乙硫氮在铁闪锌矿表面吸附的电化学行为及机理
余润兰1, 2, 邱冠周2, 胡岳华2, 覃文庆2
(1. 衡阳师范学院 化学与材料科学系, 衡阳 421008;
2. 中南大学 资源加工与生物工程学院, 长沙 410083)
摘 要: 采用光谱分析、 循环伏安及恒电位阶跃法, 研究了乙硫氮(NaD)在铁闪锌矿表面吸附的电化学行为及机理, 该机理与浮选电化学的混合电位模型并不一致。 在酸性条件下, 当电位为0~200mV时, 乙硫氮在铁闪锌矿表面电化学吸附形成双乙硫氮(D2); 当电位为410mV时, 乙硫氮与矿物发生电化学反应形成ZnD2和S0, 并产生钝化, 且表面疏水性强; 当电位大于600mV, 电极过程由自腐蚀反应控制。 在中性和碱性条件下, 铁闪锌矿表面的电极过程主要由自腐蚀阳极溶解控制。 随着pH值的增大, 表面中间态分别为Fe(OH)D2、 Fe(OH)2D和Zn(OH)D, 并随电位增大进一步氧化成Zn(OH)2、 Fe(OH)3和D2, 且矿物表面亲水性强。 在开路电位条件下, 铁闪锌矿表面存在双乙硫氮和乙硫氮金属盐, 但不能有效地附着在电极表面。
关键词: 铁闪锌矿; 乙硫氮; 吸附; 浮选电化学 中图分类号: TD923
文献标识码: A
Electrochemical adsorption behavior and mechanism of diethyldithiocarbamate on surface of marmatite
YU Run-lan1, 2, QIU Guan-zhou2, HU Yue-hua2, QIN Wen-qing2
(1. Department of Chemistry and Material Science, Hengyang Normal University,Hengyang 421008, China;
2. School of Minerals Processing and Bioengineering, Central South University,Changsha 410083, China)
Abstract: The electrochemical adsorption behavior and mechanism of diethyl dithiocarbamate (NaD) on the surface of marmatite, which are different from the mixed potential model of flotation electrochemistry, were studied by spectrum analysis, CA voltammetry and constant potential step. Under acidic condition, the dimer diethyldithiocarbamate (D2) is formed on marmatite through the electrochemical adsorption of diethyl dithiocarbamate at 0-200mV. ZnD2 and S0 are formed by electrochemical reaction and result in the passivation at 410mV, and the surface of marmatite has strong hydrophobicity. The electrode process is controlled by self-corrosive reaction at over 600mV. Under the condition of the neutral and base, the electrode process of marmatite is basically controlled by the anodic self-corrosion. With the increasing pH, the intermediates on the surface of marmatite are Fe(OH)D2, Fe(OH)2D, Zn(OH)D, respectively, which will be further oxidized into Zn(OH)2, Fe(OH)3 and D2 with the increase of the potential, and the surface of marmatite has strong hydrophilicity. There exist both dimer D2 and diethyl dithiocarbamate salts on the surface of marmatite at open-circuit potential, which can not effectively adhere on the surface of marmatite.
