复合铸造法制备的镁/铜复合材料的界面组织和力学性能
来源期刊:中国有色金属学报(英文版)2019年第6期
论文作者:徐德兴 杨长林 赵康宁 李宏祥 张济山
文章页码:1233 - 1241
关键词:Mg/Cu复合材料;复合铸造;界面结合机制;界面组织;界面力学性能
Key words:Mg/Cu bimetal composites; compound casting; interfacial bonding mechanism; interfacial microstructure; interfacial mechanical behavior
摘 要:采用复合铸造法制备镁/铜复合材料,并对其显微组织演化、相组成和界面结合强度进行研究。研究发现,界面实现冶金结合并且由两个亚层组成:靠近纯铜一侧的亚层I,厚度为30 μm,主要由Mg2Cu相组成,并且在上面随机分布一些枝状相MgCu2;靠近纯镁一侧的亚层II,厚度为140 μm,主要由层状的Mg2Cu+(Mg)纳米共晶相和少量分离的Mg2Cu组成。复合材料的平均界面剪切强度为13 MPa。本研究为镁/铜复合材料作为储氢材料的应用提供了一个新的制备方法。
Abstract: Mg/Cu bimetal composites were prepared by compound casting method, and the microstructure evolution, phase constitution and bonding strength at the interface were investigated. It is found that a good metallurgical bonding can be achieved at the interface of Mg and Cu, which consists of two sub-layers, i.e., layer I with 30 μm on the copper side composed of Mg2Cu matrix phase, on which a small amount of dendritic MgCu2 phase was randomly distributed; layer II with 140 μm on the magnesium side made up of the lamellar nano-eutectic network Mg2Cu+(Mg) and a small amount of detached Mg2Cu phase. The average interfacial shear strength of the bimetal composite is measured to be 13 MPa. This study provides a new fabrication process for the application of Mg/Cu bimetal composites as the hydrogen storage materials.
Trans. Nonferrous Met. Soc. China 29(2019) 1233-1241
De-xing XU1, Chang-lin YANG2, Kang-ning ZHAO1, Hong-xiang LI1, Ji-shan ZHANG1
1. State Key Laboratory for Advanced Metals and Materials, University of Science and Technology Beijing, Beijing 100083, China;
2. State Key Laboratory of Solidification Processing, Northwestern Polytechnical University, Xi’an 710072, China
Received 21 August 2018; accepted 18 December 2018
Abstract: Mg/Cu bimetal composites were prepared by compound casting method, and the microstructure evolution, phase constitution and bonding strength at the interface were investigated. It is found that a good metallurgical bonding can be achieved at the interface of Mg and Cu, which consists of two sub-layers, i.e., layer I with 30 μm on the copper side composed of Mg2Cu matrix phase, on which a small amount of dendritic MgCu2 phase was randomly distributed; layer II with 140 μm on the magnesium side made up of the lamellar nano-eutectic network Mg2Cu+(Mg) and a small amount of detached Mg2Cu phase. The average interfacial shear strength of the bimetal composite is measured to be 13 MPa. This study provides a new fabrication process for the application of Mg/Cu bimetal composites as the hydrogen storage materials.
Key words: Mg/Cu bimetal composites; compound casting; interfacial bonding mechanism; interfacial microstructure; interfacial mechanical behavior
1 Introduction
As an excellent hydrogen storage material, Mg/Cu bimetal composite has been paid more and more attention in recent years [1-3]. Compared with single magnesium alloy, the new composite exhibits its outstanding advantages such as better kinetics and reversibility for hydrogen reaction, higher hydrogen storage ability, and lower hydrogen desorption temperature [3,4]. So far, several studies on Mg/Cu bimetal composites, fabricated by different joint processes, have been reported. TANAKA et al [2-4] and TAKEICHI et al [5] fabricated Mg/Cu super-laminate composites (SLCs) by accumulative roll-bonding (ARB) method for the application of hydrogen storage. The micro/nano-structures from the SLCs ensure lower dehydrogenation temperature and better kinetics of hydrogen absorption/desorption. But the influence of MgO on the formation and growth of interface layers in Mg/Cu super-laminate composites cannot be negligible and the corresponding process is very complex. NONAKA et al [6] and DAI et al [7] investigated reaction diffusion kinetics in Mg-Cu system fabricated by solid-solid diffusion method. They found that two kinds of intermetallic compounds Mg2Cu and MgCu2 in the Mg-Cu diffusion couples were observed in the temperature range of 673-733 K and the growth kinetics of intermetallic phases is diffusion-controlled. However, there was a significantly large gap formed at the interface and only some local and limited metallurgical bonding could be obtained for these samples. Thus, it urgently demands to find an advanced fabrication process, which can enhance the interfacial bonding performance of Mg/Cu bimetal composites for the application of hydrogen storage.
