J. Cent. South Univ. (2016) 23: 18-26
DOI: 10.1007/s11771-016-3044-x
Room temperature gas sensor based on tube-like hydroxyapatite modified with gold nanoparticles
LUO Lan-lan(罗兰兰), LIU Yong(刘咏), TAN Yan-ni(谭彦妮),
LI Hui-xia(李会霞), ZHANG Qing(张青), LI Kun(李昆)
State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract: The main goal of this work is to explore the possibility of using Au-modified hydroxyapatite (HA) as a potential sensor material. Tube-like HA structure was fabricated with the aid of a Nafion N-117 cation exchange membrane and gold (Au) nanoparticles were added by a hydrothermal method. The morphology, structure and composition were characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS). The gas sensing properties were also investigated. Results show that Au nanoparticles are dispersed into the HA powder, which is tube-like, with rough inner and outer surfaces. Compared with pure HA, Au-modified HA exhibits improved sensing properties for NH3. 5% (mass fraction) Au-modified HA shows the highest response with relatively short response/recovery time. The response is up to 79.2% when the corresponding sensor is exposed to 200×10-6 NH3 at room temperature, and the response time and recovery time are 20 s and 25 s, respectively. For lower concentration, like 50×10-6, the response is still up to 70.8%. Good selectivity and repeatability are also observed. The sensing mechanism of high response and selectivity for NH3 gas was also discussed. These results suggest that Au-HA composite is a promising material for NH3 sensors operating at room temperature.
Key words: gas sensor; Au nanoparticles; hydroxyapatite; gas selectivity; ammonia gas
1 Introduction
Demand for chemical sensors is increasing in recent years because they play an important role in various areas like emission control, environmental protection, public safety, and human health {Mahabole, 2013 #1}{Mahabole, 2013 #1}[1]. This has stimulated considerable interest to develop simple and cost-effective chemical sensors. Up till now, the most investigated gas sensing materials are metal oxide semiconductors such as SnO2, TiO2, ZnO [2-4]. However, comparatively less attention has been directed towards the use of ceramic materials as a gas sensor. Hydroxyapatite (Ca10(PO4)6(OH)2, HA), a bioceramic material with a very flexible structure, has been extensively applied in biomaterials, adsorbents, ion exchangers and catalyst owing to its excellent biocompatibility, bioactivity, multi-adsorbing sites, superior ion exchange capacity, extraordinary chemical and thermal stability [5-7]. Moreover, HA has been received more attention as a chemical gas sensor due to its peculiar properties such as large surface P-OH which interacts with the gas molecules to be detected, highly porous structure and capability to exchange ions [1, 8]. HA also has been proposed as an effective material to achieve complementary properties and dynamic effects for hazardous gas sensing [9]. However, most gas sensors based on HA need to operate at elevated temperatures, which increases the cost of the device and limits the portability [1, 9-10].
Generally, the gas sensors with fast response, high sensitivity, selectivity and stability for detection of particular gas at low operating temperatures are being preferred in various applications. These factors can be improved by using pre-treatments like addition of catalyst/dopant, processing conditions and synthesis methods [11]. Adding dopant to a sensor matrix is one of the best known ways to enhance the sensor properties [1]. In this regard, many attempts have been carried out to improve sensing properties by doping metallic species, such as Au, Ag, Pt, Pd, Fe, and Co in base semiconductor and metal oxide systems [12-15]. The use of metal nanoparticles such as gold has attracted much attention due to their quantum size effects and high surface area, which offer great promise for catalyst, gas sensors and potential applications. Guo et al [16] have reported that the gas sensor based on ZnO nanowires functionalized by Au nanoparticles has remarkably enhanced the performance compared with pure ZnO. However, HA modified with Au nanoparticles in the application of the gas sensor has not been studied yet. Thus, an attempt was carried out in this work to study the effect of adding Au into HA on its gas sensing properties.