Key words: marmatite; diethyl dithiocarbamate; adsorption; flotation electrochemistry
电位可以调节和控制导致硫化矿物表面亲水和疏水的电化学反应, 决定了硫化矿物的浮选与抑制, 逐步发展成为电位调控浮选的新技术, 它是新世纪在矿物加工领域的重要发展方向之一[1, 2]。
脆硫锑铅矿(Pb4FeSb6S14)和铁闪锌矿(Zn1-x-FexS)是我国非常重要的、 有价的、 复杂的硫化矿物资源。 研究浮选药剂在硫化矿物表面的吸附电化学行为[3-7], 可加深对选择性浮选及电位调控浮选过程的更科学理解, 发展复杂多金属硫化矿物的浮选分离理论, 形成多金属复杂矿物电位调控浮选的创新技术, 加强矿产资源的综合利用, 提高经济效益。
文献[8, 9]已经初步报道了铁闪锌矿的腐蚀及与乙硫氮相互作用的机制。 本文作者采用光谱分析、 循环伏安及恒电位阶跃法, 进一步研究了乙硫氮在铁闪锌矿表面的吸附电化学机理。
1 实验
1.1 光谱分析
取未被氧化粒径小于150μm的铁闪锌矿矿粉1g, 分别加入到50mL pH值4.0的含0.1mol/L硫酸钠的邻苯二甲酸氢钠缓冲溶液、 pH值为6.86含0.1mol/L KNO3的磷酸盐和pH值为9.18的硼酸钠缓冲溶液中, 超声分散5min, 再加0.1mol/L乙硫氮0.5mL, 磁力搅拌10min, 澄清, 过滤, 用10mL环己烷萃取铁闪锌矿表面的疏水物质, 测定紫外光谱。 用蒸馏水洗涤过滤后的铁闪锌矿固体, 在常温下真空干燥, 在红外灯下制样, 用ATR测红外光谱。
紫外光谱分析仪为日本岛津UV3000紫外分光光度计, ATR红外测试采用美国Nicolet公司750-FTIR型傅立叶变换红外光谱仪。
1.2 电化学测试
pH值为6.86和9.18的缓冲溶液以0.10mol/L KNO3溶液作为支持电解质, 更高的pH值采用硼酸盐-氢氧化钠作缓冲溶液。 考虑到酸性条件下NO-3离子的弱氧化性可能对硫化物的氧化作用, 当pH值为4.0时, 采用0.1mol/L Na2SO4作支持电解质。 以1mmol/L乙硫氮(二乙基二硫代氨基甲酸钠, (C2H5)2NCSSNa, 用NaD表示)作为浮选捕收剂。 试剂皆为分析纯, 水为一次蒸馏水。
Fe2+以类质同像混入闪锌矿(ZnS)晶格中, 当铁含量超过6%时, 称为铁闪锌矿(Zn1-xFexS)。 经元素分析, 实验用铁闪锌矿的化学式为Zn0.77Fe0.23S。 恒电位阶跃测试的工作电极为铁闪锌矿圆柱形固体。 闪锌矿禁带能量Eg为3.60eV, 导电性差, 虽然铁的混入改善了铁闪锌矿的导电性能, 但仍不理想, 故循环伏安测试采用组成由光谱纯石墨、 矿粉和石蜡(质量比为7∶2∶1)的碳糊电极(carbon paste electrode, CPE)。 工作电极工作面的直径为15mm, 其他面都用环氧树脂密封。
电解池为三电极系统, 以铂电极做辅助电极, Ag/AgCl为参比电极, 但文中所提及的电位都已校正为相对于标准氢电极(vs SHE)的电位。 仪器为EG&G PAR公司的Potentiostat/Galvanostat Model 273A。 工作电极在通工业氮气的溶液中浸泡3h达到平衡后进行测量, 每次测量均用不同型号的砂纸逐级打磨, 最后用600#砂纸打磨成光面, 水洗以更新工作面。 测试温度恒定为25℃, 采用循环伏安测试, 且恒电位阶跃由M270系统控制。
2 结果与讨论
2.1 矿物表面疏水性物质的光谱分析
