Trans. Nonferrous Met. Soc. China 24(2014) 3343-3347
Influence of sodium 2,3-dihydroxypropyl dithiocarbonate on floatability of chalcopyrite and galena
Zheng-jie PIAO1, De-zhou WEI1, Zhi-lin LIU1,2
1. College of Resources and Civil Engineering, Northeastern University, Shenyang 110004, China;
2. Faculty of Resource and Environmental Engineering, Jiangxi University of Science and Technology, Ganzhou 341000, China
Received 27 May 2013; accepted 30 November 2013
Abstract: Sodium 2,3-dihydroxypropyl dithiocarbonate (SGX), which contains —OH and —CSS— in the molecule, was used to explore selective depression of galena from chalcopyrite in the flotation tests with ammonium dibutyl dithiophosphate (DDTP), and zeta potential and adsorption measurements were performed to study the interaction between SGX and minerals. The flotation tests of single minerals show that SGX has slight activation on chalcopyrite and strong depression on galena in the whole pH range. With SGX dosage increasing, the recovery of galena decreases rapidly, while that of chalcopyrite increases slightly. At pH=6, the copper grade and recovery of concentrate are 29.52% and 82.15% respectively when mixture of two minerals is tested. Zeta potential and adsorption measurements indicate that SGX has strong adsorption on galena and slight adsorption on chalcopyrite.
Key words: sodium 2,3-dihydroxypropyl dithiocarbonate; depression; flotation; chalcopyrite; galena
1 Introduction
In the past long time, inorganic compounds were mainly used as depressants in the separation of complex sulfide ores, which made many problems in the application, such as poor selectivity, high dosage and unfriendliness to the environment. With the development of society, because of the advantages of better selectivity, structural diversity and environmental friendliness of organic compounds, many scholars mainly focused on the study of organic depressants. XIONG et al [1], and HE et al [2] indicated that sodium glycerine xanthate (SGX) depressed arsenopyrite and pyrite strongly, but had a slight depression on marmatite by butyl xanthate. Zeta potential and adsorption isotherm showed that SGX adsorbed more strongly on arsenopyrite and pyrite than marmatite.
LIU et al [3] found that ferrochrome lignin (FCLS) depressed chalcopyrite slightly, but depressed galena strongly. IR spectrum analysis indicated that FCLS adsorbed strongly on galena while the absorption on chalcopyrite was weak. DONG [4] showed that mixtures of carboxymethyl cellulose, silicate sodium and sodium sulfite could depress galena well, but depress chalcopyrite slightly. QIN et al [5] indicated that sodium pyrophosphate (SPH) separated chalcopyrite from galena in acidic condition by O-isopropyl-N-ethyl thionocarbamate. The infrared spectral analysis indicated chemical adsorption between SPH and galena. WEI [6] had synthesized a new organic depressant PPD, and found that PPD depressed galena but activated chalcopyrite, because PPD anion could react with Pb2+ to produce hydrophilic compound, which covered on the surface of galena and hindered the collector to adsorb on the surface of galena, and the interaction was chemisorption. BULATOVIC and WYSLOUIIL [7] reported that RB-SO2-starch could separate chalcopyrite from galena by depressing galena. Some researchers [8-10] indicated that dextrin selectively depressed galena while chalcopyrite was floated with xanthate as the collector.
HUANG et al [11,12] found that chitosan, a linear structure of β-(1–4)-linked D-glucosamine, partially deacetylated from the β-(1–4)-linked N-acetyl-D- glucosamine, depressed chalcopyrite while galena was floated by xanthate. Chitosan-metal ions adsorption test, TOF-SIMS and X-ray photoelectron spectroscopy (XPS) were employed to study the interaction between chitosan and minerals, and the TOF-SIMS measurements indicated that chitosan adsorbed slightly on galena but adsorbed largely on chalcopyrite.
Because of the similar floatability of chalcopyrite and galena, the separation of two minerals is difficult. In this work, sodium 2,3-dihydroxypropyl dithiocarbonate (SGX) was studied on two minerals at different pulp pH values by ammonium dibutyl dithiophosphate (DDTP), and zeta potential and adsorption isotherms measurements were used to discuss the interaction mechanism between SGX and minerals.
2 Experimental
2.1 Materials
The mineral sample of chalcopyrite was obtained from Jiujiang, Jiangxi Province in China, and galena was obtained from Outer Mongolia. The size of sample used in flotation tests was ≤0.1 mm. Chemical composition analysis of two minerals showed that the purities of chalcopyrite and galena were 95.2% and 99.3% respectively.