Compound casting is characterized as a process of joining two metals or alloys in which a metallic melt is cast onto or around a solid metal substrate and a liquid-solid diffusion reaction zone is formed at the interface that results in bonding of the two metals [8,9]. This process could join semi-finished parts with complex structures and the process is simple and easy, and the fabrication time is also short. Importantly, by this technique good interfacial metallurgical bonding can be obtained. Up to date, a few studies have been reported to join different dissimilar and similar metallic couples such as Al/Mg [10,11], Ti/Al [12,13], Cu/Al [14,15], Al/Al [16,17] and Mg/Mg [18,19] by the compound casting process. However, joining copper and magnesium alloys together by compound casting process is still an unexplored area.
In this work, the fabrication of Mg/Cu bimetal composites using pure magnesium and pure copper by compound casting process was studied and the inter- facial microstructure and mechanical behaviors were investigated in detail. It is expected to provide a new fabrication route for the application of Mg/Cu bimetal composites as the hydrogen storage materials.
2 Experimental
2.1 Specimens preparation
Pure copper (99.96 wt.%) and pure magnesium (99.8 wt.%) were used as base materials in this study. The copper bars machined with 10 mm in diameter and 100 mm in height were mechanically polished using 240-600 grit abrasive papers to obtain clean and smooth surfaces and then treated with degreasing, alkali cleaning and acid picking before the compound casting. Similarly, pure magnesium ingots were cut into pieces and pre-treated to remove surface dirt and oxides. Subsequently, the selected pure magnesium ingots were heated in a graphite crucible located in an electrical resistance furnace and melted at 933 K. For preventing the oxidation, a protective gas mixture containing 0.2 vol.% SF6 and remainder CO2 was used during the melting process. Moreover, Mg melt was regularly stirred and the dross floating on the surface was removed. When the melt temperature was maintained at 923 K, the Mg melt was poured into the steel mold inserted the copper bar and cooled by circulating water throughout the entire process. The schematic sketches of fabricating Mg/Cu composite samples using the compound casting process and the attained Mg/Cu couple are shown in Figs. 1(a) and (b), respectively. The cross-section of Mg/Cu specimens is shown in Fig. 1(c).
2.2 Interface characterization and mechanical tests
To analyze the microstructure and bonding behavior at the interface of the Mg/Cu composites, the specimens were cut from the middle part of the samples perpendicular to the cylindrical insert with a thickness of 5 mm shown in Fig. 1(c). The interfacial microstructure and element compositions of the specimens were examined by a ZEISS EVO-18 scanning electron microscope (SEM) equipped with an energy dispersive X-ray spectrometer (EDS). To investigate more details of the interface layer, the phases on bonding interface were further observed by a Tecnai G2 F30 transmission electron microscope (TEM) operated at 300 kV.
To characterize the mechanical performance, Mg/Cu bimetal composite specimens were taken from the middle part of the cylindrical samples. Shear tests were carried out on a DDL universal material testing machine. A schematic sketch of the setup used for the push-out tests is illustrated in Fig. 2. The tested specimens were put on the flat supporting surface with a circular hole of 12 mm in diameter. Then, the tested specimens were pushed by means of a steel cylinder stub punch with 8 mm in diameter, which is concentric with the support hole and a cross-head displacement rate of 0.5 mm/min at an ambient temperature. For repeatability and accuracy, five slices of the samples were submitted to push-out test. Shear strength of the interface (τint) was calculated using the following equation [11,12]:
 (1)
(1)
where Fmax is the maximum load, r is the insert radius (5 mm), and l is the specimen thickness (5 mm). The Vickers hardness was measured across the interface layer by the micro-hardness tester at a load of 50 g and a dwell time of 15 s.

