
Reaction behavior and mechanism of anatase in digestion process of diasporic bauxite
LI Xiao-bin(李小斌), FU Wei-an(付伟岸), ZHOU Qiu-sheng(周秋生),
LIU Gui-hua(刘桂华), PENG Zhi-hong(彭志宏)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 15 December 2008; accepted 29 March 2009
Abstract: Based on the study of influence of temperature, digestion time, amount of CaO added and composition of aluminate solution on reaction behavior of pure anatase in high-pressure digestion process of bauxite, reaction mechanism of anatase was preliminarily determined. Anatase first reacts with caustic soda to produce Na2TiO3, then the resultant Na2TiO3 reacts with 3CaO·Al2O3·6H2O resulting from the reaction of CaO with sodium aluminate solution to produce CaO·2TiO2·H2O which eventually converts into CaTiO3. Higher temperature, concentration of free Na2Ok (the caustic soda uncombined with aluminate anions in the form of Na2O) and molar ratio of CaO to TiO2 are favorable to the conversion of CaO·2TiO2·H2O to CaTiO3. And Al(OH)4- shows the function of a catalyzer in the reaction of anatase with caustic soda with or without CaO added during the digestion process of diasporic bauxite.
Key words: anatase; hydroxy calcium titanate; calcium titanate; high-pressure digestion; reaction mechanism
1 Introduction
There is 2%-4% of TiO2 mainly in the form of anatase, rutile or brookite in almost all kinds of bauxite[1-3]. In the digestion process at relatively low temperature for gibbsitic bauxite, chemical reactions between these titanium-containing minerals and components of aluminate solution will not take place. But as for the digestion of diasporic bauxite in China, higher digestion temperature ranging from 210 ℃ to 270 ℃ is required. In this case, titanium-containing minerals will react with components of aluminate solution. According to the results reported in Refs.[4-5], sodium titanates with different compositions produced by the reaction of titanium-containing mineral with caustic soda appear in a dense thin film covering on the surface of bauxite particles. The film can prevent diaspore from contacting with caustic solution, which significantly lowers the extraction rate of alumina from diasporic bauxite. In order to eliminate the inhibition effect of titanium-containing mineral, lime is always added to the original slurry in the high pressure digestion process for diasporic bauxite in the alumina industry. With the addition of lime in the digestion process, titanium-containing minerals react with calcium-bearing compounds to produce calcium-titanium compounds, such as CaO·TiO2 and CaO·2TiO2·H2O[6]. These compounds usually form substantial scale which is rather difficult to be cleaned up on the surface of heat exchanger during the bauxite digestion process so as to increase energy consumption and cause wastage of equipments productivity[7].
Up to now, although some research work was carried out on the composition, crystal morphology and lattice parameters of calcium-titanium compounds in the red mud[5], it is still unclear about the formation conditions of calcium-titanium compounds and reaction mechanism of titanium-containing minerals with the additive of lime in the alkaline aluminate solution. Therefore, it is of great importance to theoretically make clear the reaction behavior and mechanism of anatase in Bayer high-pressure digestion process of diasporic bauxite for scale control and removal to reduce manufacture cost and improve equipment efficiency.
However, as there are so many other minerals besides diaspore and anatase in bauxite ore, such as limestone, kaolinite, illite, hematite, goethite, etc, the chemical reactions and influencing factors are complex during high-pressure digestion process of diasporic bauxite, which is one of the reasons that the reaction mechanism of anatase in high-pressure digestion process of bauxite has not yet been revealed clearly. So, it is necessary to employ pure titanium-containing mineral to explore the reaction mechanism of titanium-containing mineral in high-pressure digestion process of diasporic bauxite. With consideration of the presence state and the sequence of reaction ability of titanium-containing minerals with caustic soda[8], anatase, the most representative titanium-containing mineral present in diasporic bauxite in China, was chosen as the starting material in this research work.
2 Experimental
2.1 Materials
TiO2 powder was a chemical reagent in the phase of anatase; NaOH and Na2CO3 were reagents with analytically pure grade; Sodium aluminate solution was synthesized in laboratory by heating the mixture of NaOH and Al(OH)3 with analytically pure grade; and CaO was prepared by calcination of Ca(OH)2 with analytically pure grade at 800 ℃ for 2 h.