2.2 Reagents
Ammonium dibutyl dithiophosphate and terpenic oil were industrial grade products from Tieling Flotation Reagents Factory, Liaoning, China. Sodium 2,3-dihydroxypropyl dithiocarbonate was synthesized in our lab. Analytical grade HCl or NaOH was used to adjust the pH value of flotation pulp.
2.3 Flotation tests
For each flotation test, 2 g of sample was taken and ultrasonically washed for 5 min to remove any possible oxides on the mineral surface. The suspension liquid was settled, and then the upper liquid layer was decanted and the remaining part was floated, after that the floated and non-floated fractions were filtered, dried and weighed for the recovery calculation. The flotation tests were carried out in a microflotation cell with a 35 mL effective volume.
2.4 Zeta potential measurement
Nano-ZS90 apparatus (Made in England) was used to measure zeta potential of minerals. First of all, the pure minerals were ground to be smaller than 5 μm in the agate mortar for the tests. Then, a small amount of mineral powder was added to a beaker with 50 mL distilled water and cleaned for 5 min with ultrasonic generator to remove the oxidation film. At last, the reagents were added to the solution, and the suspension was stirred for 3 min with a magnetic stirring apparatus.
2.5 Adsorption isotherms measurement
Adsorption isotherms measurement was photolab 6600 (Made in German). The sample was taken into the vessel and ultrasonically washed for 5 min, after that the washing solution was decanted and added 30 mL distilled water. Then, a certain dosage of reagent was taken to the vessel and ore pulp was stirred for 1.5 h. The hypothesis was that the depleted dosage of reagent had been adsorbed onto the mineral surface. At last, the mineral solution was centrifuged, and the supernatant was used to measure the residual reagent concentration [13,14].
3 Results and discussion
3.1 Flotation of pure minerals
The effects of pH on flotation of chalcopyrite by the collector DDTP (6.5 mg/L) with or without SGX (1.9 g/L) as a function of pH value are shown in Fig. 1. As seen from Fig. 1, the recovery of chalcopyrite is above 85% without SGX depressant in the whole range of pH, and increases slightly in the presence of SGX. The increment of chalcopyrite recovery gets slowly with the increase of pH.
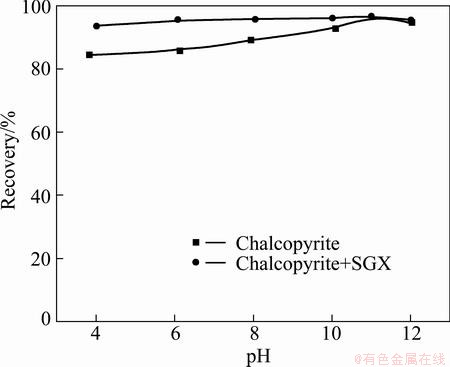
Fig. 1 Effects of pH on flotation of chalcopyrite by collector DDTP (6.5 mg/L) with or without SGX depressant (1.9 g/L)
The recovery of galena by the collector DDTP (6.5 mg/L) with or without SGX depressant (1.9 g/L) as a function of pH value is described in Fig. 2. It is shown that the recovery of galena is maintained at about 80% without SGX depressant when pH value is below 10; it is dropped to 55% at pH=11. In the presence of SGX depressant, the recovery of galena decreases slightly with pH value increasing and that is about 28% and 15% respectively when pH values are 4 and 11. According to Refs. [2,15], this is maybe attributed to the competition mechanism between DDTP and SGX on the mineral surface. Hydrophilic groups of SGX are adsorbed on the surface of galena to form a hydrophilic film, which hinders the absorption of DDTP, thus galena is depressed.
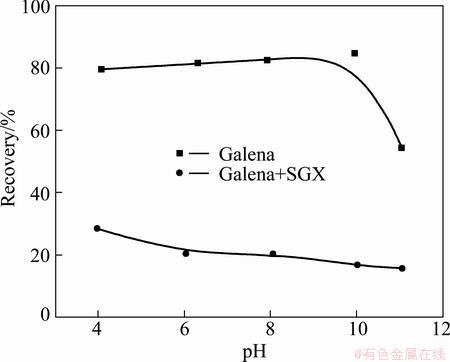
Fig. 2 Effects of pH on flotation of galena by collector DDTP (6.5 mg/L) with or without SGX depressant (1.9 g/L)
In order to find the appropriate dosage of SGX at pH 6, the effects of concentrations of SGX on flotation of chalcopyrite and galena with DDTP (6.5 mg/L) are considered. As seen from Fig. 3, in the presence of SGX depressant, the recovery of chalcopyrite increases to about 95% with increasing SGX dosage, but that of galena decreases rapidly. When the concentration is 1.9 g/L, the recoveries of chalcopyrite and galena are about 96% and 20%, respectively.