Fig. 1 Schematic sketches of mold used for casting process (a), preparing Mg/Cu couple (b) and cross-section of Mg/Cu specimens (c)

Fig. 2 Schematic sketch of setup used for push-out tests
3 Results
3.1 Interfacial chemistry
As illustrated in Fig. 3, the reaction diffusion layer formed at the interface of Mg/Cu bimetal composites can be observed, indicating a good metallurgical bonding.
From Fig. 3(a), the interface layer of Mg/Cu joints prepared by compound casting consists of two sub- layers, i.e., a layer on the copper side with a thickness about 30 μm (layer I) and a layer on the magnesium side with a thickness about 140 μm (layer II). Layer I is mainly composed of light gray phases as the matrix phase, on which a small number of white dendritic phases are randomly distributed. The magnified image of area A (Fig. 3(a)) in layer I is shown Fig. 3(b), and from the measurement it is seen that the scale of the white dendritic phase is nanoscale. Layer II is composed of the dark gray phase. As shown in Fig. 3(c), in the magnified image of area B (Fig. 3(a)) in layer II, it is shown that the nano-lamellar eutectic network was formed in layer II. This eutectic structure was frequently seen at the interface of Al/Cu couples [20-22] and Al/Mg couples [23,24] as Al2Cu+(Al) eutectic and Al12Mg17+(Mg) eutectic, respectively. From Fig. 3(c), the lamellar nano-eutectic network is too small to be confirmed by SEM.

Fig. 3 SEM micrographs of interfacial microstructures for Mg/Cu bimetal composites prepared by compound casting process

Fig. 4 Line scan spectrum of reaction interface detected by EDS
Figure 4 shows the composition variations of Mg and Cu elements at the interface of the bimetal composites. The result reveals that the bimetal composites retain the as-cast layered characteristics. In the present study, the thickness of diffusion reaction layer is defined as the interdiffusion distance of Mg and Cu elements across the interface. It is seen that continuous intermetallic layers were formed with Mg/Cu energy spectrum lines exhibiting three smooth platforms. And the content of Mg atom is obviously higher than that of Cu atom in entire interface layers. However, the energy spectrum lines of the Mg/Cu interface layer are not straight but wavy lines because of the light gray phase containing some dendritic phases in layer I and the eutectic phase in layer II. Figure 5 describes the distribution of Mg and Cu atoms in the interface zone, and red and green points represent Mg and Cu atoms, respectively. The result of atom distribution shows that the diffusion range of Cu atom is obviously larger than that of Mg atom. According to Dai’s discussion [7], the diffusion coefficient of Cu in Mg is an order of magnitude faster than that of Mg in Cu. Therefore, it can explain the reason for the fact that the thickness of layer I is much thinner than that of layer II shown in Fig. 3.