2.2 Methods
Digestion operations of anatase were carried out in high-pressure autoclaves (made in Machine Factory of Central South University, China) with mixed nitrate molten salts as heating medium. The resultant slurry from digestion process was abruptly cooled down by cold water, then filtered by vacuum pump, and washed with boiling water three times. The filter cake was dried at 95-105 ℃, and finally weighed by electrical balance with a accuracy of 0.1 g. Then 0.50 g of filter cake was put into 100 mL hydrochloric acid solution with a volume ratio of hydrogen chlorine to water of 1, then heated at 100 ℃ for 15 min in thermostat and filtered while it was hot. The concentration of titanium dissolved in hydrochloric acid was measured by H2O2 colorimeter with 7230G spectrometer (Shanghai Analytical Apparatus Company, China).
Symbol ω(Ti) represents mass ratio of TiO2 dissolved in hydrochloric acid to the total amount of TiO2 present in the anatase added. Na2Ok represents caustic soda (NaOH) in the form of Na2O in alkali aluminate solution, and free Na2Ok represents the caustic soda uncombined with aluminate anions in the form of Na2O. Na2Oc represents sodium carbonate (Na2CO3) in the form of Na2O.
3 Results and discussion
3.1 Influence of temperature and time on reaction behavior of anatase
It was reported that the apparent activation energy of de-titanization in diasporic bauxite digestion slurry is 84.4 kJ/mol in the temperature range of 180-260 ℃, and surface chemical reaction is the controlling step of the de-titanization reaction in this temperature range[9-11]. Temperature and time have great influence on reaction ability and solubility of all minerals present in bauxite, and on the composition of scale and scaling speed during the bauxite digestion process. Composition of scale formed by Ti-containing minerals varies with temperature and time, such as CaO·2TiO2·H2O, titanium-containing hydrogarnet, and perovskite.
Fig.1 shows the influence of temperature on ω(Ti). It is obviously that ω(Ti) first increases slowly to reach its maximum and then decreases sharply with the increase of temperature. When temperature is lower than 180 ℃, ω(Ti) increases with the increase of time; when temperature is higher than 180 ℃, ω(Ti) first increases and then decreases with the prolongation of time.
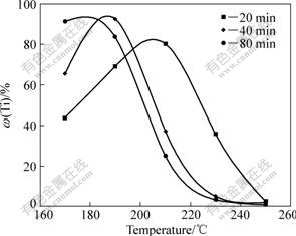
Fig.1 Influence of temperature on ω(Ti) of residue resulting from reaction of anatase with alkaline aluminate solution (ρ(Na2Ok)=233.11 g/L, ρ(Al2O3)=183.01 g/L, n(CaO)/n(TiO2)= 1.0)
Fig.2 represents XRD patterns of residue obtained at different digestion temperatures for various time. When digestion temperature is lower than 200 ℃, the main phases of residue are CaO·2TiO2·H2O and 3CaO·Al2O3·6H2O with a small amount of CaTiO3 as shown in Fig.2(a), and CaO·2TiO2·H2O converts into more stable CaTiO3 at higher digestion temperature and longer time, as shown in Fig.2(b).
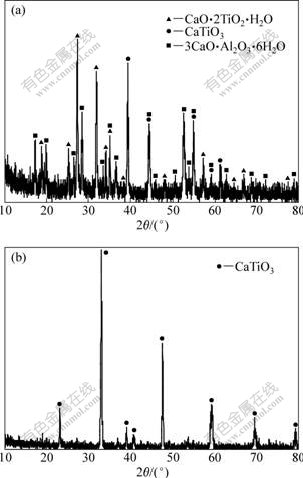
Fig.2 XRD patterns of residue prepared under different conditions (ρ(Na2Ok)=233.11 g/L, ρ(Al2O3)=183.01 g/L, n(CaO)/ n(TiO2)=1.0): (a) 170 ℃, 40 min; (b) 250 ℃, 80 min
CaTiO3 in the phase of perovskite is the most stable phase of titanium-containing compounds in residue in the digestion process of diasporic bauxite, which is also the main component of scale formed on the surface of heat exchanger in alumina refinery. Well crystallized CaTiO3 dissolves completely only in hydrofluoric acid, and with a small solubility in boiling hydrochloric acid[12-14], while poor crystallized CaTiO3, Na2TiO3 and CaO·2TiO2·H2O can completely dissolve in the boiling hydrochloric acid solution. With increasing the digestion temperature and time, more CaTiO3 with better crystallization degree is produced, which leads to the decreases of ω(Ti) rapidly. So, it should be noted that ω(Ti) is not always a reliable parameter to characterize reaction degree of titanium-containing mineral, which is different from the common knowledge.