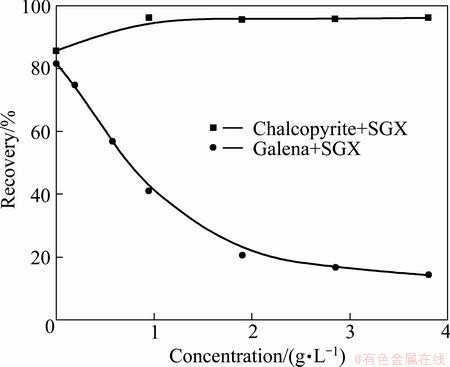
Fig. 3 Effects of concentrations of SGX on flotation of chalcopyrite and galena by collector DDTP (6.5 mg/L) at pH=6
3.2 Flotation of artificially mixed minerals
According to the single mineral flotation tests, it can be seen that chalcopyrite may be separated from galena with SGX depressant in certain condition, upon which artificially mixed samples (the mass ratio of chalcopyrite and galena is 1:1) are adopted to demonstrate that SGX is an effective depressant to separate chalcopyrite from galena.
The separation efficiency of chalcopyrite by SGX depressant (1.9 g/L) with the collector DDTP (6.5 mg/L) as a function of pH value is shown in Fig. 4. The copper grade and recovery of concentrate are increased and decreased respectively with pH value increasing. When pH value is 6, the copper grade and recovery of concentrate are 29.52% and 82.15%, respectively.
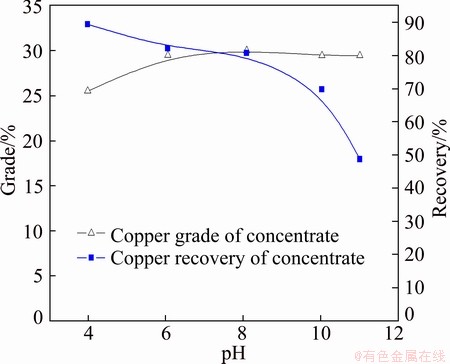
Fig. 4 Effects of pH on separation of chalcopyrite by SGX depressant (1.9 g/L) with collector DDTP (6.5 mg/L)
The effects of SGX dosage on separation efficiency of chalcopyrite with collector DDTP (6.5 mg/L) are described in Fig. 5. It can be seen that the copper grade of concentrate increases from about 24% to 31% with SGX dosage increasing, while the copper recovery of concentrate decreases from 86% to 70%. When the concentration of SGX is 1.9 g/L, the separation efficiency of chalcopyrite is preferable.
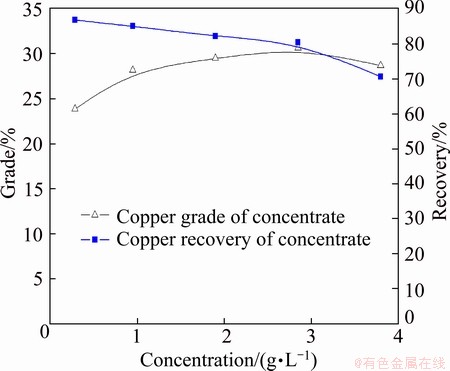
Fig. 5 Effects of concentrations of SGX on separation of chalcopyrite by collector DDTP (6.5 mg/L) at pH=6
3.3 Zeta potential measurement
The effects of SGX on zeta potential of chalcopyrite and galena are shown in Figs. 6 and 7 respectively. It is indicated that the negative charges of chalcopyrite and galena increase in the presence of SGX, and the increment of negative charges on galena is more than chalcopyrite, which proves that SGX has good flotation selectivity between chalcopyrite and galena. Because of the adsorption of SGX, the potential of minerals is negative in the whole range of pH, and the potential of minerals is positive in the absence of SGX at a low pH value, which demonstrates that there is electrostatic attraction between SGX and minerals. The negative potential of minerals is higher with SGX relative to that without SGX at a high pH value, implying the existence of other types of interaction between SGX and minerals.