环己烷萃取铁闪锌矿表面疏水性物质的紫外分光光度曲线如图1所示。 由图1可看出, 在约230、 261和280nm(肩峰)处分别存在一个紫外吸收峰; 矿物表面吸附乙硫氮的量随pH值的升高而降低, 碱性条件下的吸附量下降更快些。
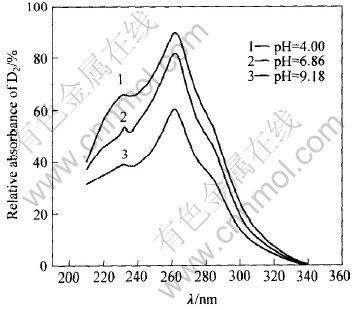
图1 铁闪锌矿与NaD相互作用后环己烷萃取固体矿物表面疏水性物质的紫外分光光谱
Fig.1 UV spectra of hydrophobe for extracted solution by cyclohexane from marmatite after interaction with NaD
为了鉴定铁闪锌矿表面的疏水性物质, 测量了NaD溶液的标准紫外光谱, 结果如图2所示。 取一定量的乙硫氮溶液, 加适量的H2O2使之氧化成双乙硫氮(D2), 未经提纯, 用环己烷萃取, 测得其紫外光谱(见图2)。 从图2可看出, 乙硫氮在256nm存在最强的吸收峰, 在280nm有一个比较强的肩峰, 在205nm峰处则对应着乙硫氮的紫外分解。 D2在230nm处的吸收峰最强, 261nm处的吸收峰次之, 280nm处的吸收峰最弱。 因此, 230nm处的吸收峰可作为鉴定双乙硫氮分子的特征紫外吸收峰, 261nm处的吸收峰则是双乙硫氮分子和乙硫氮金属盐的叠合峰。
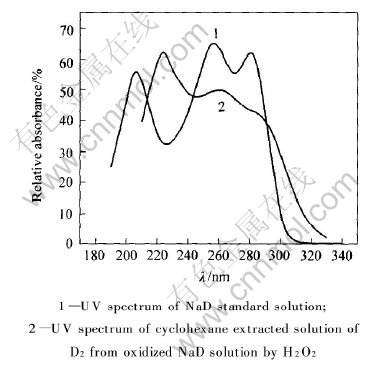
图2 NaD和D2的紫外分光光谱
Fig.2 UV spectra of NaD and D2
比较图1和2可知, 铁闪锌矿表面吸附的疏水性物质为双乙硫氮分子和乙硫氮金属盐。
与乙硫氮作用后, 铁闪锌矿的红外光谱如图3所示。 谱中存在 CH3基的反对称变角振动频率(1460±10)cm-1和对称变角振动频率在(1375±10)cm-1这两个特征峰[10], 所以, 铁闪锌矿表面存在捕收剂的吸附。 1025cm-1和1268cm-1处的吸收峰是双乙硫氮(D2)中的C-S和C—N—C键的特征吸收谱带, 1484cm-1、 1420cm-1和984cm-1分别是乙硫氮盐的υas(NCSS—)、 υs(NCSS—)和υ(NC-S)的吸收峰[11]。 铁闪锌矿表面体现双乙硫氮分子和捕收剂盐的吸附特征。 红外光谱弱是由于铁闪锌矿的红外基体散射效应大, 附着于铁闪锌矿表面物质为不稳定的中间体和双乙硫氮分子在矿物表面的附着力差, 易挥发损失所致。
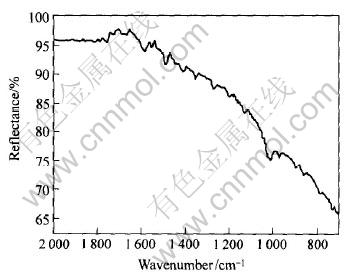
图3 与NaD作用后铁闪锌矿的红外光谱
Fig.3 Infrared spectrum of marmatite interacted by NaD
2.2 乙硫氮在铁闪锌矿表面的电化学吸附行为
图4所示为铁闪锌矿在存在乙硫氮和无乙硫氮时不同pH值情况下的循环伏安曲线。
由图4可看出, 在各pH值条件下, 矿物的自腐蚀伏安曲线仅由两个阳极峰组成。 第一个阳极峰(在400mV左右)的峰电流随pH值的改变变化不大, 没有明显的峰形状, 类似于半导体阳极溶解的伏安特性[12, 13]。 Hamilton等[14]将这种现象解释为铁离子脱离晶格, 形成缺金属富硫表层并产生钝化效应。 在更高电位下氧化为SO2-4, 使得电极的电流密度随电位的增大而持续增大。 因此, 铁闪锌矿的自氧化阳极过程在酸性条件下可表示为
Zn0.77Fe0.23S→0.77ZnS·Fe(0.23-x)S00.23(lattice)+xFe2++2xe(1)
在中性、 碱性条件下可表示为
Zn0.77Fe0.23S+(n+3x)OH-→0.77ZnS·Fe(0.23-x)S00.23(lattice)(OH)-n+xFe(OH)3+3xe(2)
