Trans. Nonferrous Met. Soc. China 22(2012) s688-s691
Microstructure of porous Cu fabricated by freeze-drying process of CuO/camphene slurry
Sung-tag OH1, Si-young CHANG2, Myung-jin SUK3
1. Department of Materials Science and Engineering, Seoul National University of Science and Technology, Seoul 139-743, Korea;
2. Department of Materials Engineering, Korea Aerospace University, Gyeonggi 412-791, Korea;
3. Department of Materials and Metallurgical Engineering, Kangwon National University, Samcheok 245-711, Korea
Received 21 May 2012; accepted 00 November 2012
Abstract: Porous Cu with macroscopically aligned channels was synthesized using a freeze-drying process. Camphene-based CuO slurry was prepared by milling at 60 °C with a small amount of dispersant. Freezing of a slurry was done at -25 °C while unidirectionally controlling the growth direction of the camphene. Pores were generated subsequently by sublimation of the camphene during drying. The green body was hydrogen-reduced at 300 °C for 30 min, and sintered in the furnace at 700 °C for 1 h under a hydrogen atmosphere. Microstructural observation reveals that all of the sintered samples are composed of only Cu phase and show macroscopic open pores with an average size of 100 μm which are aligned along its macroscopic growth direction. The internal wall of the macroscopic aligned pore shows relatively small pores due to the traces of the camphene left between the concentrated Cu particles on the internal wall. Increase in the porosity and pore size with increasing camphene content was explained by the change of the growth behavior of the camphene crystals.
Key words: porous Cu; CuO-camphene slurry; freeze-drying; hydrogen reduction; sintering
1 Introduction
Porous materials with interconnected pore channels are widely used for industrial applications such as filters, catalysis supports and preforms for metal-impregnated composites. Since high permeability and large surface are required for these applications, it is essential to control pore characteristics such as size, shape, orientation and porosity in these materials [1-3]. To fabricate porous materials with controlled pore channels, a number of fabrication techniques are developed [4,5]. One of the most common methods is phase separation followed by leaching [6]. For example, bulk glasses with interconnected pores have been fabricated by heat treatment for spinodal decomposition, followed by acid leaching of one phase. However, this technique is applicable only to a limited range of materials, and pore structure cannot be controlled.
Freeze-drying process has received increasing interest, as it can endow porous ceramics with well- defined pore structures [7,8]. In this method, slurries with lower solids contents are first frozen to obtain vehicle crystals, usually ice, and often connected with each other in dendritic shapes. Then, pore channels are produced by removing the frozen ice dendrites via freeze drying and controlled sintering. Till now, camphene has been adopted as the vehicle materials, because it can be frozen and easily sublimed near room temperature, offering more flexibility in the process [9-11].
However, the freeze-drying process has been applied only to the fabrication of porous ceramics due to the difficulty in the preparation of homogeneous slurry with metal particles. Thus, in order to fabricate porous metals with controlled pore characteristics, unique processing by using metal oxide instead of metal powder is proposed. The CuO powder is selected as the source material, which is hydrogen-reduced to metallic Cu in the sintering stage. Starting of camphene-based slurries with different contents of CuO powder produced by warm mixing, freeze-drying and sintering in hydrogen atmosphere, was used to obtain porous Cu. In this work, the dispersion stability of slurries and hydrogen reduction behavior were analyzed. Also, the dependence of the CuO content in the slurry on microstructure of porous Cu was described.
2 Experimental
Commercially available CuO powder (Kojundo Chemical Lab. Co., Japan) with an average size of 1 μm was used as the source of Cu, and camphene (C10H16, Sigma-Aldrich Co., USA) was used as the sublimable vehicle. To produce stable CuO suspensions in liquid camphene, we used for the dispersant an oligomeric polyester (Hypermer KD-4, UniQema, Belgium). CuO/ camphene slurries with various solid contents (10%, 17% and 20%, volume fraction) were prepared by ball milling at 60 °C. The warm slurries were then poured into Teflon mold at -25 °C to produce a disc 10 mm in diameter and 10 mm in thickness. After demolding, green bodies were placed in an ambient atmosphere with airflow to sublime the frozen camphene from the green bodies. The dried samples were heated up to 300oC in a hydrogen atmosphere and kept at this temperature for 30 min to induce the complete reduction of CuO to metallic Cu, followed by subsequent sintering at 700 °C for 1 h.
The dispersion stability of CuO/camphene slurries was estimated by Turbiscan (Formulaction, France). It measures any change in the sample by monitoring the transmission or backscattering of near-infrared light every 40 m along the sample cell [12]. The hydrogen-reduction behavior was analyzed by the measurement of the water vapor content in outlet gas with an in situ humidity measuring system [13]. Phase identification of the samples was determined by X-ray diffractometry (XRD, D/Max-IIIC, Rigaku Denki Co., Japan). Microstructure of the sintered bodies was observed with scanning electron microscopy (SEM, JSM-6700F, JEOL Co., Japan).
