Membrane behavior of bentonite-amended Fukakusa clay in K, Na and Ca solutions
来源期刊:中南大学学报(英文版)2016年第12期
论文作者:王恒宇 唐强 刘维 程蓉 钱寅飞
文章页码:3122 - 3131
Key words:bentonite-amended Fukakusa clay; chemico-osmotic efficiency coefficient; mechanism; membrane behavior
Abstract: Bentonite has been proven to be effective in enhancing the membrane property of clay, by which landfill liners can have better barrier performance with regard to the migration of contaminants. In this work, 5% sodium bentonite amended with locally available Fukakusa clay was utilized to evaluate the membrane behavior toward different kinds of ions: K, Na and Ca. The chemico-osmotic efficiency coefficient, ω, was obtained in electrolyte solution with different concentrations of 0.5, 1, 5, 10, and 50 mmol/L. According to the results, solute type and ion valence have a significant effect on membrane behaviors. Additionally, ω continually decreased as the Na and Ca concentrations increased, which is consistent with the Gouy-Chapman theory. The membrane behavior toward Na was similar to that toward K, according to the chemico-osmotic efficiency coefficient ω. In the case of the divalent ion Ca, the membrane behavior was lower compared to monovalent ions Na and K at the same concentration. The mechanisms of the membrane performance change were discussed with the assistance of XRD patterns, free-swelling results and SEM images.
J. Cent. South Univ. (2016) 23: 3122-3131
DOI: 10.1007/s11771-016-3378-4
TANG Qiang(唐强)1, LIU Wei(刘维)1, WANG Heng-yu(王恒宇)2, CHENG Rong(程蓉)3, QIAN Yin-fei(钱寅飞)3
1. School of Urban Rail Transportation, Soochow University, Suzhou 215131, China;
2. Research Center of Coastal and Urban Geotechnical Engineering, Zhejiang University, Hangzhou 310058, China;
3. Environmental Sanitation Administration Agency, Suzhou 215131, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract: Bentonite has been proven to be effective in enhancing the membrane property of clay, by which landfill liners can have better barrier performance with regard to the migration of contaminants. In this work, 5% sodium bentonite amended with locally available Fukakusa clay was utilized to evaluate the membrane behavior toward different kinds of ions: K, Na and Ca. The chemico-osmotic efficiency coefficient, ω, was obtained in electrolyte solution with different concentrations of 0.5, 1, 5, 10, and 50 mmol/L. According to the results, solute type and ion valence have a significant effect on membrane behaviors. Additionally, ω continually decreased as the Na and Ca concentrations increased, which is consistent with the Gouy-Chapman theory. The membrane behavior toward Na was similar to that toward K, according to the chemico-osmotic efficiency coefficient ω. In the case of the divalent ion Ca, the membrane behavior was lower compared to monovalent ions Na and K at the same concentration. The mechanisms of the membrane performance change were discussed with the assistance of XRD patterns, free-swelling results and SEM images.
Key words: bentonite-amended Fukakusa clay; chemico-osmotic efficiency coefficient; mechanism; membrane behavior
1 Introduction
Over the past several decades, increasing attention has been given to the development of affordable and effective barriers against aqueous contaminant, e.g., compacted clay used as bottom liners in landfill facilities [1-3]. It would be ideal if the clay liner could inhibit the migration of solute molecules [4] without restricting the passage of water, much like a semi-permeable membrane [1, 5]. Such membrane property exhibited by soil is called membrane behavior, by which the liners can prevent or restrict the migration of selected substances [6-9].
Membrane behavior in clay is usually ascribed to two causes: size restriction and static electro-repulsion from a diffuse double layer [10]. Size restriction is the process by which very large non-electric molecules are blocked when pore size is sufficiently small [10-11]. The diffuse double layer (DDL) is composed of the negative surface charge of the clay particles and a distribution of attracted counterions, by which anions cannot migrate through the pores due to the predominantly negative charge electrical potential of the clay particle surface. Therefore, to maintain electrical neutrality in solution, cations tend to remain with their co-ions [12-17]. Typically, the degree to which soil acts as a membrane is quantified in terms of the chemico-osmotic efficiency coefficient, ω [4-5]. ω ranges from 0 to 1 (0≤ω≤1), where 0 represents no solute restrictions and 1 represents an ‘‘ideal’’ or ‘‘perfect’’ membrane that completely restricts the movement of solutes [1,15,18].
Extensive research has been conducted in this field. Mckelvey and Milne presented the first experimental evidence of the salt filtering ability of compacted bentonite and shale material [19]. This was also the first time that the membrane behavior was discovered in soil through an experimental method. KEMPER and QUIRK [20] reported the chemico-osmotic efficiency of several different kinds of clayed soil, including bentonite, illite, and kaolinite clays. In 2002, Malusis and Shackelford measured the chemico-osmotic efficiency coefficient of GCL sodium bentonite toward KCl under different concentrations and porosity conditions [21]. TANG et al [9] conducted a serious laboratory-scale test to study the bentonite effect on membrane behavior. In all, 0, 5, 10,15 and 20% bentonite contents were utilized, and according to the test results, 5% was regarded as the optimum mixing ratio. According to Refs. [22-23], the concentration of Na, K and Ca in landfill leachate were around 2000, 1500 and 1000 mg/L, respectively. However, very little research has been conducted regarding the membrane behavior change under NaCl and CaCl2 solutions. Considering that the bottom liners, especially clay liners applied in landfill sites, are directly exposed to leachate, the objective of this study is to evaluate the membrane behavior toward NaCl, KCl and CaCl2 under different concentrations. Based on the experimental results, the mechanisms of the membrane performance change are discussed with the assistance of XRF results, free-swelling results, and SEM images.