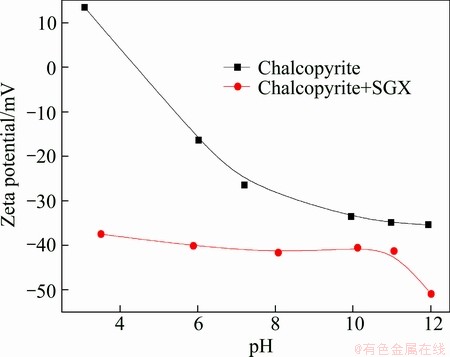
Fig. 6 Zeta potential of chalcopyrite as function of pH
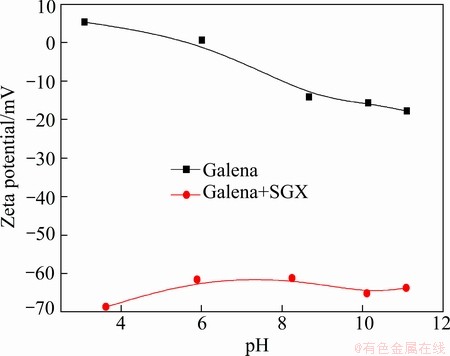
Fig. 7 Zeta potential of galena as function of pH
3.4 Adsorption isotherms measurement
The adsorbing capacity may testify the depression performance of SGX on minerals as the more the adsorption, the better the depression performance. The adsorption of SGX on chalcopyrite and galena with dosage of SGX at pH=6 is shown in Fig. 8. As seen from Fig. 8, the adsorption of SGX on galena is increased rapidly with dosage increasing, which is consistent with the results of flotation tests (Fig. 3). The adsorbing curve of galena is nearly linear when the dosage of galena is below 1.9 g/L, and this may be due to the chemisorption between SGX and galena [16,17].
Adsorption of SGX on chalcopyrite and galena as a function of pH value is described in Fig. 9. It is shown that the adsorption of SGX on galena is distinctly larger than that on chalcopyrite in the whole range of pH value, and the adsorption amounts with galena and chalcopyrite is maintained at about 25 mg/g and 5 mg/g, respectively, when pH value is above 6.
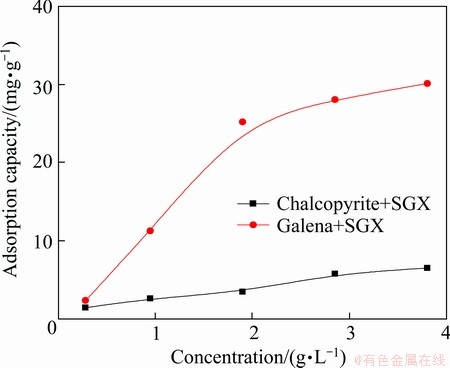
Fig. 8 Adsorption of SGX on chalcopyrite and galena as function of SGX concentration (pH=6)
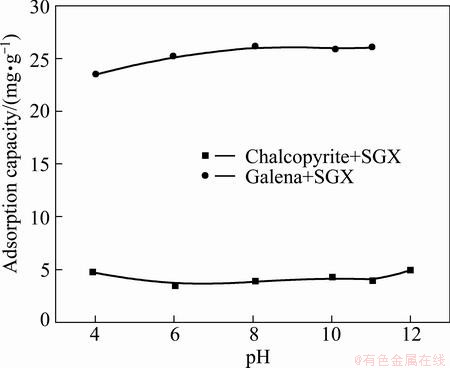
Fig. 9 Adsorption of SGX on chalcopyrite and galena as function of pH
4 Conclusions
1) Sodium 2,3-dihydroxypropyl dithiocarbonate (SGX) activates chalcopyrite slightly and depresses galena strongly with ammonium dibutyl dithiophosphate (DDTP) as the collector in the flotation of single minerals. Galena is depressed by SGX in the whole range of pH, and the recovery of galena is below 28%. The satisfied results of artificially mixed samples are that the copper grade and recovery of concentrate are 29.52% and 82.15% respectively when SGX dosage is 1.9 g/L and pH value is 6.
2) Zeta potential and adsorption measurements illustrate that SGX has a higher adsorption capacity on galena than chalcopyrite.
References
[1] XIONG Dao-ling, HU Yue-hua, QIN Wen-qing, HE Ming-fei. Synthesis of glycerine-xanthate and its depressing mechanism in separation of marmatite from arsenopyrite [J]. Journal of Central South University of Technology, 2006, 13(6): 678-682.
[2] HE Ming-fei, QIN Wen-qing, LI Wei-zhong, ZENG Ke. Pyrite depression in marmatite flotation by sodium glycerine-xanthate [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(5): 1161-1165.
