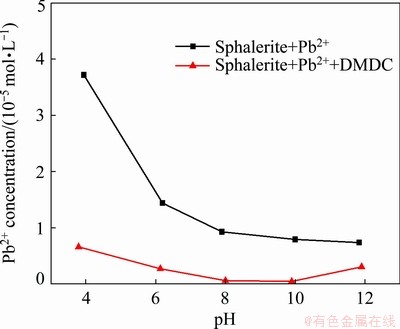
Fig. 6 Concentration of Pb2+ in flotation slurry as function of pH
3.3 Contact angle measurement results
The wettability of the mineral surface could be measured by the contact angle [33,34]. The larger the contact angle, the stronger the hydrophobicity and the better the floatability of mineral. In order to study the effect of ZnSO4 and DMDC on the hydrophobicity of mineral surfaces, the contact angles of galena and sphalerite surfaces under different reagent conditions were measured, and the results are illustrated in Fig. 7 (some contact angle diagrams were omitted). The contact angle of galena was measured to be 74.37° in the absence of any depressant (Picture 1). This indicated that galena had good hydrophobicity at pH 9. However, the contact angle of sphalerite was measured to be only 42.97° without Pb2+. The small contact angle indicated that the sphalerite exhibited a weak floatability. This was consistent with the results of previous research [3]. After the un-activated sphalerite was treated by Pb2+, it is shown that the contact angle of sphalerite increased to 62.49° (Picture 3), indicating that the hydrophobicity of sphalerite surface increased and sphalerite could be activated by Pb2+ [5,6]. After being treated by ZnSO4 and DMDC, the contact angles of sphalerite decreased to 42.71° and 39.97°, respectively. This indicated that ZnSO4 and DMDC could be adsorbed on the sphalerite surface to decrease the hydrophobicity of the mineral surface. Figure 7 shows that the contact angle of sphalerite was 37.17° (Picture 4) with the addition of combined depressant, indicating that the surface of sphalerite was more hydrophilic with the combined depressant than the single depressant. After galena treated by depressants, the contact angles were still above 67° (Picture 2), indicating that galena still had a lower hydrophobicity than sphalerite after the same sequential treatment by ZnSO4 and DMDC. The results are consistent with those of flotation, that ZnSO4 and DMDC can depress sphalerite but not galena.
3.4 XPS evaluation
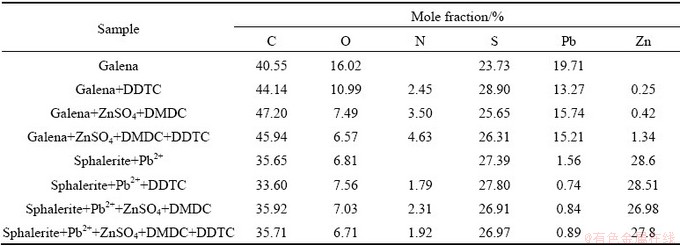
XPS test was employed to confirm the chemical species of galena and sphalerite with different reagents. Table 3 shows the relative contents of elements on galena and sphalerite. It was shown that the N 1s was observed on the surface of galena and sphalerite after adding collector DDTC and combined depressant ZnSO4+DMDC separately, indicating that the DDTC and DMDC could be chemically adsorbed on the surface of galena and sphalerite. After galena was treated by combined depressant ZnSO4+ DMDC and collector DDTC, the contents of N 1s increased from 3.5% to 4.63% on the galena surface, indicating that ZnSO4+DMDC and DDTC can be co-adsorbed on the galena surface. However, the relative content of N 1s decreased from 2.31% to 1.92% on the sphalerite surface, indicating that competitive adsorption between depressant and DDTC occurred on the sphalerite surface and this could reduce the adsorption of DDTC. For sphalerite, the relative content of Pb decreased to 0.84% from 1.56% after adding ZnSO4+DMDC. The results indicated that the adsorption of DMDC on sphalerite decreased the actives sites, which could reduce the activation of lead ions.