3.2 Influence of composition of sodium aluminate solution on reaction behavior of anatase
When concentration of Na2Ok or free Na2Ok is fixed, influence of concentration of Al2O3 on reaction behavior of anatase is shown in Fig.3(a). ω(Ti) is almost invariable with the increase of concentration of Al2O3 while concentration of free Na2Ok is fixed. However, when concentration of Na2Ok is fixed, ω(Ti) decreases with the increase of concentration of Al2O3. It may be explained that the concentration of free Na2Ok decreases with the increase of concentration of Al2O3 in the alkaline aluminate solution. And it is the same case in Fig.3(b). The reason will be discussed later in detail.
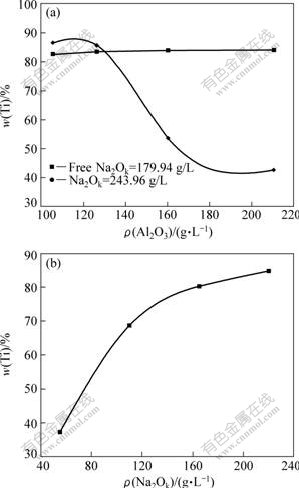
Fig.3 Influence of composition of sodium aluminate solution on ω(Ti): (a) 180 ℃, 30 min, n(CaO)/n(TiO2)=1.0; (b) ρ(Al2O3)=183.01 g/L, 180 ℃, 30 min, n(CaO)/n(TiO2)=1.0
3.3 Influence of molar ratio of CaO to TiO2 on reaction behavior of anatase in sodium aluminate solution
During the high-pressure digestion process, Na2TiO3 isolates diaspore from caustic soda so as to inhibit digestion of alumina in the diasporic bauxite. Calcium aluminate hydrate (3CaO·Al2O3·6H2O) is rapidly produced once CaO is added to the aluminate solution, then other reactions may take place. As for the familiar compounds, CaO·2TiO2·H2O and CaTiO3, theoretical molar ratios of CaO to TiO2 are 0.5 and 1.0. Research on the phase transformation of silicon-containing and titanium-containing minerals in high-pressure digestion proves that TiO2 mainly converts into CaO·2TiO2·H2O when molar ratio of CaO to TiO2 is less than 0.76, and that CaO·2TiO2·H2O becomes unstable and changes into more stable calcium-titanium compound gradually if more CaO is added[15-19].
Fig.4 indicates the relationship between ω(Ti) and molar ratio of CaO to TiO2. ω(Ti) increases with the increase of molar ratio of CaO to TiO2 with relatively low concentration of free Na2Ok, whereas ω(Ti) first increases and then decreases with the increase of molar ratio of CaO to TiO2 under the condition of high concentration of free Na2Ok. For example, ω(Ti) decreases from 84.70% to 35.63% with molar ratio correspondingly ranging from 0.5 to 3.0 when concentration of free Na2Ok is fixed at 220 g/L. Fig.5 shows the XRD pattern of residue obtained under the following conditions: the molar ratio of CaO to TiO2 is 3, and the concentration of free Na2Ok is 220 g/L. It can be revealed that CaO·2TiO2·H2O changes into more stable CaTiO3 which is hard to dissolve in hydrochloric acid.
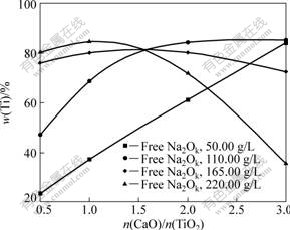
Fig.4 Influence of molar ratio of CaO to TiO2 on ω(Ti) (ρ(Al2O3)=180.94 g/L, 180 ℃ for 30 min)
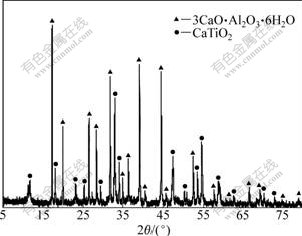
Fig.5 XRD pattern of residue prepared with high molar ratio of CaO to TiO2 and high concentration of free Na2Ok (ρ(Al2O3)=180.94 g/L, n(CaO)/n(TiO2)=3.0, ρ(free Na2Ok)=220 g/L, 180 ℃, 30 min)
3.4 Reaction mechanism of anatase in high-pressure digestion process
In order to explore the reaction laws and its mechanism of titanium-containing mineral during high-pressure digestion process of diasporic bauxite, digestion experiments of anatase in different solutions were carried out at 180 ℃ for 60 min. Experimental results are listed in Table 1.