在高电位或pH值大于9.18时
Zn0.77Fe0.23S+6.23H2O =0.77Zn(OH)2+0.23Fe(OH)3+SO2-4+8.23e+10.23H+(3)
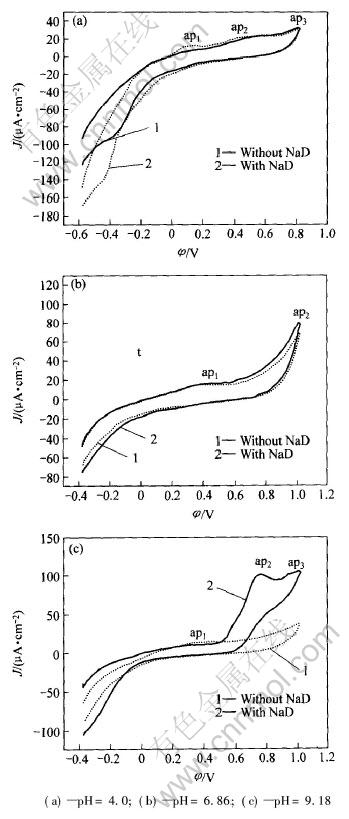
图4 铁闪锌矿电极在不同pH值缓冲溶液中的循环伏安曲线
Fig.4 Voltammogram curves of marmatite electrode in buffer solutions with different pH values
当乙硫氮存在时, 各pH值条件下的铁闪锌矿伏安曲线见图4中的2线。 当pH为 4.0时, 循环伏安曲线在0~0.2V存在阳极峰ap1, 对应反应(4), 即乙硫氮氧化成双乙硫氮D2。
2D-=D2+2e φ0=-0.015V
HD=H++D- Ka=10-5.6
φ=0.3154-0.059lgc(HD)-0.059pH(4)
在约410mV处存在ap2峰, 对应着乙硫氮在矿物表面发生电化学反应, 形成了乙硫氮金属盐和元素硫, 并产生钝化现象; 更高电位(大于600mV)的ap3峰为矿物自腐蚀反应产生了SO2-4, 对应反应(3), ap2峰的反应为
Zn0.77Fe0.23S+1.54HD =0.77ZnD2+0.23Fe2++S0+1.54H++2e(5)
当pH为6.86时, 无论体系中是否存在捕收剂, 在约0.4V时存在一个几乎相同又不明显的阳极峰(ap1), 没有明显的峰形状, 在0~0.2V处却没有明显的乙硫氮氧化成二聚分子的电流峰, 这是由于ap1峰的电极过程由Fe2+离子脱离晶格形成缺金属羟基化富硫层所致[9, 14]。 阳极峰ap2对应着形成SO2-4。
当pH为9.18时, 在约0.7V左右出现了明显的S2O2-3电流峰, 这是由于随着碱性增强, 铁闪锌矿的氧化作用增强, 缺金属富硫中间态的稳定性更差, 且ZnD2受羟基化水解的严重影响, 不能紧密附着在铁闪锌矿表面, 乙硫氮与铁闪锌矿表面直接发生电化学反应形成了S2O2-3峰(ap2), 且在更高电位下形成了SO2-4峰(ap3)所致。
当乙硫氮的浓度为1.0mmol/L, 按式(4)可计算出pH为4.00、 6.86和9.18时乙硫氮氧化成双硫氮的可逆电位φr(D2/D-)值为0.257V、 0.163V、 0.162V, 而铁闪锌矿的自腐蚀电位(φcorr)约为0.255V[9]。 当pH值为4.0时, φcorr≈φr(D-/D2), 则反应式(1)和(4)同时发生; 当pH值为6.86和9.18时, φcorr>φr(D-/D2), 按照混合电位模型, 铁闪锌矿表面的疏水物质应该是双乙硫氮分子[15]。 但是, 无论pH为4.00, 6.86还是9.18, 光谱分析都能检测到双乙硫氮分子和乙硫氮金属盐。
为弄清乙硫氮在铁闪锌矿表面的吸附电化学行为及作用机理, 本文作者通过恒电位阶跃法测得了铁闪锌在不同pH值的0.1mol/L KNO3溶液中、 存在或不存在乙硫氮时的电容和电阻随电位的变化曲线(见图5和6)。
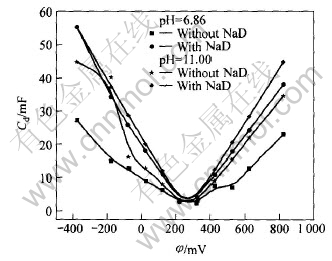
图5 铁闪锌矿在不同pH值0.1mol/L KNO3溶液中电容随电位的变化曲线
Fig.5 Change curves of capacitance with potential for marmatite electrode in 0.1mol/L KNO3 solution with different pH values