3 Results and discussion
One of the key issues involved in successfully achieving porous Cu with controlled pore characteristics is the parameter of homogeneous CuO/camphene slurry with dispersion stability. To analyze the effect of dispersant addition on the dispersion stability, the backscattering percent of slurries was measured by using Turbiscan, as shown in Fig. 1. In this experiment, the sample cell containing CuO powders suspended in an aqueous camphene solution was placed in the instrument and its sedimentation behavior is monitored every 3 min. The backscattering detector receives the light scattered by the sample from the incident beam where backscattering intensity versus the height in the sample at periodic times is obtained. As shown in Fig. 1(a), the backscattering percent of the CuO/camphene slurry is changed with increasing time, whereas that of the slurry with dispersant keeps constant value (Fig. 1(b)). Thus, it is suggested that the homogeneous CuO/camphene slurry with suitable stability can be achieved by the addition of dispersant.

Fig. 1 Backscattering intensity versus height in sample at different times for CuO/camphene slurry without (a) and with (b) dispersant
Humidity curve for the hydrogen reduction process of the CuO powder during heat-up to 600 °C with a heating rate of 10 °C/min are presented in Fig. 2. Sharp increase in humidity curve is observed at 240 °C. After this increase the curve decreased slowly to a temperature of about 300 °C and then remained at the same value. This peak resulted from the water vapor formation by reduction of CuO. Thus, the peak temperature appears to be a reduction temperature of CuO in the present system and coincides with the reported value [14].
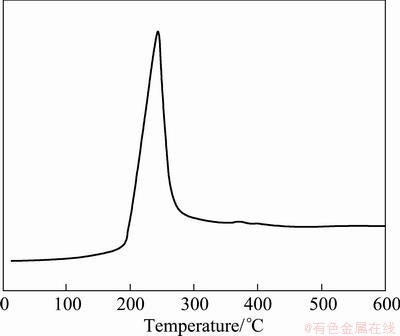
Fig. 2 Humidity curve for hydrogen reduction of CuO powder during heat-up to 600 °C with heating rate of 10 °C/min
Figure 3 shows the X-ray diffraction pattern of the initial and the reduced CuO powder. Before hydrogen reduction, the powder showed only peaks associated with the CuO phase (Fig. 3(a)), indicating that the camphene did not react with CuO powders. On the other hand, the CuO powder reduced at 300 °C for 30 min in hydrogen atmosphere was composed only of Cu phase without any reaction phases (Fig. 3(b)). This result agreed with that of hygrometry measurement in Fig. 2. On the basis of this result, the reduction schedule of CuO green body, prepared by freeze-drying process of CuO/camphene slurry, was established.

Fig. 3 XRD patterns of samples before (a) and after (b) reduction in hydrogen atmosphere for 30 min
Typical SEM images of the cross section parallel to the macroscopic camphene growth direction for the sample with 10% CuO, sintered at 700 °C, is shown in Fig. 4(a). It is observed that macroscopic open pores with an average size of 100 μm have uniformly formed over the entire sample. These pores are generated from sublimation of the camphene and were aligned along its macroscopic growth direction. Figure 4(b) shows an internal wall of the macroscopic aligned pore. It should be noted that the microstructure of the internal wall has relatively small pores of a few micron dimensions.

Fig. 4 SEM images of cross section (a) of porous Cu and magnified image (b) of internal wall of macroscopic aligned pore shown in Fig. 4(a)
It was reported in the solidification system of the liquid with foreign particles that solid particles are rejected by the solid-liquid interface and concentrated on the spaces between dendrite arms or neighboring dendrites [9,15,16]. Thus, it is reasonable that the formation of small pores occurs during freeze-drying due to the traces of the camphene left between the concentrated Cu particles on the internal wall.
As shown in Fig. 5, as the initial CuO content increased, the pores became smaller, as is often the case with the freeze drying method [10,17]. As the composite particulate concentration increased with solid loading and the content of camphene in the frozen body reduced, the pore structure became microporous, interconnected, and homogenous. In this case, more nucleation sites are available for the camphene crystals to grow and fewer camphene molecules sublimated, leading to a progressive reduction in porosity with loading, as shown in Fig. 5.

Fig. 5 Typical SEM images of porous Cu bodies produced using different CuO contents
4 Conclusions
1) Porous Cu bodies with a unique structure composed of complex macropores and micropores were fabricated by freezing CuO/camphene slurries and sublimating the camphene. The homogeneous slurries with suitable stability can be achieved by the addition of dispersant. After heat treatment in hydrogen atmosphere at 300oC for 30 min, the CuO powders were completely converted to metallic Cu without any reaction phase.