Recently, along with the increasing of the world awareness about environmental problems and human safety, the need to detect low concentration of ammonia (NH3) has greatly increased in many fields, such as food technology, chemical engineering, medical diagnosis, environmental protection, monitoring of car interiors and industrial processes [17]. Ammonia is a colorless gas with a distinct odor and is very harmful to the human body. Exposure to higher concentration of ammonia can cause bronchiolar and respiratory failures. Occupational Safety and Health Administration (OSHA) has set an acceptable 8 h exposure limit of 25×10-6 and a short-term (15 min) exposure level of 35×10-6 for human beings [18]. Most NH3 gas sensors are based on metal oxide films, which have a limited maximum sensitivity and need a high working temperature.
The main goal of this work is to explore the possibility of using Au-modified HA as a potential sensor material for ammonia. Our approach focuses on the influence of the Au adding on the structural, morphologic properties of HA, as well as repeatability and reproducibility, response and recovery time of HA sensor for NH3 gas.
2 Materials and methods
2.1 Chemicals
All the chemicals (Ca(NO3)2·4H2O, NH4H2PO4, HAuCl4·4H2O, NH3·H2O, C6H5Na3O7) were of analytical grade and used without further purification. Nafion N-117 cation exchange membrane was purchased from Alpha Awsare. Deionized water (DI water, Millipore) was used as solvent in all experiments.
2.2 Preparation of HA and Au-HA
The tube-like HA was prepared by the method similar to that described in our previous work [19]. Calcium nitrate and ammonium dihydrogen phosphate solutions were prepared according to the theoretical ratio of Ca-to-P close to 1.67. The pH value of NH4H2PO4 solution was adjusted to 11 with NH3·H2O.
A hydrothermal method was used to prepare the gold modified hydroxyapatite, denoted as Au-HA. The required amounts of HAuCl4·4H2O and sodium citrate for synthesizing 2%, 5%, 10% (by mass) Au-HA (named as 2Au-HA, 5Au-HA, 10Au-HA, respectively) were dissolved in deionized water and the pH value was adjusted to 7-8 with NH3·H2O. After HA powder was added, the mixture was kept under mildly stirring at room temperature for 1 h. After that, the solution was transferred into a Teflon-lined autoclave and heated at 120 °C for 1 h. Finally, the resultant powder was washed by deionized water and dried in vacuum oven at 90 °C for 6 h.
2.3 Characterization of materials
The morphology and phase structure were investigated by using field emission scanning electron microscope (FESEM; NOVA NANOSEM 230, USA), field emission transmission electron microscope (FETEM; JEOL JEM-2100F, Japan), powder X-ray diffraction meter (XRD; D/ruax 2550PC, Japan) by using Cu Kα as the source, and X-ray photoelectron spectroscope (XPS; Pekrin-Elmer pHI-5400, USA).
2.4 Test of gas sensing properties
The as-prepared HA and Au modified tube-like HA were mixed with deionized water to form a paste. The paste was then coated on a ceramic tube to form a sensing film on which a pair of Au electrodes was previously printed, and then a Ni-Cr heating wire was inserted in the tube to form a side-heated gas sensor. The structure and schematic diagram of the sensor are shown in Figs. 1(a) and (b).
Gas sensing properties were measured at room temperature (25 °C) by a CGS-8 intelligent gas-sensing analysis system (Beijing Elite Tech Co., Ltd., China) (Fig. 1(c)). The measuring schematic is shown in Fig. 1(d). The responses to ammonia gas of all the samples (HA, 2Au-HA, 5Au-HA and 10Au-HA) were tested. In order to investigate the selectivity of the sensor, the responses of 5Au-HA to different concentrations of different gases (ethanol, methanol, heptane, methylbenzene, and acetone) were also measured at room temperature. The response to different concentration of water vapor was also tested in order to evaluate the influence of water vapor on the results. Saturated target gas (liquid state) was injected into a glass vacuumed test chamber (20 dm3 in volume) for low concentration (≤100×10-6) and a vacuumed test bottle (1 dm3 in volume) for high concentration (>100×10-6) by a syringe through a rubber plug. The following formula was used to calculate the volume of target gas to get certain concentration of target gas:
Q=V×C×M/(22.4×d× ρ)×10-9×(273+TR)/(273+TB)
where Q is the volume of target gas; V is the volume of the test chamber; C is the target gas concentration that we need; M is the molecular weight; d is the density of target gas; ρ is the purity of target gas; TR is the temperature of the test environment; TB is the temperature in the test chamber.