2 Experimental
2.1 Soils and solutions
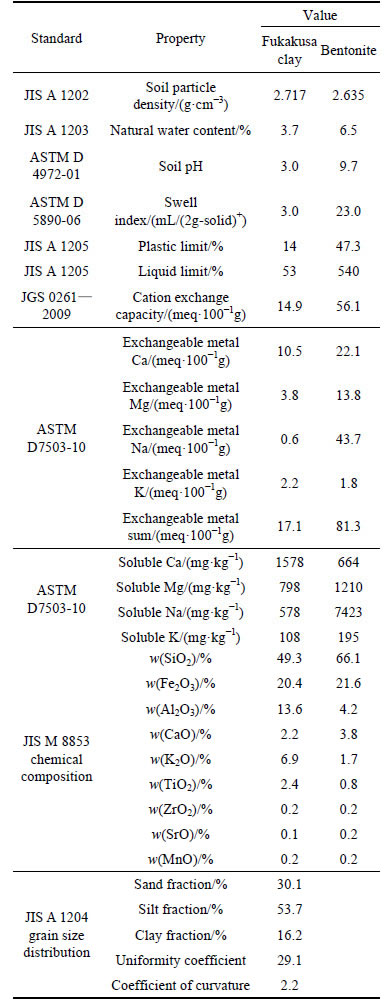
The materials employed in this study were: 1) locally available natural clay known as Fukakusa clay (FC) which was obtained from Kyoto, Japan; and 2) commercial sodium bentonite, originally from Wyoming, U. S., which was purchased from Hojun Co. Ltd. Table 1 lists select physical and chemical properties of Fukakusa clay and bentonite [22].
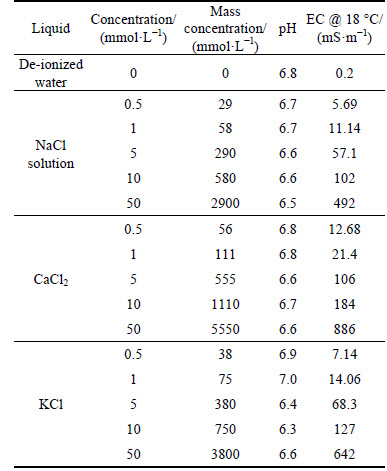
De-ionized water and various concentrations of KCl, CaCl2, and NaCl solutions were used in this work. De-ionized water (DIW) was prepared from tap water by using a water distillation apparatus (RFD240NA, Advantec, Japan). According to Ref. [22], the concentration ranges of Na, K and Ca in landfill leachates were 5-4000 mg/L (Na), 5-3000 mg/L (Ca), 5-3000 mg/L (K) [22]. Considering the compacted clay, bottom liner material will be exposed to landfill leachate directly, therefore five concentrations of KCl, NaCl2 and CaCl2 (0.5, 1, 5, 10, and 50 mmol/L) were selected to simulate the real condition. KCl, NaCl2 and CaCl2 (Guaranteed reagent, Wako Ltd., Japan) were dissolved in the DIW, then diluted to target concentrations. The pH and electrical conductivity (EC) of the above solutions were measured by a pH/ion/cond.-meter (F-55, Horiba, Japan), and the results are shown in Table 2.
2.2 Membrane behavior test apparatus and procedures
The membrane behavior test apparatus applied in this work are shown in Fig. 1. The specimen was locked inside the cylinder by top cap and base pedestal to maintain constant thickness. Porous stones covered both sides to prevent clogging. Ports were equipped and connected with pipes and peristaltic tube pumps (SMP-23AS, As one, Japan), respectively, at both sides to form two independent circulation loops, and the pressure transducer (PTI-S-JC300-22AQ-T, Swagelok, German) was installed at the top side to measure the actual chemico-osmotic pressure.
Table 1 Properties of Fukakusa clay and bentonite
FC was mixed with 5% bentonite following the optimum water content of 23.2% (JIS A 1210). After stirring for 10 min (KM-800, Kenmix, Japan), the sample was covered with a polyethylene membrane and allowed to stand for 12 h to ensure that the water was well distributed.
Table 2 Measured chemical properties of solutions
The specimens were prepared in three stages: specimen assembly, saturation, and flushing. First, each sample was compacted by three layers directly in the column for later membrane behavior tests with an inner diameter of 100 mm and a height of 30 mm following ASTM D 698-12. All of the specimens were then submerged into the DIW inside a vacuum chamber connected to a pump (LMP100, Welch, Japan) for saturation for one day. After this, specimens were permeated with DIW with the hydraulic gradient at around 135 for flushing, which was done to remove soluble salts from the specimens in order to enhance the potential of the membrane behavior [23-25]. During the flushing stage, the outflow volume, duration, and hydraulic gradient were recorded for use in the hydraulic conductivity calculation following Darcy’s law.