[3] LIU Run-qing, SUN Wei, HU Yue-hua. Study on organic depressant FCLS for separation of chalcopyrite and galena [J]. Mining and Metallurgical Engineering, 2009, 29(3): 29-32. (in Chinese)
[4] DONG Yan-fei. Study on the electrochemistry mechanism of flotation separating copper-lead sulphide minerals [D]. Changsha: Central South University, 2011. (in Chinese)
[5] QIN Wen-qing, WEI Qian, JIAO Fen, LI Ning, WANG Pei-pei, KE Li-fang. Effect of sodium pyrophosphate on the flotation separation of chalcopyrite from galena [J]. International Journal of Mining Science and Technology, 2012, 22(3): 345-349.
[6] WEI Ming-an. Fundamental research on flotation separation of chalcopyrite and galena [D]. Shenyang: Northeastern University, 2008. (in Chinese)
[7] BULATOVIC S, WYSLOUZIL D M. Selection and evaluation of different depressants systems for flotation of complex sulphide ores [J]. Minerals Engineering, 1995, 8(1): 63-76.
[8] VALDIVIESO A L, LOPEZ A A S, SONG S, MARTINEZ H A G, ALMADA S L. Dextrin as a regulator for the selective flotation of chalcopyrite, galena and pyrite [J]. Canadian Metallurgical Quarterly, 2007, 46(3): 301-309.
[9] DRZYMALA J, KAPUSNIAK J, TORNASIK P. Removal of lead minerals from copper industrial flotation concentrates by xanthate flotation in the presence of dextrin [J]. International Journal of Mineral Processing, 2003, 70(1-4): 147-155.
[10] LIU Qi, ZHANG Ya-hui. Effect of calcium ions and citric acid on the flotation separation of chalcopyrite from galena using dextrin [J]. Minerals Engineering, 2000, 13(13): 1405-1416.
[11] HUANG Peng, CAO Ming-li, LIU Qi. Using chitosan as a selective depressant in the differential flotation of Cu-Pb sulfides [J]. International Journal of Mineral Processing, 2012, 106-109: 8-15.
[12] HUANG Peng, CAO Ming-li, LIU Qi. Adsorption of chitosan on chalcopyrite and galena from aqueous suspensions [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2012, 409: 167-175.
[13] MORRIS G E. The adsorption characteristics of polymeric depressants at the talc-water interface [D]. Adelaide: University of South Australia, 1996.
[14] DUBIOS M, GILLES K A, HAMILTON J K, REBERS P A, SMITH F. Colorimetric method for determination of sugars and related substances [J]. Analytical Chemistry, 1956, 28(3): 350-356.
[15] WANG Dian-zuo. Molecular design of reagents for mineral and metallurgical processing [M]. Changsha: Central South University of Technology Press, 1996. (in Chinese)
[16] NATARAJAN R, SHARMA J, NIRDOSH I. Adsorption of N-hydrocinnamoyl-N-phenylhydroxylamine on pure minerals [J]. Adsorption-Journal of the International Adsorption Society, 2010, 16(6): 541-548.
[17] XIONG Dao-ling. The synthesis of xanthate orgnic depressant and its depressing mechanisms in zinc-iron sulfide minerals [D]. Changsha: Central South University, 2006. (in Chinese).
2,3-二羟基丙基二硫代碳酸钠对铜铅硫化矿可浮性的影响
朴正杰1,魏德洲1,刘智林1,2
1. 东北大学 资源与土木工程学院,沈阳 110004;
2. 江西理工大学 资源与环境学院,赣州 341000
摘 要:在丁铵黑药浮选体系中,研究含有—OH和—CSS—的2,3-二羟基丙基二硫代碳酸钠(SGX)对黄铜矿和方铅矿的抑制效果,并通过动电位和吸附量的测试,探讨抑制剂SGX与矿物的相互作用机理。浮选试验研究结果表明:在整个pH范围内,抑制剂SGX对黄铜矿有活化作用,而对方铅矿有很强的抑制作用。随着抑制剂SGX用量的增加,方铅矿的回收率迅速下降,而黄铜矿的回收率有小幅度的升高。在矿浆pH为6的条件下,人工混合矿浮选得出的精矿中铜的品位和回收率分别为29.52%和82.15%。通过动电位和吸附量的测试结果可知,抑制剂SGX在方铅矿表面有较强的吸附,而在黄铜矿表面吸附很弱。
关键词:2,3-二羟基丙基二硫代碳酸钠;抑制;浮选;黄铜矿;方铅矿
(Edited by Hua YANG)
Foundation item: Project (2012BAB01B03) supported by National Key Technologies R&D Program of China
Corresponding author: De-zhou WEI; Tel: +86-24-83673863; E-mail: dzwei@mail.neu.edu.cn
DOI: 10.1016/S1003-6326(14)63475-0