The saturated target gas was mixed with air (relative humidity was about 25%) by two fans in the analysis system. After the sensor resistances reached a new constant value, the test chamber was opened to recover the sensors in air. All the measurements were performed in a laboratory fume hood. The sensor resistance and response values were acquired by the analysis system automatically. The response value (β) can be defined as
 (1)
(1)
where Rair and Rgas denote the electric resistances of the senor in air and in the target gas, respectively. The time taken by the sensor to achieve 90% of the total resistance change was defined as the response time in the case of adsorption or the recovery time in the case of desorption.
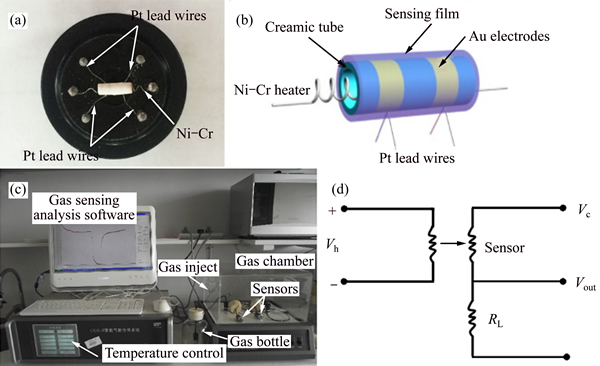
Fig. 1 completed gas sensing device (a), ceramic tube (b), CGS-8 intelligent gas-sensing analysis system (c) and measuring schematics (d)
3 Results and discussion
3.1 Structural and morphological characteristics
Figure 2 shows the XRD patterns of pure HA and Au-HA with various Au concentrations. The XRD pattern of pure HA shows well-defined diffraction peaks (JCPDS No. 09-0432). In Au modified HA, the crystal phase of HA was well maintained and the diffraction peaks corresponding to Au are also indicated. It is observed that the intensities of all characteristics peaks of Au increase with the concentration increasing.
Figure 3(a) shows the morphology of pure HA, which is of tube-like structure with a diameter of 2.5-5.5 μm. The morphology of the structure can be considered to be a result of the self-assembly of nanorods [20]. After the adding of Au, the morphology of 5Au-HA, as shown in Fig. 3(b), almost keeps the same with the HA. The outer and inner surfaces of pure tube-like HA are shown in Figs. 3(c) and (d). Both the outer and inner surfaces are rough, covered by needle-like and tabular crystal aggregates. TEM image of 5Au-HA (Fig. 3(e)) shows that the agglomerates are composed of nanorods with a length of 20-100 nm and a width of 10-30 nm. Many particles in a size of 5 nm adhere individually on the nanorods. The SAED pattern shown in Fig. 3(f) indicates that the HA powders are polycrystalline in structure. In 10% Au modified HA, large particles (about 25-93 nm) are observed (Fig. 3(g)). The increase of gold content to 10% results in the agglomeration of gold nanoparticles.
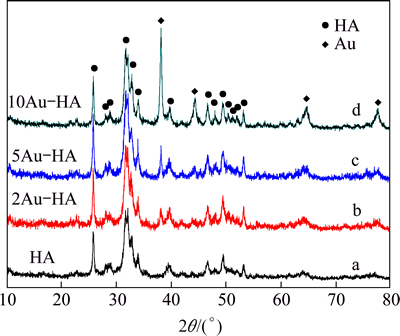
Fig. 2 XRD patterns of pure HA (a), 2Au-HA (b), 5Au-HA (c) and 10Au-HA (d)
XPS technique is used for qualitative determination of surface composition of the materials. Figure 4 shows the survey XPS narrow scan spectra of the as-prepared Au-HA materials. The main peaks at 347, 531.1, 133.3 and 285 eV can be readily assigned to the binding energy of Ca 2p, O 1s, P 2p and C 1s, respectively. In the XPS spectrum of Au-HA, the binding energy of Au (4f5/2, 87.3 eV), Ca (2p, 347 eV), O (1s, 531 eV) and P (2p, 133.3 eV) can be clearly found. XPS narrow scan spectra of Au element are presented in Figs. 4(b)-(d). The values are in good agreement with the previous report for Au0 [21], indicating the formation of gold nanoparticles on the surface of HA.