Before the membrane behavior test, DIW was circulated over both the top and bottom surfaces at a constant circulation rate (about 205 mL/d) for 6 d to establish a steady baseline pressure prior to introducing different concentration solutions. The membrane behavior tests consisted of five individual stages. In each stage, one of the five electrolytes, with KCl, NaCl, and CaCl2 concentrations of 0.5, 1, 5, 10 and 50 mmol/L, was sequentially infused into the top porous stone of the specimen, while the bottom surface was flushed with DIW. The solution at the top of the specimens represented the leachate of the landfill, while the flushing boundary at the bottom represented the aquifer underneath. The concentration in the leachate was generally higher than that in the groundwater. Therefore, the columns were expected to simulate the real condition of landfill liner, through the established concentration across the specimen [6, 26]. Each stage of the test was conducted until a stable chemico-osmotic pressure was observed across the specimen. The circulation loop at the bottom provided outflow for sample collection to measure NaCl and CaCl2 concentrations by ICP (ICPS-8000, Shimadzu, Japan), and EC and pH were measured twice per day. To improve the reliability of the test results, the experiments were carried out in a room with a control temperature of (18±1) °C.
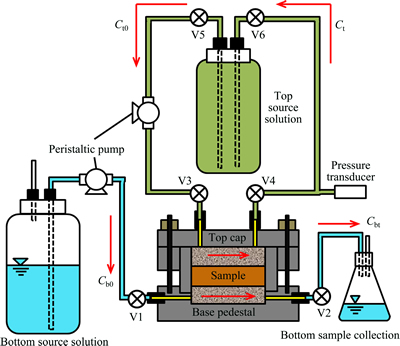
Fig. 1 Test apparatus for membrane behavior test
2.3 Calculation of membrane efficiency
Throughout the membrane behavior tests, the thickness and volume remain constant, and the infused liquid is equal to the outflow, which is to prevent the source solution and the DIW from entering or exiting the specimens. The chemico-osmotic efficiency coefficient ω can be defined as [21, 27-29]
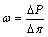 (1)
(1)
where △P is the actual chemico-osmotic pressure across the specimen, and △π is the theoretical chemico-osmotic pressure across an ideal semi-permeable membrane [30]. For a single salt system, △π can be approximated using the van’t Hoff equation based on the solution concentration difference as [31-32]
△π=vRT△C (2)
where ν is the number of ions in one salt molecule; R is the universal gas constant, 8.314 J×K/mol; T represents the thermodynamic temperature of the membrane behavior testing system in K; and △C is the concentration difference across the specimen, which can be rewritten as [21]
△C=Ct-Cb (3)
where Ct and Cb represent solute concentration at the top and bottom sides, respectively. In this work, the source solution is circulated at the top surface to provide an initial upper concentration of Ct0>0, while the bottom surface is flushed by DIW to create a bottom concentration of Cb0≈0. Thus, the chemico-osmotic efficiency coefficient ω0, in terms of KCl solution, can be expressed as [21]
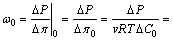
 (4)
(4)
where △π0 exists under a perfect flushing boundary condition when the circulation rate is sufficiently large so that the boundary KCl concentrations caused by diffusion are negligible. However, in practice, the diffusion and insufficient circulation rate at the bottom sides may result in a time-dependent reduction of △π [33]. Thus, the average chemico-osmotic efficiency coefficient ωave is more accurate to describe the actual membrane behavior, which can be written as [24]
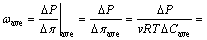
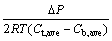 (5)
(5)
where Ct,ave and Cb,ave are the average KCl concentrations across the top and bottom of the specimen boundaries, respectively.
3 Results
3.1 Specimen flushing
Figures 2(a)-(c) present the flushing stage, and the results are summarized in Table 3. The flushing stage lasts for 70 d, until the EC of outflow is lower than 3.6 mS/m and the pH is close to 7.0 (neutral). As shown in Table 3, the initial EC values of outflows are 685 and 734 mS/m, which are almost 150-200 times higher than the target value. With continual permeation with DIW, the EC of outflow decreases gradually and is eventually reduced to 2.5 and 2.9 mS/m. According to flushing progress, to make outflow pH much closer to 7.0 through flushing, approximately 1 or 2 additional months are required, as shown in Fig. 2(b). To reduce the total duration of the membrane behavior test, the flushing stage is stopped after the outflow pH of the two specimens increase to 6.2 and 6.4, respectively.
Figure 2(c) presents the measured hydraulic conductivities of the two specimens toward DIW during the flushing stage. Compared to the initial results, the hydraulic conductivities decrease slightly with time, which was also observed by TANG et al [9]. This time-dependent decrease could be attributed to the concentration decrease of leaching flow during the flushing stage, while a lower concentration resulted in lower hydraulic conductivity [34]. This finding is also proved by the flushing results, in which the hydraulic conductivities decrease around the 15th-20th days, while the EC decreases sharply by about 80% at the same time, as shown in Fig. 2(a). After 70 days’ flushing, the hydraulic conductivities gradually stabilize around 1.01×10-9 m/s and 0.95×10-9 m/s, which is very close to reaching the requirement of the upper limit of hydraulic conductivity for the landfill liner directly [35-36].