Table 1 Reaction behavior of anatase in different solutions
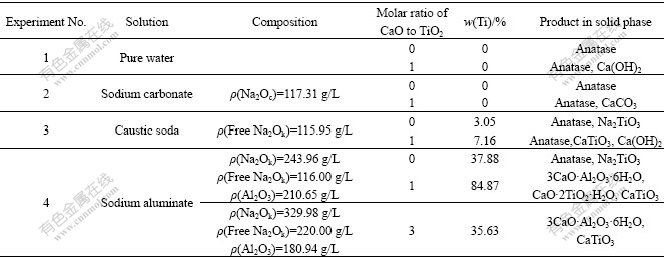
From results listed in Table 1 and the reaction behaviors of anatase discussed above, reaction mechanism of anatase in sodium aluminate solution during the high-pressure digestion process can be presumably determined as follows.
1) Neither soda (Na2CO3) nor Ca(OH)2 can react with anatase under the experimental conditions. However, anatase can react with NaOH to form Na2TiO3 which may further react with Ca(OH)2 to form CaTiO3 if CaO is added to the system, as shown in reactions (1) and (2):
2NaOH+TiO2=Na2TiO3+H2O (1)
Ca(OH)2+Na2TiO3=CaTiO3+2NaOH (2)
2) Aluminate anion seems to remarkably catalyze reactions of anatase with caustic soda solution. Results of experiment 3 and experiment 4 show that, under the almost same concentrations of free Na2Ok (Na2Ok 116.00 g/L), ω(Ti) increases from 3.05% to 37.88% in the presence of aluminate anion without CaO, and ω(Ti) also increases from 7.16% to 84.87% in the presence of aluminate anion when CaO is added with molar ratio of CaO to TiO2 of 1. So, the conclusion can be drawn that aluminate anions plays catalysis role in the reaction process. The catalysis mechanism of aluminate anions could be explained as follows:
2NaAl(OH)4+TiO2=Na2TiO3+2Al(OH)3+H2O (3)
NaOH+Al(OH)3=NaAl(OH)4 (4)
The total reaction can be expressed as
2NaOH+ TiO2=Na2TiO3+H2O (5)
3) Hydroxy calcium titanate (CaO·2TiO2·H2O) is the product of reaction of Na2TiO3 with calcium aluminate hydrate (3CaO·Al2O3·6H2O) resulting from the reaction of CaO added with sodium aluminate solution, as shown in chemical reactions (6) and (7):
3Ca(OH)2+2NaAl(OH)4=3CaO·Al2O3·6H2O+2NaOH (6)
3CaO·Al2O3·6H2O+6Na2TiO3+6H2O=
3[CaO·2TiO2·H2O]+2NaAl(OH)4+10NaOH (7)
Calcium titanate (CaTiO3) may be formed from two ways. One is from reaction of Na2TiO3 and Ca(OH)2, and the other is from reaction of CaO·2TiO2·H2O with 3CaO·Al2O3·6H2O and NaOH, as shown in chemical reaction (8):
3[CaO·2TiO2·H2O]+3CaO·Al2O3·6H2O+2NaOH=
6CaTiO3+2NaAl(OH)4+6H2O (8)
According to the theoretical calculation, reaction (8) may take place only if molar ratio of CaO to TiO2 is more than 0.5. Result from experiment 4 in Table 1 also indicates that more CaO·2TiO2·H2O converts into CaTiO3, which results in the decrease of ω(Ti), if molar ratio of CaO to TiO2 is 3. From the discussion mentioned above, conclusion can be made that elevating the digestion temperature, prolonging the duration time, increasing concentration of free Na2Ok and molar ratio of CaO to TiO2 favor the conversion of CaO·2TiO2·H2O to CaTiO3.
4 Conclusions
1) In sodium aluminate solution, aluminate anion has a catalysis function for reaction of anatase with caustic soda.
2) During high-pressure digestion process of diasporic bauxite, the reaction degree of anatase depends on the concentration of free Na2Ok rather than the concentration of Al2O3, so long as there is aluminate anions in alkali solution.
3) Anatase first reacts with free Na2Ok to form Na2TiO3 which further reacts with 3CaO·Al2O3·6H2O to produce CaO·2TiO2·H2O, and CaO·2TiO2·H2O eventually changes into CaTiO3. Moreover, higher temperature, longer time, higher concentration of free Na2Ok and molar ratio of CaO to TiO2 all favor the conversion of CaO·2TiO2·H2O to CaTiO3.