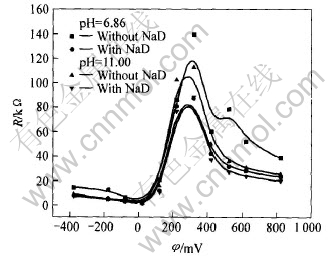
图6 铁闪锌矿在不同pH值0.1mol/L KNO3溶液中电阻随电位的变化曲线
Fig.6 Change curves of resistance with potential for marmatite electrode in 0.1mol/L KNO3 solution with different pH values
恒电位阶跃的电流—时间的关系可表示为
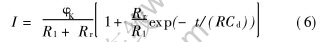
式中 R=R1Rr/(R1+Rr), 相当于溶液电阻R1和电化学反应电阻Rr的并联, φK是相对平衡电极电位的恒电位值, Cd是双电层微分电容, 则

式中 Ir为电流—时间出现平台时的电流; Δφ为阶跃幅值。 通过式(7)、 (8)和(9)就可以获得相应电阻及电容值。
存在捕收剂时的界面电容总是大于无捕收剂时的界面电容。 随着pH值的增大, 界面电容随之增大, 极化电阻随之减小。 在约300mV时, 二者的电容差别不大, 且存在一个极化电阻的极大值。 存在乙硫氮时的极化电阻比无乙硫氮时的极化电阻小, 乙硫氮促进阳极反应。 因此, 在中性和碱性条件下, 乙硫氮和OH-是铁闪锌矿的腐蚀剂, 乙硫氮与矿物的电化学反应所形成的疏水性产物不能有效吸附在矿物表面以抑制阳极过程, 或者矿物阳极过程和界面结构主要由矿物表面电化学反应形成产物的羟基化程度决定。
3 结论
在酸性条件下, 当φ≤φr(D-/D2) ≈φcorr时, 乙硫氮在铁闪锌矿表面化学吸附Zn1-xFexSOH+3—D-(ads), 并继续放电形成D2的电流峰; 当φ≥φr(D-/D2)≈φcorr时, 发生电化学反应生成了ZnD2和元素硫, 并产生钝化现象。 E大于600mV的电极过程由自腐蚀反应控制。 因此, 矿物表面在0~0.6V时具有疏水性, 即具有可浮选性能。
在中性和碱性条件下, 由于矿物表面易羟基化, 乙硫氮不能有效吸附在矿物表面, 电极过程由自氧化反应控制, 乙硫氮为腐蚀剂, 并促进矿物表面的氧化。 随着pH值的不同, 电化学反应形成Zn(OH)D、 Fe(OH)D2和Fe(OH)2D等不稳定的中间态。 随着电位和pH值的增大, Zn(OH)D、 Fe(OH)D2和Fe(OH)2D等会进一步氧化成Fe(OH)3和D2。 光谱分析测得的捕收剂盐、 D2分别为电化学反应的中间态及分解产物, 伏安行为不会出现独立的D2峰。 尽管羟基化的捕收剂盐具有一定的疏水性, D2也是疏水的, 但它们不能有效地附着在矿物表面, 可浮选性能会较差。
REFERENCES
[1]王淀佐. 浮选理论的新进展[M]. 北京: 科学出版社, 1992. 70-138.
WANG Dian-zuo. New Developments of Flotation Theory[M]. Beijing: Science Press, 1992. 70-138.
[2]王淀佐, 顾帼华, 刘如意. 方铅矿-石灰-乙硫氮体系电化学调控浮选[J]. 中国有色金属学报, 1998, 8(2): 321-326.
WANG Dian-zuo, GU Guo-hua, LIU Ru-yi. Potential adjustment flotation of galena-lime-diethyldithiocarbamate System[J]. The Chinese Journal of Nonferrous Metals, 1998, 8(2): 321-326.