3.2 Boundary results during membrane behavior tests
Figures 3(a)-(b) show the top and bottom boundary concentrations during the membrane behavior test. The tests consist of five stages where the CaCl2 and NaCl concentrations for the top circulation increase from 0.5 mmol/L to 50 mmol/L. It is apparent that Ca and Na concentrations inside the top circulation decrease at the end of almost every stage (Ct
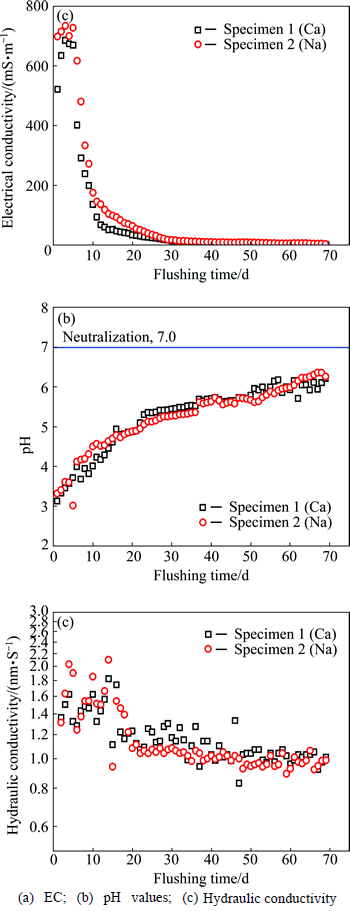
Fig. 2 Specimens’ flushing:
Table 3 EC, pH and hydraulic conductivity (k) before and after flushing
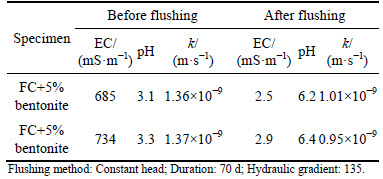
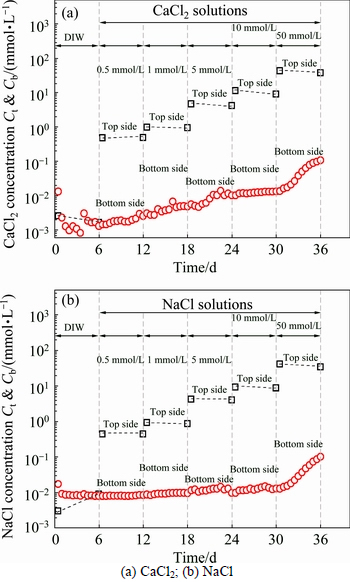
Fig. 3 Boundary concentrations during membrane behavior test:
Figures 4(a)-(b) present the boundary pH values during the membrane behavior test. The pH values at the top side decrease after every stage, which also indicates the occurrence of adsorption and diffusion. Thus, some acidic salts origin from the ion-exchange adsorption is leached out from the specimen, since the diffusion process results in the decrease of pH values. In the case of pH at the bottom side, the decrease trends can be observed at the beginning from a concentration difference higher than 5 mmol/L. This phenomenon can be attributed to the leaching out of acidic substance origin from diffusion and ion-exchange adsorption.
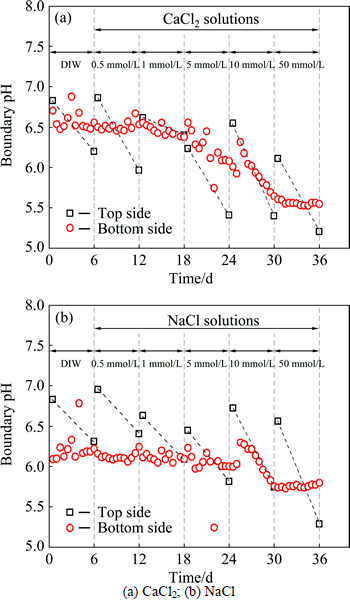
Fig. 4 Boundary pH values during membrane behavior test:
3.3 Chemico-osmotic pressure
Figures 5(a)-(b) show the actual values of △P of the two specimens. DIW was circulated at both the top and bottom sides of the specimens (Ct=Cb=0) during the first 6 d to obtain the baseline pressures of 0.42 kPa and 0.76 kPa. The baseline pressures were also observed by TANG et al [9] and MALUSIS and SHACKELFORD [21]. TANG et al [9] ascribed this to the leaching out of the remaining soluble salts in specimens, while this was attributed to slight differences in the hydraulic resistance of the porous stones at the opposite ends of the specimens by MALUSIS and SHACKELFORD [21].
From the figures, it is apparent that the changes in chemico-osmotic pressure under CaCl2 and NaCl are significantly different. In the case of CaCl2, when the concentration difference increases from 0 to 0.5 mmol/L, then from 0.5 to 1 mmol/L, the introduction of electrolyte results in an immediate and rapid increase in the chemico-osmotic pressure. However, with the continuous increase of concentration difference to 5 mmol/L, 10 and 50 mmol/L, the chemico-osmotic pressure experiences a slight decrease. In the case of NaCl, the chemico-osmotic pressure experiences a rapid increase immediately when introducing electrolyte at the beginning of every stage. For both specimens, the time required for the chemico-osmotic pressure to equilibrate is dependent upon the solute concentration: less than 1 d for the 0.5, 1 and 5 mmol/L solutions, and about 3 d for the 10 and 50 mmol/L solutions.
