DOI: 10.11817/j.ysxb.1004.0609.2020-35873
Mg-Nd/Ni储氢合金微观组织及吸放氢动力学
解立帅1, 2,徐 慢1,章晓波1, 2
(1. 南京工程学院 材料科学与工程学院,南京 211167;
2. 江苏省先进结构材料与应用技术重点实验室,南京 211167)
摘 要:采用熔剂保护法制备了不同Nd含量的Mg-Nd二元及Mg-Nd-Ni三元储氢合金,通过高能球磨对铸态合金进行组织细化,制备镁基纳米复合储氢颗粒,采用X射线衍射仪(XRD)、扫描电子显微镜(SEM)、透射电子显微镜(TEM)、自动Sievert设备(PCT)及差示扫描量热仪(DSC)等系统研究了Ni、Nd添加及活化过程中氢压对合金微观组织及吸放氢动力学的影响。结果表明:Ni、Nd含量会影响球磨后合金的颗粒尺寸,Mg12Nd与H反应生成的NdH3会稳定存在,NdH3明显促进Mg的吸氢,而Mg2Ni明显改善MgH2的放氢。活化过程中超高的氢压可明显细化第二相颗粒,在3 MPa氢压下活化后,合金中NdH3相的颗粒尺寸在50~200 nm之间,而在8 MPa氢压下活化后,NdH3的颗粒尺寸在10 nm左右。相较于在3 MPa氢压下活化的合金颗粒,在8 MPa氢压下活化后样品的吸放氢速率得到明显改善。
关键词:镁合金;储氢材料;高能球磨;吸放氢速率
文章编号:1004-0609(2020)-10-2340-10 中图分类号:TG139.7 文献标志码:A
镁作为储氢材料具有储氢量高(7.6%,质量分数)、价格便宜、可逆性好等显著优点而被认为是最有希望实现大规模储氢应用的材料,但MgH2在低温下 (<200 ℃)几乎不放氢,成为限制镁基储氢材料规模化应用的关键问题之一[1-3]。通过合金化、组织细化以及添加催化剂等方法在不同程度上改善了镁的吸放氢动力学,降低了其吸放氢温度[4-10]。
细化镁基储氢材料可显著改善其吸放氢动力学,细小的颗粒尺寸可以缩短氢原子的扩散距离,大量的晶界、相界可以作为吸放氢过程中新相的形核位点及氢原子的扩散通道,加速吸放氢过程。采用物理气相沉积制备的直径为30~50 nm的镁纳米线在300 ℃,30 min内吸氢量可达7.6%[11]。将MgH2与MgO[12]、SiC[13]等硬度较高的颗粒高能球磨后,MgH2的颗粒及晶粒尺寸均明显减小,MgH2的放氢温度甚至降至200 ℃以下,当Mg-Ni-Ce合金被细化至非晶态时,其储氢量会高于对应的晶体材料[14]。
在镁中添加金属单质、金属氢化物、碳材料等催化剂,通过促进氢的解离/重组、加速氢的扩散、削弱Mg—H键等作用,可有效改善镁基储氢合金的吸放氢热动力学[15-18]。HUANG等[19]在Mg-Al储氢合金中添加V、Y后,合金初始放氢温度分别下降了80、60 K,Ni与CeH2.73加入后可使Mg的放氢温度降低100 K,同时具有良好的循环稳定性[20]。稀土元素与氢具有极强的亲和力,生成的稀土氢化物(REHx)在镁基合金吸放氢过程中可稳定存在,催化镁的吸放氢过程,此外,与外部添加的催化剂相比,Mg-RE合金在吸放氢过 程中原位生成的催化相具有更好的分散性和更小的尺寸[21]。YONG等[22]通过制备Mg-Ce-Nd合金发现NdHx的形成可降低MgH2的形核能垒从而加速Mg的吸氢。ZHANG等[23]采用熔体快淬法制备Mg-Nd-Ni-Cu合金发现Nd的加入明显促进合金非晶化,同时显著改善合金的吸放氢动力学及循环效率。另据报道,NdHx/NdOx可一定程度改善镁基材料吸放氢的热力学性能及抗氧化性能[24]。Mg-Ni-Y三元合金经首次吸氢,可生成尺寸小于100 nm的YH2与Mg2Ni催化相,显著改善镁的吸放氢速率[25],但考虑到储氢量的要求,合金化元素的量应控制在合适范围内。