Fig. 3 SEM images of pure HA (a), 5Au-HA (b), outer surface of pure HA(c), inner surface of pure HA(d), TEM image of 5Au-HA (e), selected area electron diffraction(SAED) pattern of 5Au-HA(f), and TEM image of 10Au-HA (g)
3.2 Gas sensing properties
The gas-sensing properties of materials were performed at room temperature. Figure 5 shows the responses of pure and Au-HA to different concentrations of NH3. It can be seen that the response value increases with increasing the concentration of ammonia. In the low concentration range (from 10×10-6 to 50×10-6)(Fig. 5(b)), the increase in the response depends linearly on the concentration, while the response increases slowly in the range of (50-2000)×10-6, and gradually saturates at above 1000×10-6. The 5Au-HA shows the highest response in all the samples. The response of 5Au-HA increases with increasing NH3 concentration below 1000×10-6 (the response value is about 86.7%), and finally reaches the saturation at about 2000×10-6 (the response value is about 90.1%). The response of 10Au-HA is lower than that of 5Au-HA, which may be due to the agglomeration of gold nanoparticles (Fig. 3(g)).One of the main factors affecting the reaction kinetics is the size of Au nanoparticles. The Au nanoparticles in the range of 2-4 nm in size were found to be the most active for many catalytic applications [22]. In this work, the agglomeration of gold nanoparticles leads to the decrease of the sensing performance of 10Au-HA.
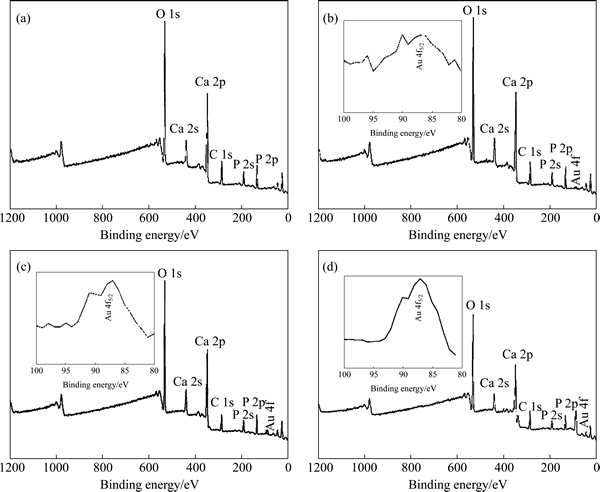
Fig. 4 XPS general spectra of pure HA (a), 2Au-HA (b), 5Au-HA (c), 10Au-HA (d) powders (XPS narrow scan spectra of Au element are presented for samples with Au concentration of 2% (b), 5% (c) and 10% (d))
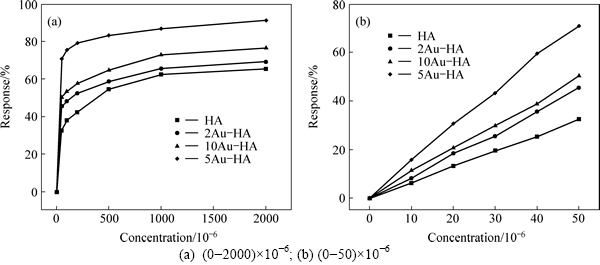
Fig. 5 Responses of pure and Au-modified HA to different concentrations of NH3 at room temperature:
The curves of the response versus time of pure and Au-HA to 200×10-6 NH3 are shown in Fig. 6. It can be seen that although the response value increases by adding Au, the response and recovery time are similar. The response and recovery time of 5Au-HA sensor are about 20 s and 25 s, respectively. Figure 7 shows the reproducibility and response at room temperature of the gas sensor based on 5Au-HA material for a concentration of NH3 of 1000×10-6. This gas sensor exhibits a very high sensitivity of about 86.7%. Moreover, the gas sensor based on 5Au-HA shows good reproducibility with relatively minor deviations for five straight times. The NH3 sensing properties of Au-HA are compared with those of other gas sensor materials, which are summarized from literatures reported in recent years [23-29], as shown in Table 1. Compared with other materials, Au-HA in this work demonstrated better combination sensor properties for detection of ammonia gas, with good reproducibility, competitive detection limit, shorter response and recovery time, as well as low working temperature.