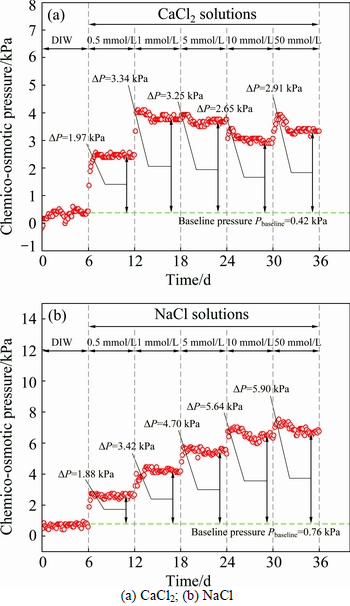
Fig. 5 Measured chemico-osmotic pressure across specimens during the membrane behavior tests:
4 Discussion
The comparison of boundary concentrations at the bottom side during the membrane behavior test are displayed in Fig. 6. In Fig. 6(a), it is clear that the concentrations of Ca and Na at the outflow of the bottom side exhibit similar trends. The solute concentration remained stable and started to increase until the concentration difference increased to 50 mmol/L. As shown in Fig. 6(b), the EC of outflow for K, Ca and Na increase when concentration is higher than 5 mmol/L. The EC increases almost 12 d earlier than the solute concentration of the outflow, which can be attributed to the ion-exchange adsorption and some soluble salts that are generated. In addition, it has to be mentioned that compared with the pure clay, the bentonite amended Fukakusa clay has better barrier performance which effectively retarded the migration of the solute since the existence of membrane behavior.It was rational to predict that as the liner material, the service life of the bentonite amended Fukakusa clay can be greatly prolonged.
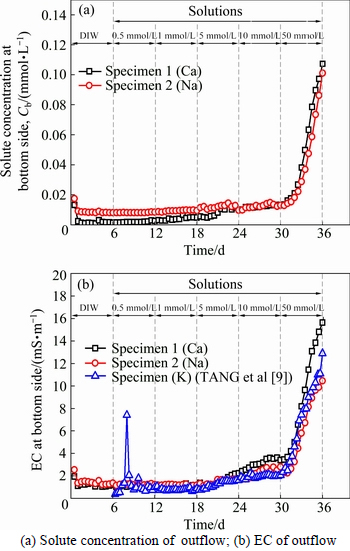
Fig. 6 Comparison of boundary conditions of outflow at bottom side during membrane behavior test:
Table 4 presents the membrane behavior test results. The chemico-osmotic efficiency coefficient ω0 and average chemico-osmotic efficiency coefficient ωave decrease as the concentration difference increases. As shown in Table 4, since the continual solute diffusion from the source solution into the specimen and adsorption at the top side (Ct,ave
In Fig. 7, the values of ωave are compared against different ions in this study with that of previous literature for Nelson Farm Clay (NFC) and sodium bentonite [9, 21, 40]. Although the relative positions of the trend lines differ, the general trends are the same. Membrane behaviors ωave decrease as concentrations increase, which are likely attributed to two factors: the diffuse double layer (DDL) and inter-particle pores [10].
Table 4 Summary of membrane behavior tests results
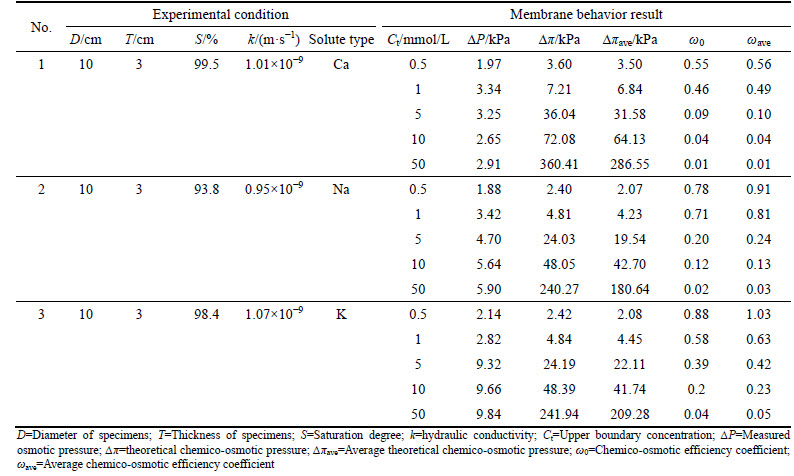
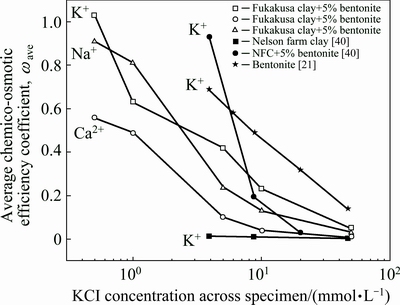
Fig. 7 Chemico-osmotic efficiency coefficient ωave as a function of concentration
Bentonite minerals consist of tetrahedral and octahedral layers, which provide many hydration sites since the crystal lattice [41]. According to Ref. [42], the water-bounded molecules are immobile and behave like a solid phase to obstruct the passage of solution. As the immobile water phase becomes thicker with an increase in bound water molecules, the effective pore space for free flow is reduced. However, when the solution concentration increases, more exchange sites at the DDL are occupied by cations instead of water molecules, causing a stronger attraction force between the soil particle and its DDL. Compared with Na, K solution, Ca solution would cause higher shrinkage since the greater atomic weight, and resulted in the narrower inter-layer space and responding decrease in the DDL thickness, finally the lower membrane behavior exhibited in Ca solutions. According to Ref. [43], the hydration shell surrounding the cations in a DDL consists of about six water molecules for dilute solutions, but is reduced to about three water molecules in concentrated solutions. According to the Gouy-Chapman theory (DDL theory), the thickness of the DDL decreases as the solute concentration increases, which leads to the increase in inter-particle pore size [21]. Thus, it is rational to explain the decrease of membrane behavior as a solute concentration increase.