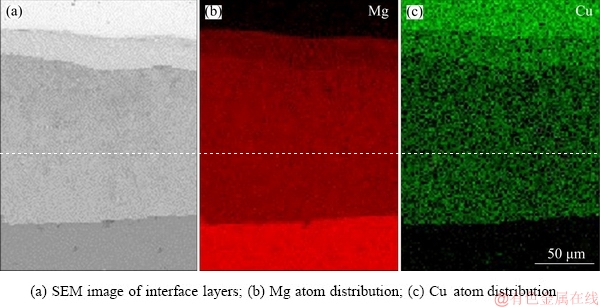
Fig. 5 Area scan spectrum maps of reaction interface by EDS

Fig. 6 STEM micrograph (a) and bright-field (BF) image (d) of interface layer I, SAED patterns of intermetallic phases marked with spectra 1 (b) and 2 (e) in (a), and chemical analysis by EDS marked with spectra 1 (c) and 2 (f)
To investigate more details of the local micro- structures between these two joints, the intermetallic phases in the interface zone were analyzed by scanning transmission electron microscopy (STEM) and selected area electron diffraction (SAED). Figures 6(a) and (d) show the STEM micrograph and the bright-field (BF) image of interface layer I, respectively. Figures 6(b) and (e) respectively show the related SAED patterns of the intermetallic phases in Fig. 6(a) marked by spectra 1 and 2. The white dendritic phase marked by spectrum 1 is MgCu2, which has a face-centered-cubic (FCC) structure with a lattice constant of a=0.7 nm. From EDS analysis shown in Fig. 6(c), the composition of the white dendritic phase is estimated to be 32.58at.%Mg- 67.42at.%Cu. The gray phase marked with spectrum 2, which has an orthorhombic structure with a lattice constant of a=0.528 nm, b=0.906 nm and c=1.835 nm, is identified as Mg2Cu. From EDS analysis shown in Fig. 6(f), the composition of the gray phase is estimated to be 62.47at.%Mg-37.53at.%Cu. The reason for the higher Cu concentration is due to a small amount of copper dissolved into the Mg2Cu. Figures 7(a) and (d) respectively show the STEM micrograph and the bright-field (BF) image of layer II shown in Fig. 3. The nano-eutectic network structure was further identified, and some detached gray phases were found. Figures 7(b) and (e) show the related SAED patterns of the intermetallic phases in Fig. 7(a) marked with spectra 1, 2 and 3. The SAED patterns recorded from detached phase and the gray phase revealed that they are the Mg2Cu phase. From EDS analysis shown in Fig. 7(c), the composition of the light gray phase marked by spectrum 2 is estimated to be 67.25at.%Mg-32.75at.%Cu, which is like Mg2Cu and the detached phase has also a similar composition. The dark phase marked by spectrum 3, which has a hexagonal-close-packing (HCP) structure, is identified as Mg. The composition of the dark phases is determined to be more than 98.71 at.% Mg. From the area scan spectrum maps of the interface layer II shown in Fig. 8, Cu element is only distributed on the nano-lamellar network structure, indicating the formation of Mg2Cu+(Mg) phase. It is reported that Mg and Mg2Cu can react with hydrogen through the following reactions, which are almost fully reversible [25-27]:
Mg+H2 MgH2 (2)
MgH2 (2)
2/3Mg2Cu+H2 MgH2+1/3MgCu2 (3)
MgH2+1/3MgCu2 (3)
The activation energy for the decomposition of hydrides of Mg2Cu is lower than that of Mg, which is 200 and 289 kJ/mol, respectively [25]. Moreover, the nanostructured hydrogen-active phases of Mg2Cu and Mg exhibit enhanced hydrogen absorption kinetics possibly due to the small grain size [27,28]. Thus, it can be possible for the current Mg/Cu bimetal composite to be applied as a potential hydrogen storage material. The corresponding studies on the hydrogen-storage property will be carried out in our group.
3.2 Interfacial mechanical properties
Microhardness measured across the bonding interface is shown in Fig. 9. The hardness curve is stepped, and the hardness of the interface layers is significantly higher than that of the base metals. The base metals, e.g., pure magnesium and pure copper, have average hardness values of HV 35 and HV 62, respectively, while the interface layers I and II exhibit average hardness values of HV 310 and HV 180, respectively. The higher hardness of interface layer is due to the formation of intermetallic compound Mg2Cu in layer I and the eutectic phase Mg2Cu+(Mg) in layer II during casting. The interface transition zone with a width of 170 μm is in accordance with Fig. 3.