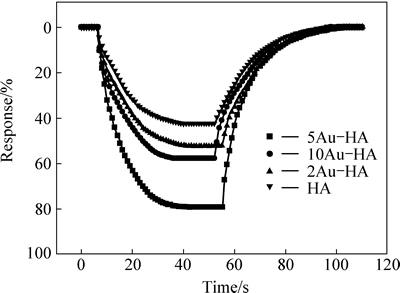
Fig. 6 response curves of pure HA and Au-HA to 200×10-6 NH3 at room temperature
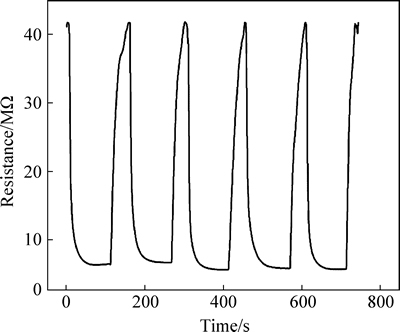
Fig. 7 Reproducibility and response of gas sensor based on 5Au-HA versus time for a concentration of NH3 of 1000×10-6 at room temperature
Figure 8 shows the responses of 5Au-HA to different concentrations of different gases (ethanol, methanol, heptane, methylbenzene, and acetone) and water vapor. The results show that the Au-HA can adsorb many organic molecules in the testing atmosphere. However, the response to NH3 (86.7%) is much higher than to other gases (lower than 55%). So, the Au-HA demonstrates a high selectivity for NH3 gas. The sensor has very low response to water vapor (<6%) and the response reaches the highest value (5.8%) when the concentration of H2O is 500×10-6, with no change at the concentration of 1000×10-6 and 2000×10-6. So, the influence of humidity or the water vapor in the atmosphere on the gas sensing response can be excluded.
3.3 Discussion of gas sensing mechanism
HA is generally considered to be ionic conductor, and its conduction mechanism is believed to be related with OH- or H+. The former usually plays the main part at elevated temperature while the later plays the leading role at room temperature [30]. Moreover, the surface of hydroxyapatite can be characterized by P-OH groups and/or several ionic species like Ca2+, 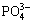 and OH- along with porosity. The protons (H+), oxide ions (O2-) and the lattice hydroxyl ions (OH-) play an important role in determining the reactivity with gas molecules, and contribute to the conductivity [10].
and OH- along with porosity. The protons (H+), oxide ions (O2-) and the lattice hydroxyl ions (OH-) play an important role in determining the reactivity with gas molecules, and contribute to the conductivity [10].
Table 1 NH3 sensing property of 5Au-HA compared with other gas sensor reported in recently literature
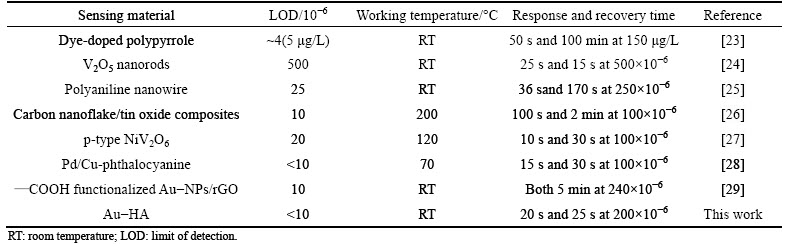
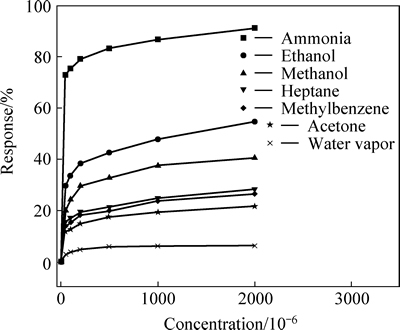
Fig. 8 Responses of 5Au-HA to different concentrations of different gases and water vapor
As shown in Fig. 9, the possible mechanism for the adsorption of NH3 gas on HA surface is illustrated as follows:
1) The excellent gas sensing properties of HA can be attributed to the unique tube-like structure, which has higher specific area. The tube structure allows the gas molecules to diffuse rapidly throughout the inner channel and the surface, resulting in a fast gas response. Selective physisorption of HA to ammonia is one of the key factors. A variety of active sites available for ammonia molecule sorption exist on both outer and inner surface of the tube-like HA. This selective physisorption to ammonia affects electron transport through the HA arrays. The following discussion will explain the selective physisorption of HA to ammonia.