[3]Buckley A N, Woods R. Chemisorption—the thermodynamically favored process in the interaction of thio collectors with sulfide minerals[J]. Int J Miner Process, 1997, 51(1): 15-26.
[4]YU Run-lan, HU Yue-hua, QIU Guan-zhou, et al. An electrochemical study of DDTC adsorption jamesonite[J]. Electrochemistry, 2004, 10(2): 145-153.
[5]余润兰, 邱冠周, 胡岳华, 等. 脆硫锑铅矿与捕收剂作用的界面电化学[J]. 中国有色金属学报, 2004, 14(1): 127-131.
YU Run-lan, QIU Guan-zhou, HU Yue-hua, et al. Interface electrochemistry of interaction of collector with jamesonite[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(1): 127-131.
[6]余润兰, 邱冠周, 胡岳华, 等. 脆硫锑铅矿在乙硫氮-饱和Ca(OH)2体系中的电化学[J]. 中国有色金属学报, 2004, 14(10): 1763-1769.
YU Run-lan, QIU Guan-zhou, HU Yue-hua, et al. Electrochemistry of jamesonite in system of diethyldithiocarbamate and saturated Ca(OH)2[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(10): 1763-1769.
[7]Woods R. Chemisorption of thios on metal and metal sulfides[A]. Bockris J O M, Conway B E, White R E. Proceedings of Modern Aspects of Electrochemistry[C]. New York: Plenum Press, 1997. 401-453.
[8]余润兰, 胡岳华, 邱冠周, 等. 循环伏安法研究铁闪锌矿的腐蚀及与捕收剂的相互作用[J]. 矿冶工程, 2004, 24(1): 41-43.
YU Run-lan, HU Yue-hua, QIU Guan-zhou, et al. A voltammetric study of corrosion and interaction of marmatite with collector[J]. Mining and Metallurgical Engineering, 2004, 24(1): 41-43.
[9]余润兰, 邱冠周, 胡岳华, 等. 铁闪锌矿的腐蚀电化学研究[J]. 中国腐蚀与防护学报, 2004, 24(4): 226-229.
YU Run-lan, QIU Guan-zhou, HU Yue-hua, et al. Corrosion electrochemical study of marmatite[J]. Journal of Chinese Society for Corrosion and Protection, 2004, 24(4): 226-229.
[10]吴瑾光. 近代傅立叶变换红外光谱技术及应用(上册)[M]. 北京: 科学技术文献出版社, 1994.
WU Jin-guang. Technology and application of Modern Fourier Transform Infrared (FT-IR) Spectrum(First Volume)[M]. Beijing: Scientific Technology and Literature Press, 1994.
[11]Bellamy L J. 复杂分子的红外光谱[M]. 黄维恒, 译. 北京: 科学出版社, 1975. 425-445.
Bellamy L J. Infrared Spectrum of Complicated molecules[M]. HUANG Wei-heng, tral. Beijing: Science Press, 1975. 425-445.
[12]Mishra K K, Asare K O. Aspects of the interface electrochemistry of semiconductor pyrite[J]. J Electrochem Soc, 1988, 135(10): 2502-2509.
[13]Wei Q, Asare K O. Semiconductor electrochemistry of particle pyrite: dissolution via hole and electron pathways[J]. J Electrochem Soc, 1996, 143(10): 3193-3198.
[14]Hamilton I C, Wood R. A voltammetric study of the surface oxidation of sulfide minerals[A]. Forssbery K S E. Developments in Mineral Processing of Flotation of Sulfide Minerals[C]. Netherland: Elsevier, 1985. 259-285.
[15]冯其明, 陈荩. 硫化矿物浮选电化学[M]. 长沙: 中南工业大学出版社, 1992.
FENG Qi-ming, CHEN Jin. Flotation Electrochemistry of Sulfides[M]. Changsha: Central South University of Technology Press, 1992.
(编辑李艳红)
基金项目: 国家自然科学基金资助项目(50234010)
收稿日期: 2005-02-25; 修订日期: 2005-07-13
作者简介: 余润兰(1965-), 男, 副教授, 博士.
通讯作者: 余润兰, 电话: 13187234582; E-mail: YRL715@sina.com