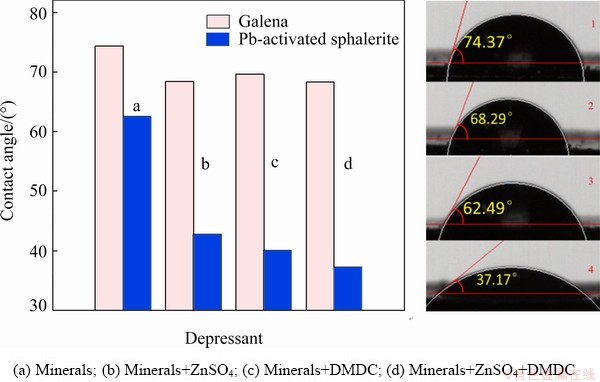
Fig. 7 Contact angles of galena and Pb-activated sphalerite surfaces pretreated with different depressants
Table 3 Mole fractions of elements on surfaces of galena and sphalerite
The narrow spectra of Pb on galena surface and Zn on sphalerite surface were illustrated in Fig. 8. After adding ZnSO4+DMDC and DDTC in sequence, the chemical shifts of Pb 4f5/2 and 4f7/2 on galena surface were 0.19 and 0.19 eV comparing with the shifts that are only adding ZnSO4+DMDC. While for sphalerite, the chemical shifts of Zn 2p1/2 and 2p3/2 on galena surface were only 0.11 and 0.11 eV. More chemical shifts of Pb on galena surface indicated that the adsorption of DDTC on galena surface was intenser than that on sphalerite surface. This was likely due to the competitive adsorption of ZnSO4+DMDC and DDTC on sphalerite surface, which reduce the adsorption of DDTC and results in an obvious reduction in the recovery of sphalerite.
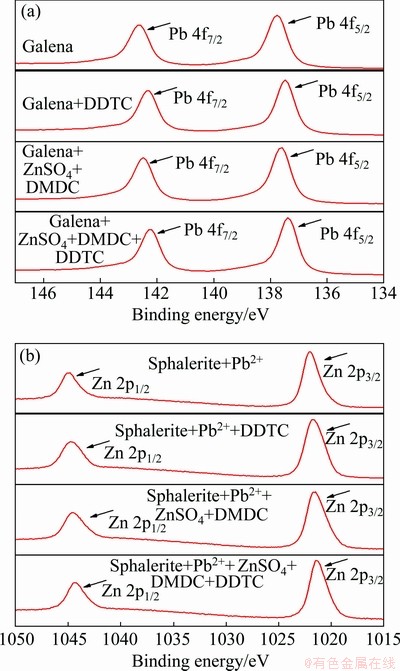
Fig. 8 XPS narrow spectra of Pb on galena (a) and Zn on sphalerite (b)
4 Conclusions
(1) Combined depressant ZnSO4+DMDC could achieve good flotation separation of sphalerite from galena at pH 9. A satisfactory flotation performance was achieved in the flotation of mixed mineral. The concentrate with a grade of 79.89% Pb and 3.84% Zn and a recovery of 90.23% Pb and 5.64% Zn was obtained with the addition of 2×10-4 mol/L combined depressants.
(2) UV-vis spectroscopy and ICP tests indicate that the DMDC has a strong complexing capacity with lead ions, which could reduce the activation of Pb2+.
(3) Contact angle test indicates that the single depressant ZnSO4 or DMDC could increase the hydrophilicity of sphalerite surface and the surface of sphalerite is more hydrophilic with the combined depressant ZnSO4+DMDC. However, the combined depressant has a small effect on the galena surface.
(4) XPS analysis indicates that the DMDC could remove Pb2+, and the combined depressant can prevent collector adsorbing on the Pb-activated sphalerite surface by a competitive adsorption method, while the combined depressant and collector are co-adsorbed on the galena surface.
References
[1] LI Jian-min, SONG Kai-wei, LIU Dian-wen, ZHANG Xiao-lin, LAN Zhuo-yue, SUN Yun-li, WEN Shu-ming. Hydrolyzation and adsorption behaviors of SPH and SCT used as combined depressants in the selective flotation of galena from sphalerite [J]. Journal of Molecular Liquids, 2017, 231: 485-490.
[2] LIU Jian, WANG Yu, LUO De-qiang, CHEN Lu-zheng, DENG Jiu-shuai. Comparativestudy on the copper activation and xanthate adsorption on sphalerite and marmatite surfaces [J]. Applied Surface Science, 2018, 439: 263-271.
[3] KHMELEVA T N, SKINNER W, BEATTIE D A. Depressing mechanisms of sodium bisulphite in the collectorless flotation of copper-activated sphalerite [J]. International Journal of Mineral Processing, 2005, 76(1): 43-53.
[4] SARVARAMINI A, LARACHI F, HART B. Collector attachment to lead-activated sphalerite—Experiments and DFT study on pH and solvent effects [J]. Applied Science Surface, 2016, 37: 459-472.