Fig. 7 STEM micrograph (a) and bright-field (BF) image (d) of interface layer II, SAED patterns of intermetallic phases marked with spectra 1 and 2 (b) and 3 (e) in (a), and chemical analysis by EDS marked with spectra 2 (c) and 3 (f)
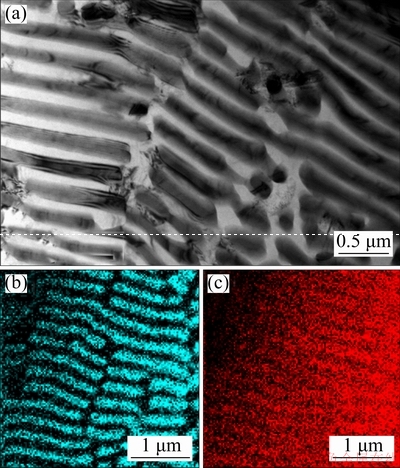
Fig. 8 STEM micrograph (a) and area scan spectra of elements Cu (b) and Mg (c) of interface layer II by EDS

Fig. 9 Microhardness across bonding interface of Mg/Cu bimetal composites
Shear strength can be used to evaluate the bonding quality at the interface. Figure 10(a) exhibits shear strength-displacement curve for the Mg/Cu interface. The shear strength increases linearly with the displacement increment, then decreases after reaching a maximum load, without showing apparent plastic deformation. The maximum strength value obtained from the current Mg/Cu bimetal composites is 13 MPa, which is close to the value reported by ZHANG et al [29]. To observe the crack initiation, the characteristic SEM image of Mg/Cu bimetal composites after push-out test interrupted at 80% of maximum load is shown in Fig. 10(b). It can be found that the crack is located at the interface between layer I and layer II with an orientation parallel to the load direction, and the crack lips stick to each other under loading. Therefore, it can be concluded that the crack propagation is mainly related to brittle intermetallic Mg2Cu phases formed in the interface layers. In addition, a few cracks generated in the casting process, as shown in the two black ellipses in Fig. 3(a), have also some influence on the final shear strength of the samples.

Fig. 10 Stress-distance curve of Mg/Cu interface obtained by push-out tests (a) and characteristic SEM image of Mg/Cu bimetal composites after push-out test interrupted at 80% of maximum load (b)
4 Discussion

Fig. 11 Schematic drawings showing formation process of Mg/Cu interface layers during compound casting
The bimetal composites fabricated by compound casting process include three types of interface bonding states, i.e., the surface layer of the insert partially melted and metallurgically bonded with the casting (Type 1), only a mechanical joint achieved (Type 2), or insert fully melted and lost its shape (Type 3) [30]. Figure 11 shows a schematic view of the formation process of the interface layers. As the magnesium melt encounters the copper insert and surrounds it, the outer layer of the insert starts getting melted. As shown in Fig. 3, the interface between copper and layer I is irregular due to the partial melting of the surface of copper. The concentration of copper atoms in the magnesium melt near the surface of copper bar increased sharply and began to form compounds on the copper surface. According to Ref. [31], the primary phases formed in Mg/Cu interface layer are Mg2Cu due to the lowest melting point among all the compounds in the phase diagram. In this work, it was also determined that Mg2Cu was firstly formed on the Cu surface. The flow of melt around the copper bar due to the filling of the mold and the convection of the melt, can facilitate the detachment of the Mg2Cu phases formed on the Cu surface during the contact between Cu insert and the Mg melt. From the Mg-Cu binary phase diagram (Fig. 12) [6,7], the melting point of the Mg2Cu compounds is 841 K, which is lower than pouring temperature of Mg melt (923 K), so some Mg2Cu compounds were melted and then spread out due to capillary pressure. When the temperature decreases to 758 K, the eutectic compounds Mg2Cu+(Mg) can be formed due to L→Mg2Cu+Mg eutectic transformation. Due to the cooling of the circulating water throughout the pouring process, the eutectic compounds are difficult to grow. Thus, the lamellar nano-eutectic network was formed in layer II shown in Figs. 3 and 7. Moreover, from the reaction of the unmelted Mg2Cu matrix with Cu, some dendritic phase MgCu2 can be generated in layer I as follows [32]:
3Cu+ Mg2Cu→2MgCu2 (4)
Thus, from the above discussion, the interface layer of Mg/Cu bimetal composites prepared by compound casting process consists of two sub-layers: layer I on the copper side composed of Mg2Cu matrix phase and some dendritic MgCu2 phases and layer II on the magnesium side made up of the lamellar nano-eutectic network Mg-Mg2Cu and some detached Mg2Cu phase. This microstructural characterization of the interface is different from the early investigations due to different manufacturing processes [3,7,29].