References
[1] ZHAO Heng-qin, LI Jie, WANG Li-zhuo, LIU Ye-xiang. Review of current situation on bauxite resources and production technology of alumina in China [J]. Conservation and Utilization of Mineral Resources, 2001(5): 38-42. (in Chinese)
[2] YANG Zhong-yu. Technology for alumina production [M]. Beijing: Metallurgical Industry Press, 1993: 176-179. (in Chinese)
[3] ALDABERGENOVA S B, GHICOV A, ALBU S, MACAK J M, SCHMUKI P. Smooth titania nanotubes: Self-organization and stabilization of anatase phase [J]. Journal of Non-Crystalline Solids, 2008, 354(19/25): 2190-2194.
[4] Миронов М В, Паэухин В А. Reaction behavior of titanium oxide in the caustic soda and sodium aluminate solution [J]. Nonferrous Metallurgy, 1959(1): 83-90. (in Russian)
[5] деревянкин в а, кузнецов с и, шабалина о к. Influence of titanium oxide and silicon added on the leaching speed of alumina hydrate [J]. Applied Chemistry, 1961, 34(7): 1456-1461. (in Russian)
[6] CHEN Wan-kun, PENG Guan-cai. Technology of intensified digestion process for diaspore [M]. Beijing: Metallurgical Industry Press, 1997: 104-109. (in Chinese)
[7] PALMER S J, FROST R L, NGUYEN T. Hydrotalcites and their role in coordination of anions in Bayer liquors: Anion binding in layered double hydroxides [J]. Coordination Chemistry Reviews, 2009, 253(1/2): 250-267.
[8] BI Shi-wen, YANG Yi-hong, LI Dian-feng. Digestion of bauxite by Bayer process [M]. Beijing: Metallurgical Industry Press, 1996: 148-153. (in Chinese)
[9] YANNIS P, YANNIS T. Reaction kinetics for the leaching of iron oxides in diasporic bauxite from the Parnassus-Giona Zone (Greece) by hydrochloric acid [J]. Hydrometallurgy, 1987, 19(2): 259-266.
[10] YIN Zhong-lin, BI Shi-wen, GU Song-qing. Study of reaction kinetics of Ti-containing minerals in preheating process of bauxite slurry [J]. Mining and Metallurgical Engineering, 2005(4): 54-57. (in Chinese)
[11] REDDY B R, MISHRA S K, BANERJEE G N. Kinetics of leaching of a gibbsitic bauxite with hydrochloric acid [J]. Hydrometallurgy, 1999, 51(1): 131-138.
[12] BI Shi-wen, LI Dian-feng, YANG Yi-hong. The study on the reaction of rutile in Bayer liquor [C]//Light Metals. Warrendale, Pennsylvania, 1996: 43-48.
[13] GU Song-qing, CAO Rong-jiang, CHENG Xin-min. CaO·2TiO2·H2O in Bayer digesting process [J]. Chinese Journal of Rare Metals, 1987, 6(3): 161-166. (in Chinese)
[14] PAWLEK F,KHEIRI M J,KAMMEL H C R. The leaching behavior of bauxite during mechanochemical treatment [C]//Light Metals. Warrendale, Pennsylvania, 1992: 91-95.
[15] AI Zi-jin. Study on behavior of Si and Ti during indirectly heating process for bauxites from different areas [J]. Nonferrous Metals: Extractive Metallurgy, 1995(6): 33-36. (in Chinese)
[16] CHEN Li, LI Man. Phase transformation of Si-containing and Ti-containing minerals during high-pressure leaching process for bauxites [J]. Nonferrous Metals: Extractive Metallurgy, 1991(5): 25-29. (in Chinese)
[17] CHEN Li, LI Man. Phase transformation of Si-containing and Ti-containing minerals during high-pressure leaching process for bauxite from Guizhou [J]. Light Metals, 1991(6): 18-22. (in Chinese)
[18] CHEN Li, LI Yong. The effect of lime on high pressure digestion of bauxite in Guizhou [J]. Journal of Guizhou University of Technology, 1995, 24(1): 44-47. (in Chinese)
[19] LI Yong. The effect of lime in the process of digestion of Guizhou diaspore bauxite [J]. Journal of Guizhou University of Technology, 1996, 25(5): 78-82. (in Chinese)
Foundation item: Project(2005CB6237-02) supported by the National Basic Research Program of China
Corresponding author: ZHOU Qiu-sheng; Tel/Fax: +86-731-88830453; E-mail: csqszhou@163.com
DOI: 10.1016/S1003-6326(09)60111-4
(Edited by YANG Hua)