According to Ref. [4], the hydration of bentonite during the swelling process involves four basic interaction mechanisms: (i) hydrogen bonding, (ii) dipole-charged surface attraction, (iii) van der Waals attraction, and (iv) hydration of exchangeable cations. For bentonite, hydration of exchangeable cations is most important, and this occurs as the positively charged ions attract water dipoles, which form a hydration shell surrounding the cation [44]. Because the cations are restrained by the layered charge field, their water in the hydration shell is restrained as well. Secondary layers of water can H-bond with the primary layer of hydration water, forming multiple hydration shells. Beyond two or three layers, the water of these shells behaves as bulk water [44]. However, such layers when adsorbed by bentonite are particularly sensitive to changes in the composition of the pore fluid, which influences the thickness significantly. The electrolyte with high concentration causes the adsorbed layer to collapse, which results in the increase of interlayer space, finally leading to the decrease of chemico-osmotic efficiency coefficient as concentration increases [45]. The soil microstructure was also partially influenced by the saline permeant since the existence of geo-bacteria [28, 46]. Even with a limited amount of nutrition, the microbes can maintain their fundamental metabolism, which help to block the inter-particle pores [7]. When permeated with highly concentrated salt, the microbial cell might be lysed due to the chemo-osmotic effect on the cell membrane [8], and this help to demonstrate the concentration-dependent decrease of membrane behavior as shown in Fig. 7.
To further research the mechanism, standard swelling tests were conducted following ASTM D 5890-06, and the results are presented in Fig. 8. According to the results, the Fukakusa clay almost never expands no matter the concentration of surrounding solution. The phenomenon was also observed and proved by Tang, in which the swelling index of Fukakusa clay towards KCl solution were around 3 mL/2g-solid [22].In the case of bentonite, it is clear that the swelling volume under Na solution is higher than others, especially at elevated concentration. Such phenomenon was summarized as the influence of valence by SHACKELFORD et al [34], and the similar trend of swelling volume decreasing as the valence of cation increased was also observed by JO et al [47]. For sodium bentonite, replacement of sodium in the exchange complex with other ions affects the thickness of the adsorbed layer, thus swelling the volume of bentonite [48]. The higher valence cation showed the larger effect on the swelling capacity, which is consistent with the Gouy-Chapman and Stern-Gouy theories [34]. The similiar phenomena were also observed in Fig. 6, and proved by RUHL and DANIEL’s research, in which Ca was found more aggressive compared with other monovalent ions [44, 49]. Thus, the inter-particle pore increases as valence increases, which causes the decrease of membrane behavior of Na and K>Ca, as shown in Fig. 7.
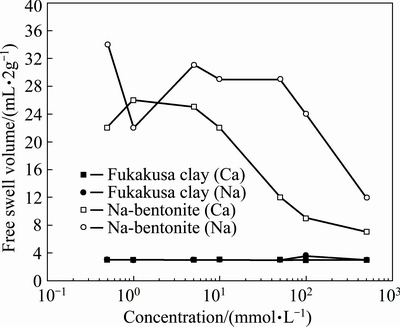
Fig. 8 Free swell of Fukakusa clay and bentonite toward Na and Ca solutions
KATSUMI et al [48] thought bentonite cannot swell sufficiently inside the electrolyte solution, and the increase in inter-particle pores allows more solute to pass, which is also attributed to the decrease of membrane behavior at elevated concentration. Figure 9 shows scanning electron microscope (SEM) (JSM-5510LV, JEOL, Japan) images at the top side of specimens after the membrane behavior test. Figures 9(a)-(b) display results based on this study, while (c) displays results cited from TANG [22] for comparison. From the image, it’s apparent that the size of soil clusters is significantly different, e.g., the average size of soil clusters under monovalent ions Na and K condition is almost 30 μm. And, in the case of divalent ion Ca condition, the average size of soil clusters is less than 10 μm. This indicates that divalent ions can cause greater shrinking of the soil clusters, and some observable pores can be noted at the surface of the specimens under Ca, which is the permeable path during the diffusion, and which also causes the decrease in membrane behavior.
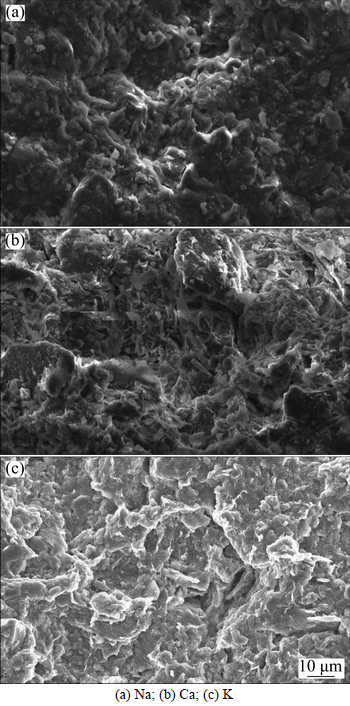
Fig. 9 SEM images of specimens after membrane behavior test:
5 Conclusions
A series of lab-scale experiments was conducted on bentonite-amended Fukakusa clay composite materials. According to the test results, solute type has a significant effect on membrane behaviors. Compared with monovalent ions (Na+, K+), introduction of divalent ions (Ca2+) resulted in bigger inter-particle pores and greater shrinkage of adsorption layer, thus leading to lower membrane performance of the bentonite-amended Fukakusa clay. Considering all of above, membrane behavior can greatly improve the barrier performance of compacted clay, also can help effectively retard the migration of the contaminant.
References
[1] TANG Q, KATSUMI T, INUI T, LI ZZ. Membrane behavior of bentonite amended compacted clay [J]. Soils and Foundations (JGS), 2014, 54(3): 329-344.