Fig. 12 Phase diagram of Mg-Cu binary alloys
5 Conclusions
(1) Mg/Cu bimetal composites, with good metallurgical bonding and relatively uniform interface thickness of about 170 μm, were prepared.
(2) The interfacial formation in the compound casting process is diffusion-controlled, which consists of two sub-layers: layer I on the copper side composed of Mg2Cu matric phases, on which a small amount of dendritic MgCu2 phases are randomly distributed and layer II on the magnesium side made up of the lamellar nano-eutectic network Mg2Cu+(Mg) and some detached Mg2Cu phases.
(3) The hardness curve of the bonding interface is stepped. The average hardness values of the interface layer I and layer II are HV 310 and HV 180, respectively, which are much higher than those of both base metals (Mg and Cu). The shear strength of the interface is only 13 MPa due to the formation of brittle phase Mg2Cu.
(4) Current work provides a new fabrication process for the application of Mg/Cu bimetal composites as the hydrogen storage materials.
References
[1] TAKAMURA H, KAKUTA H, GOTO Y, WATANABE H, KAMEGAWA A, OKADA M. High-pressure synthesis and energetics of MgCu with a CsCl-type structure [J]. Journal of Alloys and Compounds, 2005, 404-406: 372-376.
[2] TANAKA K, NISHINO D, HAYASHI K, IKEUCHI S, KONDO R, TAKESHITA H T. Formation of Mg2Cu at low temperature in Mg/Cu super-laminate composites during initial hydrogenation [J]. International Journal of Hydrogen Energy, 2017, 42(35): 1-9.
[3] TANAKA K, SHIBATA K, KURUMATANI K, IKEUCHI S, KIKUCHI S, KONDO R, TAKESHITA H T. Formation mechanism of micro/nano-structures through competitive reactions in Mg/Cu super-laminate composites during initial hydrogenation [J]. Journal of Alloys and Compounds, 2015, 645(S): s72-s75.
[4] TANAKA K, TAKEICHI N, TANAKA H, KURIYAMA N, UEDA T T, TSUKAHARA M, MIYAMURA H, KIKUCHI S. Investigation of micro-structural transition through disproportionation and recombination during hydrogenation and dehydrogenation in Mg/Cu super-laminates [J]. Journal of Materials Science, 2008, 43(11): 3812-3816.
[5] TAKEICHI N, TANAKA K, TANAKA H, UEDA T T, KAMIYA Y, TSUKAHARA M, MIYAMURA H, KIKUCHI S. Hydrogen storage properties of Mg/Cu and Mg/Pd laminate composites and metallographic structure [J]. Journal of Alloys and Compounds, 2007, 446-447: 543-548.
[6] NONAKA K, SAKAZAWA T, NAKAJIMA H. Reaction diffusion in Mg-Cu system [J]. Materials Transactions, JIM, 1995, 36(12): 1463-1466.
[7] DAI Jia-hong, JIANG Bin, ZHANG Jian-yue, YANG Qing-shan, JIANG Zhong-tao, DONG Han-wu, PAN Fu-sheng. Diffusion kinetics in Mg-Cu binary system [J]. Journal of Phase Equilibria and Diffusion, 2015, 36(6): 613-619.
[8] PAPIS K J M, LOEFFLER J F, UGGOWITZER P J. Light metal compound casting [J]. Science in China Series E: Technological Sciences, 2009, 52(1): 46-51.
[9] HU Yuan, CHEN Yi-qing, LI Li, HU Huan-dong, ZHU Zi-ang. Microstructure and properties of Al/Cu bimetal in liquid–solid compound casting process [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(6): 1555-1563.