[5] RASHCHI F, SUI C, FINCH J A. Sphalerite activation and surface Pb ion concentration [J]. International Journal of Mineral Processing, 2002,67(1-4): 43-58.
[6] MOREY M S, GRANO S R, RALSTON J, PRESTIDGE C A, VERITY B. The electrochemistry of Pb2+ activated sphalerite in relation flotation [J]. Minerals Engineering, 2001, 14(9): 1009-1017.
[7] LIANG Yi-qiang, LIU Peng, SONG Tao, KAN Sai-qiong. Study on calcium-free ion flotation process for separation of high sulfur lead-zinc ores at low basicity [J]. Nonferrous Metals (Minerals Processing Section), 2019(5): 71-75. (in Chinese)
[8] WEI Zong-wu, ZHANG Han, SHI Xing-jun. Flotation technology research of a Pb-Zn polymetallic ore in Guangxi [J]. Modern Mining, 2019, 35(8): 86-89. (in Chinese)
[9] FENG Qi-ming, ZHOU Rong. Flotation separation of sphalerite from Pb-Zn-S bulk concentrate activated by cupric sulfate [J]. Mining and Metallurgical Engineering, 2011, 31(5): 32-34. (in Chinese)
[10] EL-SHALL H E, ELGILLANI D A, ABDEL-KHALEK N A. Role of zinc sulfate in depression of lead-activated sphalerite [J]. International Journal of Mineral Processing, 2000, 58(1): 67-75.
[11] KHMELEVA T N, CHAPELET J K. Depression mechanisms of sodium bisulphite in the xanthate-induced flotation of copper activated sphalerite [J]. International Journal of Mineral Processing, 2006, 79(1): 61-75.
[12] SEKE M D, PISTORIUS P C. Effect of cuprous cyanide, dry and wet milling on the selective flotation of galena and sphalerite [J]. Minerals Engineering, 2006, 19(1): 1-11.
[13] DRZYMALA J, KAPUSNIAK J, TOMASIK P. Removal of lead minerals from copper industrial flotation concentrates by xanthate flotation in the presence of dextrin [J]. International Journal of Mineral Processing, 2003, 70(1): 147-155.
[14] CHEN Jian-hua, LIANG Mei-lian, LAN Li-hong. Depression effect of azo organic depressants on sulphide [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(11): 2239-2247.
[15] HUANG Peng, CAO Ming-li, LIU Qi. Adsorption of chitosan on chalcopyrite and galena from aqueous suspensions [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2012, 409(17): 167-175.
[16] HUANG Peng, CAO Ming-li, LIU Qi. Selective depression of sphalerite by chitosan in differential Pb-Zn flotation [J]. International Journal of Mineral Processing, 2013, 122: 29-35.
[17] HUANG Peng, CAO Ming-li, LIU Qi. Selective depression of pyrite with chitosan in Pb-Fe sulfide flotation [J]. Minerals Engineering, 2013, 46-47(3): 45-51.
[18] LIU Jian, WANG Yu, LUO De-qiang, ZENG Yong. Use of ZnSO4 and SDD mixture as sphalerite depressant in copper flotation [J]. Minerals Engineering, 2018, 121: 31-38.
[19] QIN Wen-qing, JIAO Fen, SUN Wei, WANG Xing-jie, LIU Bei, WANG Jun, ZENG Ke, WEI Qian, LIU Kai. Effects of sodium salt of N,N-dimethyldi-thiocarbamate on floatability of chalcopyrite, sphalerite, marmatite and its adsorption properties [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2013, 421(11): 181-192.
[20] LIU Rui-zeng, QIN Wen-qing, JIAO Fen, WANG Xing-jie, PEL Bin, YANG Yong-jun, LAI Chun-hua. Flotation separation of chalcopyrite from galena by sodium humate and ammonium persulfate [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(1): 265-271.
[21] WANG Dao-wei, JIAO Fen, QIN Wen-qing, WANG Xing-jie. Effect of surface oxidation on the flotation separation of chalcopyrite and galena using sodium humate as depressant [J]. Separation Science and Technology, 2018, 53(6): 961-972.
[22] KAR B, SAHOO H, RATH S S, DAS B. Investigations on different starches as depressants for iron ore flotation [J]. Minerals Engineering, 2013, 49: 1-6.
[23] XIA Liu-yin, ZHONG Hong, LIU Guang-yi, WANG Shuai. Utilization of soluble starch as a depressant for the reverse flotation of diaspore from kaolinite [J]. Minerals Engineering, 2009, 22(6): 560-565.