[2] CHAPUIS R P. The 2000 R M. Hardy Lecture: Full-scale hydraulic performance of soil–bentonite and compacted clay liners [J]. Canadian Geotechnical Journal, 2002, 39: 417-439.
[3] SHELLEY T, DANIEL D. Effect of gravel on hydraulic conductivity of compacted soil liners [J]. Journal of Geotechnical and Geoenvironmental Engineering (ASCE), 1993, 119: 54-68.
[4] MITCHELL J K, SOGA K. Fundamentals of soil behavior [M]. 3rd ed. New York: John Wiley & Sons, 2005.
[5] MANASSERO M, DOMINIJANNI A. Modelling the osmosis effect on solute migration through porous media [J]. Geotechnique, 2003, 53: 481-492.
[6] TANG Q, KIM H J, ENDO K, KATSUMI T, INUI T. Size effect on lysimeter test evaluating the properties of construction and demolition waste leachate [J]. Soils and Foundations (JGS), 2015, 55(4): 720-736.
[7] THULLNER M, ZEYER J, KINZELBACH W. Influence of microbial growth on hydraulic properties of pore networks [J]. Transport in Porous Media, 2002, 49: 99-122.
[8] HAND V L, LLOYD J R, VAUGHAN D J, WILKINS M J, BOULT S. Experimental studies of the influence of grain size, oxygen availability and organic carbon availability on bioclogging in porous media. Environmental Science &Technology, 2008, 42: 1485-1491.
[9] TANG Qiang, KATSUMI T, INUI T, LI Zhen-ze. Effect of mixing ratio on membrane behavior of clay-bentonite composite material [C]//New Advances in Geotechnical Engineering, Proceedings of the 5th China-Japan Geotechnical Symposium, Beijing: CJGS, 2013: 494-498.
[10] van IMPE P O. Consolidation, contaminant transport and chemico-osmotic effects in liner materials. A theoretical and experimental study [D]. Italy: Universita Degli Studi di Ancona, 2002.
[11] GRATHWOHL P. Diffusion in natural porous media, contaminant transport, sorption/desorption and dissolution kinetics [M]. Norwell, MA, USA: Kluwer Academic Publishing, 1998.
[12] DOMINIJANNI A, MANASSERO M. Modelling the swelling and osmotic properties of clay soils. Part II: The physical approach [J]. International Journal of Engineering Science, 2012b, 51: 51-73.
[13] FRITZ S J, MARINE I W. Experimental support for a predictive osmotic model of clay membranes [J]. Geochimica et Cosmochimica Acta, 1983, 47: 1515-1522.
[14] FRITZ S J. Ideality of clay membranes in Osmotic processes: A review [J]. Clays and Clay Minerals, 1986, 34: 214-223.
[15] KEIJZER T J S, KLEINGELD P J, LOCH J P G. Chemical osmosis in compacted clayey material and the prediction of water transport, Geoenvironmental engineering, contaminated ground: Fate of pollutants and remediation [M]. YONG R N, THOMAS H R, eds. London: Thomas Telford, 1997: 199-204.
[16] MARINE I W, FRITZ S J. Osmotic model to explain anomalous hydraulic heads [J]. Water Resources Research, 1981, 17: 73-82.
[17] SHACKELFORD C D. Membrane behavior in geosynthetic clay liners [C]//Geo-Frontiers 2011. Dallas, TX, 2011, Reston, VA: (CD-ROM). ASCE, 1961-1970.
[18] BARBOUR S L, FREDLUND D G. Mechanisms of osmotic flow and volume change in clay soils [J]. Canadian Geotechnical Journal, 1989, 26: 551-562.
[19] MCKELVEY J G, MILNE I H. The flow of salt solutions through compacted clay [J]. Clays and Clay minerals, 1962, 9: 248-259.
[20] KEMPER W D, QUIRK J P. Ion mobilities and electric charge of external clay surfaces inferred from potential differences and osmotic flow [J]. Soil Science Society of America, Proceedings, 1972, 36: 426-433.
[21] MALUSIS M A, SHACKELFORD C D. Chemico-osmotic efficiency of a geosynthetic clay liner [J]. Journal of Geotechnical and Geoenvironmental Engineering (ASCE), 2002, 128: 97-106.
[22] TANG Qiang. Factors affecting waste leachate generation and barrier performance of landfill liners [D]. Japan: Kyoto University, 2013.
[23] BAUN D L, CHRISTENSEN T H. Speciation of heavy metals in landfill leachate: A review [J]. Waste Manage Res, 2004, 22: 3-23.
[24] KANG J B, SHACKELFORD C D. Clay membrane testing using a flexible-wall cell under closed-system boundary conditions [J]. Applied Clay Science, 2009, 44: 43-58.
[25] KANG J B, SHACKELFORD C D. Consolidation enhanced membrane behavior of a geosynthetic clay liner [J]. Geotextiles and Geomembranes, 2011, 29: 544-556.
[26] DOMINIJANNI A, MANASSERO M. Modelling the swelling and osmotic properties of clay soils. Part I: The phenomenological approach [J]. International Journal of Engineering Science, 2012, 51: 32-50.
[27] GROENEVELT P H, ELRICK D E. Coupling phenomena in saturated homo-ionic montmorillonite: II. Theoretical [J]. Soil Science Society of America Journal, 1976, 40: 820-823.