2) HA exhibits good hydrophilic and can have hydrogen bonding interaction of 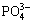 with H2O of the aqueous layer (Eq. (2)). NH3 that is a polar molecule is easy to bond with H2O through hydrogen bond. Thus, when being exposed to NH3 gas, the NH3 molecule will be adsorbed on the surface of HA by hydrogen bonding with H2O molecules that are absorbed on the HA surface (Eq. (3)). The formation of hydrogen bond between NH3 and H2O molecules was confirmed by the coadsorption study of NH3 and H2O using infra-red reflection absorption spectroscopy [25, 31].
with H2O of the aqueous layer (Eq. (2)). NH3 that is a polar molecule is easy to bond with H2O through hydrogen bond. Thus, when being exposed to NH3 gas, the NH3 molecule will be adsorbed on the surface of HA by hydrogen bonding with H2O molecules that are absorbed on the HA surface (Eq. (3)). The formation of hydrogen bond between NH3 and H2O molecules was confirmed by the coadsorption study of NH3 and H2O using infra-red reflection absorption spectroscopy [25, 31].
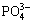 +2H2O
+2H2O HO—H…O—PO2—O…H—O—H(2)
HO—H…O—PO2—O…H—O—H(2)
H2O+NH3 H3N…H—O—H (3)
H3N…H—O—H (3)
3) Improved sorption property by gold nanoparticles. The surface of gold nanoparticles has a negative charge, with a high electron density. The redox potential of gold nanoparticle is 1.68 V, so it has a strong ability to seize electrons. On the other hand, the nitrogen atom of NH3 molecule has a lone-pair electron. NH3 is a strong electron donor. So, the electrons could be transferred from ammonia to gold nanoparticles, resulting in a significant change in the conductivity of the sensor. Additionally, Au nanoparticles play a role of catalytic activator by increasing the gas adsorption sites. The chemical and field effects of Au nanoparticles can lead to surface defects of gas-sensing materials, which also have a significant impact on the improvement of sensing properties [16]. In general, the above effects contribute to the excellent response and selectivity of Au-HA for NH3 gas.
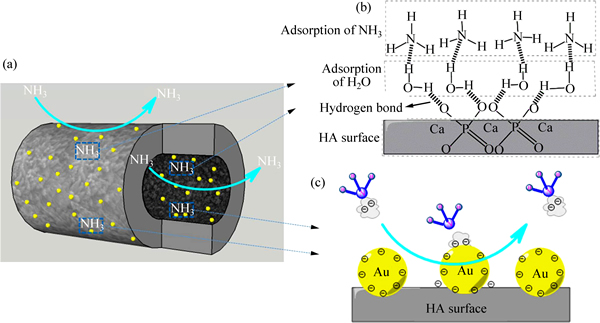
Fig. 9 Mechanism diagram of NH3 gas response with Au-HA
The sensing mechanism of other gases depends on its adsorption behavior. Different gases may bond with HA by different function groups, leading to different sensing behaviors. This part needs to be further studied in the future.
4 Conclusions
Pure HA and Au-HA powders with tube-like 3D morphologies are successfully prepared. Au nanoparticles are added into HA successfully by the hydrothermal treatment. Both HA and Au-HA exhibit a good sensitivity to several kinds of gases at room temperature, and have the highest response to NH3. Adding Au nanoparticles can significantly improve the gas sensing properties of HA at room temperature. The Au-HA with 5% (mass fraction) Au has the best gas sensing properties with the highest response value of 90.1% at a NH3 concentration of 2000×10-6, and the response value of 70.8% at a NH3 concentration of 50×10-6. The mechanism of high response and selectivity to NH3 may be a combined effect, including the function of Au nanoparticles, special structure of tube-like HA and its unique surface properties. In conclusion, the Au-HA sensor exhibits good analytical performance to NH3 in terms of high sensitivity and reproducibility, low detection limit, and high selectivity.