[24] VALDIVIESO A L, CERVANTES T C, SONG S, CABRERA A R, LASKOWSKI J S. Dextrin as a non-toxic depressant for pyrite in flotation with xanthates as collector [J]. Minerals Engineering, 2004, 17(9-10): 1001-1006.
[25] WANG Ji-zhen, BAI Jun-zhi, YIN Wan-zhong, LIANG Xiao. Flotation separation of scheelite from calcite using carboxyl methyl cellulose as depressant [J]. Minerals Engineering, 2018, 127: 329-333.
[26] LONG Tao, FENG Qi-ming, LU Yi-ping, ZHANG Guo-fan, OU Le-ming, PAN Gao-chan. Depression and dispersion effect of carboxy methyl cellulose on flotation of layered magnesium-silicates [J]. The Chinese Journal of Nonferrous Metals, 2011, 21(5): 1145-1150. (in Chinese)
[27] KHMELEVA T N, SKINNER W, BEATTIE D A. Depressing mechanisms of sodium bisulphite in the collectorless flotation of copper-activated sphalerite [J]. International Journal of Mineral Processing, 2005, 76: 43-53.
[28] GREDELJ S, ZANIN M, GRANO S R. Selective flotation of carbon in the Pb-Zn carbonaceous sulphide ores of Century Mine, Zinifex [J]. Minerals Engineering, 2009, 22(3): 279-288.
[29] FUERSTENAU M C, MILLER J D, KUHN M C. Chemistry of flotation [M]. Australia: Society of Mining Engineers of AIME, 1985.
[30] CHEN Pan, ZHAI Ji-hua, SUN Wei, HU Yue-hua, YIN Zhi-gang. The activation mechanism of lead ions in the flotation of ilmenite using sodium oleate as a collector [J]. Minerals Engineering, 2017, 111: 100-107.
[31] CHEN Pan, ZHAI Ji-hua, SUN Wei, HU Yue-hua, YIN Zhi-gang, LAI Xiang-sheng. Adsorption mechanism of lead ions at ilmenite/water interface and its influence on ilmenite flotability [J]. Journal of Industrial and Engineering Chemistry, 2017, 53: 285-293.
[32] LIU Biao, WANG Xu-ming, DU Hao, LIU Jing, ZHENG Shi-li, ZHANG Yi, JAN D M. The surface features of lead activation in amyl xanthate flotation of quartz [J]. International Journal of Mineral Processing, 2016, 151: 33-39.
[33] YAN Xiao-ci, LUO Ming-dao. Interface chemistry [M]. Beijing: Chemical Industry Press, 2005. (in Chinese)
[34] MA Yi-wen, HAN Yue-xin, ZHU Yi-min, LI Yan-jun, LIU Hao. Flotation behaviors and mechanisms of chalcopyrite and galena after cyanide treatment [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(12): 3245-3252.
崔艳芳,焦 芬,覃文庆,董留洋,王 旭
中南大学 资源加工与生物工程学院,长沙 410083
摘 要:通过浮选试验、离子络合测试、接触角测试和XPS分析,研究组合抑制剂硫酸锌(ZnSO4)和二甲基二硫代氨基甲酸钠(DMDC)对闪锌矿的抑制机理。浮选试验表明,与单一抑制剂ZnSO4和DMDC相比,组合抑制剂ZnSO4+DMDC对闪锌矿有更好的选择性抑制效果。离子络合试验表明,DMDC对铅离子或其羟基络合物有很强的络合能力。接触角测试证明,与单一抑制剂ZnSO4和DMDC相比,组合抑制剂ZnSO4+DMDC使闪锌矿表面更加亲水。XPS分析表明,组合抑制剂通过竞争吸附阻止捕收剂在铅离子活化后的闪锌矿表面吸附,而在方铅矿表面,组合抑制剂与捕收剂产生共吸附。
关键词:吸附行为;接触角;络合;方铅矿;闪锌矿;组合抑制剂
(Edited by Xiang-qun LI)
Foundation item: Projects (51974364, 51904339) supported by the National Natural Science Foundation of China; Project (2018TP1002) supported by the Hunan Province for Clean and Efficiency Utilization of Strategic Calcium-containing Mineral, China
Corresponding author: Fen JIAO; Tel: +86-13549683403; E-mail: jfen0601@126.com
DOI: 10.1016/S1003-6326(20)65400-0