[28] LI Z, KATSUMI T, INUI T, TAKAI A. Fabric effect on hydraulic conductivity of kaolin under different chemical and biochemical conditions [J]. Soils and Foundations, 2013, 53(5): 680-691.
[29] Van O E, HALE A H, MODY F K, ROY S. Transport in shales and the design of improved water-based shale drilling fluids [J]. SPEDC, 1996, 11: 137-146.
[30] OLSEN H W, YEARSLEY E N, NELSON K R. Chemicoosmosis versus diffusion-osmosis [J]. Transportation Research Record, 1990, 1288: 15-22.
[31] METTEN U. Desalination by reverse osmosis [M]. Cambridge, MA.: M.I.T. Press, 1966.
[32] TINOCO I, SAUER K, WANG J C. Physical chemistry [M]. Upper Saddle River, N.J.: Prentice-Hall, 1995.
[33] MALUSIS M A, SHACKELFORD C D, OLSEN H W. A laboratory apparatus to measure chemico-osmotic efficiency coefficients for clay soils [J]. Geotechnical Testing Journal, 2001, 24: 229-242.
[34] SHACKELFORD C D, BENSON C H, KATSUMI T, EDIL T B, LIN L. Evaluating the hydraulic conductivity of GCLs permeated with non-standard liquids [J]. Geotextiles and Geomembranes, 2000, 18: 133-161.
[35] KAMON M, KATSUMI T. Clay liners for waste landfill [C]//Clay Science for Engineering. Rotterdam: Balkema, 2001: 29-45.
[36] KATSUMI T, ISHIMORI H, OGAWA A, MARUYAMA S, FUKAGAWA R. Effects of water content distribution on hydraulic conductivity of prehydrated FCLs against calcium chloride solutions [J]. Soil and Foundations (JGS), 2008, 48: 407-417.
[37] TANG Q, TANG Xiao-wu, LI Zhen-ze, CHEN Yun-min, KOU Nai-yu, SUN Zu-feng. Adsorption and desorption behaviour of Pb(II) on a natural kaolin: Equilibrium, kinetic and thermodynamic studies [J]. Journal of Chemical Technology and Biotechnology, 2009, 84: 1371-1380.
[38] TANG Qiang, TANG Xiao-wu, HU Man-man, LI Zhen-ze, CHEN Yun-min, LOU Peng. Removal of Cd(II) from aqueous solution with activated Firmiana Simplex Leaf: Behaviors and affecting factors [J]. Journal of Hazardous Materials, 2010, 179: 95-103.
[39] TANG Q, TANG Xiao-wu, LI Zhen-ze, WANG Yan, HU Man-man, ZHANG Xiang-jie, CHEN Yun-min. Zn(II) removal with activated firmiana simplex leaf: Kinetics and equilibrium studies [J]. Journal of Environmental Engineering (ASCE), 2002, 138: 190-199.
[40] KANG J B, SHACKELFORD C D. Membrane behavior of compacted clay liners [J]. Journal of Geotechnical and Geoenvironmental Engineering (ASCE), 2010, 136: 1368-1382.
[41] YONG R N, PUSCH R, NAKANO M. Containment of High-level radioactive and hazardous solid wastes with clay barriers [M]. London and New York: Spon Press, Taylor & Francis, 2010.
[42] KATSUMI T, ISHIMORI H, OGAWA A, YOSHIKAWA K, HANAMOTO K, FUKAGAWA R. Hydraulic conductivity of nonprehydrated geosynthetic clay liners permeated with inorganic solutions and waste leachates [J]. Soil and Foundations (JGS), 2007, 47: 79-96.
[43] SPOSITO G. The surface chemistry of soils [M]. New York: Oxford University Press, 1984.
[44] Di EMIDIO G. Hydraulic and Chemico-osmotic performance of polymer treated clays [D]. Belgium: Ghent University, 2010.
[45] MESRI G, OLSON R E. Mechanisms controlling the permeability of clays [J]. Clay and Clay Minerals, 1971, 19: 151-158.
[46] TANG Q, WANG H Y, CHEN H, TANG X W. A Characterization study of hydraulic conductivity of compacted clay and fine sand treated with landfill leachate and nutrient solution [J]. Electronic Journal of Geotechnical Engineering, 2015, 20(12): 1-14.
[47] JO H, KATSUMI T, BENSON C H, EDIL T B. Hydraulic conductivity and swelling of non-prehydrated GCLs permeated with single species salt solutions [J]. Journal of Geotechnical and Geoenvironmental Engineering (ASCE), 2001, 127: 557-567.
[48] KATSUMI T, ISHIMORI H, ONIKATA M, FUKAGAWA R. Long-term barrier performance of modified bentonite materials against sodium and calcium permeant solutions [J]. Geotextiles and Geomembranes, 2008, 26: 14-30.
[49] RUHL J L, DANIEL D E. Geosynthetic clay liners permeated with chemical solutions and leachates [J]. Journal of Geotechnical and Geoenvironmental Engineering (ASCE), 1997, 123: 369-381.
(Edited by DENG Lü-xiang)
Foundation item: Projects(51179168, 51308310) supported by National Natural Science Foundation of China; Project(LQ13E080007) supported by Zhejiang Provincial Natural Science Foundation of China; Project supported by the Scientific Research Foundation for the Returned Overseas Chinese Scholars (Chinese State Education Ministry)
Received date: 2015-08-08; Accepted date: 2015-11-10
Corresponding author: WANG Heng-yu, PhD; Tel: +86-512-67601052; E-mail: 10912019@zju.edu.cn