References
[1] MAHABOLE M P, MENE R U, KHAIRNAR R S. Gas sensing and dielectric studies on cobalt doped hydroxyapatite thick films [J]. Advanced Materials Letters, 2013, 4(1): 46-52.
[2] CHOI Y J, HWANG I S, PARK J G, CHOI K J, PARK J H, LEE J H. Novel fabrication of an SnO2 nanowire gas sensor with high sensitivity [J]. Nanotechnology, 2008, 19: 095508.
[3] YANG T Y, LIN H M, WEI B Y, WU C Y, LIN C K. UV enhancement of the gas sensing properties of nano-TiO2 [J]. Review Advance Materials Science, 2003, 4: 48-54.
[4] XU J, PAN Q, SHUN Y A, TIAN Z. Grain size control and gas sensing properties of ZnO gas sensor [J]. Sensors and Actuators B: Chemical, 2000, 66: 277-279.
[5] ELLIOTT J C. Structure and chemistry of the apatites and other calcium orthophosphates [M]. Amsterdam: Elsevier, 1994.
[6] HUANG J, WANG L C, LIU Y M, CAO Y, HE H Y, FAN K N. Gold nanoparticles supported on hydroxylapatite as high performance catalysts for low temperature CO oxidation [J]. Applied Catalysis B: Environmental, 2011, 101: 560-569.
[7] DOM N M, ROMEROS F, CENTENO M, ODRIOZOLA J. Gold/hydroxyapatite catalysts: Synthesis, characterization and catalytic activity to CO oxidation [J]. Applied Catalysis B: Environmental, 2009, 87: 245-251.
[8] FUJII E, KAWABATA K, ANDO K, TSURU K, HAYAKAWA S, OSAKA A. Synthesis and structural characterization of silica-hybridized hydroxyapatite with gas adsorption capability [J].Journal of the Ceramic Society of Japan, 2006, 114(9): 769-773.
[9] MENE R U, MAHABOLE M P, KHAIRNAR R. Surface modification of cobalt doped hydroxyapatite thick films via swift heavy ion irradiations for CO and CO2 gas sensing application [C]// Proceedings IMCS. 2012: 1180-1183.
[10] MENE R U, MAHABOLE M P, SHARMA R, KHAIRNAR R S. Enhancement in CO gas sensing properties of hydroxyapatite thick films: Effect of swift heavy ion irradiation [J]. Vacuum, 2011, 86: 66-71.
[11] ZHANG D, LI C, LIU X, HAN S, TANG T, ZHOU C. Doping dependent NH3 sensing of indium oxide nanowires [J]. Applied Physics Letters, 2003, 83: 1845-1847.
[12] ZHU J. Photocatalytic properties of thin films of ruthenium metallopolymers/gold nanoparticle: Polyoxometalate composites using visible excitation [J]. Journal of Central South University, 2013, 20: 2657-2662.
[13] WANG X, ZHANG J, ZHU Z, ZHU J. Effect of Pd2+ doping on ZnO nanotetrapods ammonia sensor [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2006, 276: 59-64.
[14] RAMGIR N S, HWANG Y K, JHUNG S H, KIM H K, HWANG J S, MULLA I S, CHANG J S. CO sensor derived from mesostructured Au-doped SnO2 thin film [J]. Applied Surface Science, 2006, 252: 4298-4305.
[15] TIEN L, SADIK P, NORTON D, VOSS L, PEARTON S, WANG H, KANG B, REN F, JUN J, LIN J. Hydrogen sensing at room temperature with Pt-coated ZnO thin films and nanorods [J]. Applied Physics Letters, 2005, 87: 222106.