2) The sintered bodies showed macroscopic open pores with an average size of 100 μm, and internal wall of the macroscopic aligned pore had relatively small pores of a few micron dimensions.
3) As the CuO content increases, the pores becomes smaller. These results indicate that freeze-drying process using CuO powder as the source material of metallic Cu is a promising method of fabricating porous Cu with controlled pore characteristics.
Acknowledgment
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (grant number 2009-0089508).
References
[1] NAKAJIMA H. Fabrication, properties and application of porous metals with directional pores [J]. Progress in Materials Science, 2007, 52(7): 1091-1173.
[2] LIU P S, LIANG K M. Functional materials of porous metals made by P/M, electroplating and some other techniques [J]. Journal of Materials Science,2001, 36(21): 5059-5072.
[3] BANHART J. Manufacture, characterization and application of cellular metals and metal foams [J]. Progress in Materials Science, 2001, 46(6): 559-632.
[4] YANG D H, HUR B Y. YANG S R. Study on fabrication and foaming mechanism of Mg foam using CaCO3 as blowing agent [J]. Journal of Alloys and Compounds, 2008, 461(1-2): 221-227.
[5] SUK M J, KWON Y S. Manufacture of metal foams and porous metal, and their application [J]. Journal of Korean Powder Metallurgy Institute, 2001, 8(4): 215-222.
[6] NAKANISHI K, SOGA N. Phase separation in gelling silica-organic polymer solutions: systems containing poly (sodium styrenesulfonate) [J]. Journal of American Ceramic Society, 1991, 74(10): 2518-2530.
[7] FUKASAWA T, ANDO M, OHJI T, KANZAKI S. Synthesis of porous ceramics with complex pore structure by freeze-dry processing [J]. Journal of American Ceramic Society, 2001, 84(1): 230-232.
[8] FUKASAWA T, DENG Z-Y, ANDO M, OHJI T, KANZAKI S. Synthesis of porous silicon nitride with unidirectionally aligned channels using freeze-drying process [J]. Journal of American Ceramic Society, 2002, 85(9): 2151-2155.
[9] ARAKI K, HALLORAN J W. Porous ceramic bodies with interconnected pore channels by a novel freeze casting technique [J]. Journal of American Ceramic Society, 2005, 88(5): 1108-1114.
[10] YOON B H, KOH Y H, PARK C S, KIM H E. Generation of large pore channels for bone tissue engineering using camphene-based freeze casting [J]. Journal of American Ceramic Society, 2007, 90(6): 1744-1752.
[11] MALLICK K K. Freeze casting of porous bioactive glass and bioceramics [J]. Journal of American Ceramic Society, 2009, 92(S1): s85-s94.
[12] MENGUAL O, MEUNIER G,  I, PUECH K, SANBRE P. Turbiscan MA 2000: Multiple light scattering measurement for concentrated emulsion and suspension instability analysis [J]. Talanta, 1999, 50(2): 445-456.
I, PUECH K, SANBRE P. Turbiscan MA 2000: Multiple light scattering measurement for concentrated emulsion and suspension instability analysis [J]. Talanta, 1999, 50(2): 445-456.
[13] KIM D G, OH S T, JEON H, LEE C H, KIM Y D. Hydrogen-reduction behavior and microstructural characteristics of WO3-CuO powder mixtures with various milling time [J]. Journal of Alloys and Compounds, 2003, 354(1-2): 239-242.
[14] FIERRO G, LOJACONO M, INVERSI M, PORTA P, LAVECCHIA R, CIOCI F. A study of anomalous temperature-programmed reduction profiles of Cu2O, CuO and CuO-ZnO catalysts [J]. Journal of Catalysis, 1994, 148(2): 709-721.
[15] UHLMANN D R, CHALMERS B, JACKSON K A. Interaction between particles and a solid-liquid interface [J]. Journal of Applied Physics,1964, 35(10): 2986-2993.
[16] DEVILLE S, MAIRE E, BERNARD-GRANGER G, LASALLE A, BOGNER A, GAUTHIER C, LELOUP J, GUIZARD C. Metastable and unstable cellular solidification of colloidal suspensions [J]. Nature Materials, 2009, 8(12): 966-972.
[17] YOON B H, CHOI W Y, KIM H E, KIM J H, KOH Y H, Aligned porous alumina ceramics with high compressive strengths for bone tissue engineering [J]. Scripta Materialia, 2008, 58(7): 537-540.
(Edited by LONG Huai-zhong)
Corresponding author: Sung-tag OH; Tel: +82-2-9706631; E-mail: stoh@seoultech.ac.kr
DOI: 10.1016/S1003-6326(12)61787-7