[10] LIU J C, HU J, NIE X Y, LI H X, DU Q, ZHANG J S, ZHUANG L Z. The interface bonding mechanism and related mechanical properties of Mg/Al compound materials fabricated by insert molding [J]. Materials Science and Engineering A, 2015, 635: 70-76.
[11] EMAMI S M, DIVANDARI M, ARABI H, HAJJARI E. Effect of melt-to-solid insert volume ratio on Mg/Al dissimilar metals bonding [J]. Journal of Materials Engineering and Performance, 2013, 22(1): 123-130.
[12] NIE X Y, LIU J C, LI H X, DU Q, ZHANG J S, ZHUANG L Z. An investigation on bonding mechanism and mechanical properties of Al/Ti compound materials prepared by insert moulding [J]. Materials & Design, 2014, 63: 142-150.
[13] NIE X Y, ZHAO K N, LI H X, DU Q, ZHANG J S, ZHUANG L Z. Comparisons of interface microstructure and mechanical behavior between Ti/Al and Ti-6Al-4V/Al bimetallic composites [J]. China Foundry, 2015, 12: 1-8.
[14] HUANG Hua-gui, DONG Yi-kang, YAN Meng, DU Feng-shan. Evolution of bonding interface in solid–liquid cast-rolling bonding of Cu/Al clad strip [J]. Transactions of Nonferrous Metals Society of China, 2017, 27(5): 1019-1025.
[15] CHU Di, ZHANG Jian-yu, YAO Jin-jin, HAN Yan-qiu, WU Chun-jing. Cu–Al interfacial compounds and formation mechanism of copper cladding aluminum composites [J]. Transactions of Nonferrous Metals Society of China, 2017, 27(11): 2521-2528.
[16] JIANG Wen-ming, JIANG Zai-liang, LI Guang-yu, WU Yao, FAN Zi-tian. Microstructure of Al/Al bimetallic composites by lost foam casting with Zn interlayer [J]. Materials Science and Technology, 2018, 34(4): 487-492.
[17] HAN Xing, SHAO Bo, ZUO Ke-sheng, JIANG Lin, ZHANG Hai-tao, HE Li-zi, QIN Ke, CUI Jian-zhong. Microstructure and properties at bonding interface of AA4045/AA3003 aluminum alloy cladding billet prepared by semi-continuous casting [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(3): 658-664.
[18] ZHAO K N, LI H X, LUO J R, LIU Y J, DU Q, ZHANG J S. Interfacial bonding mechanism and mechanical properties of novel AZ31/WE43 bimetal composites fabricated by insert molding method [J]. Journal of Alloys and Compounds, 2017, 729: 344-353.
[19] ZHAO K N, LIU J C, NIE X Y, LI Y, LI H X, DU Q, ZHUANG L Z, ZHANG J S. Interface formation in magnesium–magnesium bimetal composites fabricated by insert molding method [J]. Materials & Design, 2016, 91: 122-131.
[20] LI Han-yan, CHEN Wen-ge, DONG Long-long, SHI Ying-ge, LIU Jie, FU Yong-qing. Interfacial bonding mechanism and annealing effect on Cu-Al joint produced by solid-liquid compound casting [J]. Journal of Materials Processing Technology, 2018, 252: 795-803.
[21] ZARE G R, DIVANDARI M, ARABI H. Investigation on interface of Al/Cu couples in compound casting [J]. Materials Science and Technology, 2013, 29(2): 190-196.
[22] HU Yuan, CHEN Yi-qing, LI Li, HU Huan-dong, ZHU Zi-ang. Microstructure and properties of Al/Cu bimetal in liquid–solid compound casting process [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(6): 1555-1563.
[23] JIANG Wen-ming, LI Guang-yu, FAN Zi-tian, WANG Long, LIU Fu-chu. Investigation on the interface characteristics of Al/Mg bimetallic castings processed by lost foam casting [J]. Metallurgical and Materials Transactions A, 2016, 47(5): 2462-2470.
[24] JIANG Wen-ming, FAN Zi-tian, LI Guang-yu, YANG Li, LIU Xin-wang. Effects of melt-to-solid insert volume ratio on the microstructures and mechanical properties of Al/Mg bimetallic castings produced by lost foam casting [J]. Metallurgical and Materials Transactions A, 2016, 47(12): 6487-6497.