[16] GUO J, ZHANG J, ZHU M, JU D, XU H, CAO B. High-performance gas sensor based on ZnO nanowires functionalized by Au nanoparticles [J]. Sensors and Actuators B: Chemical, 2014, 199: 339-345.
[17] TANG H, YAN M, ZHANG H, LI S, MA X, WANG M, YANG D. A selective NH3 gas sensor based on Fe2O3–ZnO nanocomposites at room temperature [J]. Sensors and Actuators B: Chemical, 2006, 114: 910-915.
[18] MANI G K, RAYAPPAN J B B. A highly selective and wide range ammonia sensor—nanostructured ZnO:Co thin film [J]. Materials Science and Engineering B, 2015, 191: 41-50.
[19] ZHANG Y, LIU Y, JI X, BANKS C E, ZHANG W. Sea cucumber-like hydroxyapatite: cation exchange membrane-assisted synthesis and its application in ultra-sensitive heavy metal detection [J]. Chemical Communication, 2011, 47: 4126-4128.
[20] ZHANG Y, LI K, ZHANG Q, LIU W, LIU Y, BANKS C E. Multi-dimensional hydroxyapatite (HAp) nanocluster architectures fabricated via Nafion-assisted biomineralization [J]. New Journal of Chemistry, 2015, 39(1): 750-754.
[21] SANGPOUR P, AKHAVAN O, MOSHFEGH A, ROOZBEHI M. Formation of gold nanoparticles in heat-treated reactive co-sputtered Au-SiO2 thin films [J]. Applied surface science, 2007, 254: 286-290.
[22] HARUTA M. Nanoparticulate gold catalysts for low-temperature CO oxidation [J]. Journal of New Materials for Electrochemical Systems, 2004, 7: 163-172.
[23] TAVOLI F, ALIZADEH N. Optical ammonia gas sensor based on nanostructure dye-doped polypyrrole [J]. Sensors and Actuators B: Chemical, 2013, 176: 761-767.
[24] DHAYAL RAJ A, PAZHANIVEL T, SURESH KUMAR P, MANGALARAJ D, NATARAJ D, PONPANDIAN N. Self assembled V2O5 nanorods for gas sensors [J]. Current Applied Physics, 2010, 10: 531-537.
[25] TUAN C V, TUAN M A, HIEU N V, TRUNG T. Electrochemical synthesis of polyaniline nanowires on Pt interdigitated microelectrode for room temperature NH3 gas sensor application [J]. Current Applied Physics, 2012, 12: 1011-1016.
[26] LEE S K, CHANG D, KIM S W. Gas sensors based on carbon nanoflake/tin oxide composites for ammonia detection [J]. Journal of Hazardous Materials, 2014, 268: 110-114.
[27] BALAMURUGAN C, LEE D W. A selective NH3 gas sensor based on mesoporous p-type NiV2O6 semiconducting nanorods synthesized using solution method [J]. Sensors and Actuators B: Chemical, 2014, 192: 414-422.
[28] MACIAK E, PUSTELNY T. An optical ammonia (NH3) gas sensing by means of Pd/CuPc interferometric nanostructures based on white light interferometry [J]. Sensors and Actuators B: Chemical, 2013, 189: 230-239.
[29] XIA X, GUO S, ZHAO W, XU P, YU H, XU T, LI X. Carboxyl functionalized gold nanoparticles in situ grown on reduced graphene oxide for micro-gravimetric ammonia sensing [J]. Sensors and Actuators B: Chemical, 2014, 202: 846-853.
[30] NAGAI M, NISHINO T. Surface conduction of porous hydroxyapatite ceramics at elevated temperatures [J]. Solid State Ionics, 1988, 28: 1456-1461.
[31] SHINGAYA Y, KUBO H, ITO M. Coadsorption of ammonia and electrolyte anions on a Pt(111) electrode [J]. Surface Science, 1999, 427/428: 173-178.
(Edited by YANG Hua)
Foundation item: Project(51272289) supported by the National Natural Science Foundation of China
Received date: 2015-03-30; Accepted date: 2015-04-10
Corresponding author: TAN Yan-ni, Lecture, PhD; Tel: +86-731-88877669; E-mail: tanyanni@csu.edu.cn