[25] SELVAM P, VISWANATHAN B, SWAMY C S, SRINIVASAN V. Studies on the thermal characteristics of hydrides of Mg, Mg2Ni, Mg2Cu and Mg2Ni1-xMx (M=Fe, Co, Cu or Zn; 0 < x < 1) alloys [J]. International Journal of Hydrogen Energy, 1988, 13(2): 87-94.
[26] REILLY J J, WISWALL R H. Reaction of hydrogen with alloys of magnesium and copper [J]. Inorganic Chemistry, 1967, 6(12): 2220-2223.
[27] LEI J P, HUANG H, DONG X L, SUN J P, LU B, LEI M K, WANG Q, DONG C, CAO G Z. Formation and hydrogen storage properties of in situ prepared Mg-Cu alloy nanoparticles by arc discharge [J]. International Journal of Hydrogen Energy, 2009, 34(19): 8127-8134.
[28] SHAO Huai-yu, WANG Yun-tao, XU Hai-ruo, LI Xing-guo. Preparation and hydrogen storage properties of nanostructured Mg2Cu alloy [J]. Journal of Solid State Chemistry, 2005, 178(7): 2211-2217.
[29] ZHANG Jian, SHEN Qiang, LUO Guo-qiang, WANG Yi-yu, LI Mei-juan, ZHANG Lian-meng. Microstructural characterization of the Mg/Cu/Al diffusion bonded joint [J]. Journal of Physics: Conference Series, 2013, 419(1): 1-4.
[30] NOGUCHI T, HORIKAWA N, NAGATE H, NAKAMURA T, SATO K. Application of flow and solidification simulation in cast-in insertion processing [J]. International Journal of Cast Metals Research, 2013, 18(4): 214-220.
[31] HONG Q Z, D'HEURLE F M. The dominant diffusing species and initial phase formation in Al-Cu, Mg-Cu, and Mg-Ni systems [J]. Journal of Applied Physics, 1992, 72: 4036-4040.
[32] ARCOT B, MURARKA S P, CLEVENGER L A, HONG Q Z, ZIEGLER W, HARPER J M E. Intermetallic formation in copper/magnesium thin films—Kinetics, nucleation and growth, and effect of interfacial oxygen [J]. Journal of Applied Physics, 1994, 76(9): 5161-5170.
徐德兴1,杨长林2,赵康宁1,李宏祥1,张济山1
1. 北京科技大学 新金属材料国家重点实验室,北京 100083;
2. 西北工业大学 凝固技术国家重点实验室,西安 710072
摘 要:采用复合铸造法制备镁/铜复合材料,并对其显微组织演化、相组成和界面结合强度进行研究。研究发现,界面实现冶金结合并且由两个亚层组成:靠近纯铜一侧的亚层I,厚度为30 μm,主要由Mg2Cu相组成,并且在上面随机分布一些枝状相MgCu2;靠近纯镁一侧的亚层II,厚度为140 μm,主要由层状的Mg2Cu+(Mg)纳米共晶相和少量分离的Mg2Cu组成。复合材料的平均界面剪切强度为13 MPa。本研究为镁/铜复合材料作为储氢材料的应用提供了一个新的制备方法。
关键词:Mg/Cu复合材料;复合铸造;界面结合机制;界面组织;界面力学性能
(Edited by Wei-ping CHEN)
Foundation item: Project (51671017) supported by the National Natural Science Foundation of China; Project (FRF-GF-17-B3) supported by the Fundamental Research Funds for the Central Universities, China; Project supported by the Beijing Laboratory of Metallic Materials and Processing for Modern Transportation, China; Project (SKLSP201835) supported by the Fund of the State Key Laboratory of Solidification Processing in NWPU, China
Corresponding author: Hong-xiang LI; Tel: +86-10-62332350; E-mail: hxli@skl.ustb.edu.cn
DOI: 10.1016/S1003-6326(19)65030-2