基于上述讨论,本研究以Nd、Ni为合金化元素,制备不同Nd含量的Mg-Nd二元及Mg-Nd-Ni三元合金,为保证高的储氢量,Mg的质量分数均在85%以上。采用高能球磨细化铸态合金,并在不同氢压下进行活化,系统研究了合金的微观组织演化及吸放氢动力学性能,通过控制活化过程中的氢压,制备出具有超细纳米结构的镁基复合储氢材料,从而显著提高了镁基储氢合金的吸放氢速率。
1 实验
1.1 样品制备
采用熔剂保护法制备了Mg-Nd二元及Mg-Nd-Ni三元合金,合金成分设计为Mg-5Nd、Mg-10Nd、Mg-15Nd和Mg-10Nd5Ni(质量分数,%)。实验用原材料为99.9% Mg锭、99.95% Nd锭和Mg-55%Ni中间合金,熔剂成分为55% KCl、28% CaCl2、15% BaCl2以及2% CaF2[26]。将按合金成分比例称好的镁锭和Mg-Ni中间合金置于石墨坩埚中,用熔剂把镁锭和Mg-Ni中间合金均匀覆盖,在温度设为900 ℃的坩埚电阻炉中熔化,待金属熔化后迅速加入称量好的钕块,充分搅拌。在整个熔炼过程中,间断加入熔剂以防止金属熔液的氧化与蒸发,待合金完全熔化后关闭电源,合金熔体随炉冷却至室温后取出,冷却时间约为24 h。采用电感耦合等离子体发射光谱(ICP)对所制备的四个合金进行了化学成分测试,测试结果为Mg-5.12Nd、Mg-10.47Nd、Mg-14.56Nd和Mg-9.59Nd5.13Ni(质量分数,%),说明本实验中采用熔剂保护法制备的合金成分控制较好。然后将铸态合金机械破碎并手工研磨成颗粒,为保证不同合金球磨实验的一致性,所有样品均采用筛网筛选尺寸在90 μm至300 μm的研磨颗粒作为高能球磨原始颗粒。采用高能球磨(Spex 8000M)细化合金颗粒,球磨时间为2 h,球料比为20:1,转速为875 r/min,采用氩气保护。
1.2 吸放氢性能测试
采用基于体积法原理设计的Sieverts型PCT Pro2000设备对高能球磨后的样品进行了活化及吸放氢性能测试,活化工艺为在350 ℃下进行三次循环吸放氢,初始吸氢氢压为3 MPa,放氢氢压为0.002 MPa,每次吸放氢时间均为1 h。此外,为了研究活化过程中氢压对催化相形成及合金吸放氢动力学的影响,球磨后的Mg-15Nd合金也在8 MPa氢压下进行了吸放氢活化,其他活化条件与上述相同。然后采用PCT对活化后合金的吸氢动力学进行了测试,吸氢温度为300 ℃,吸氢氢压为3 MPa,采用差示扫描量热仪(DSC,STA 449 F5)对活化后合金的放氢性能进行了测试,升温区间为50~450 ℃,升温速率为5 ℃/min,吹扫气及保护气均为氩气。
1.3 微观组织表征
采用扫描电子显微镜(SEM,配备能谱仪EDS)及透射电子显微镜(TEM, FEI Tecnai F30 G2)对铸态、球磨后及吸放氢测试后样品的微观组织进行了表征,并采用EDS分析铸态合金相组成,采用X射线衍射仪(XRD)对铸态合金及活化后颗粒的相组成进行了表征。
2 结果与讨论
2.1 合金微观组织
图1所示为4种不同成分铸态合金的SEM-BSE像,表1所示为图1中合金对应位置的EDS结果。由图1(a)、(c)和(e)可见,采用熔剂保护法制备的Mg-Nd二元合金均具有亚共晶组织,由初生枝晶Mg(黑色)及共晶组织(灰色)组成,随Nd含量增加,共晶组织所占比例也逐渐增加。图1(b)、(d)和(f)所示为二元合金共晶组织的微观形貌,由图可见,Mg与Mg12Nd交替排布,Mg-15Nd合金组织最为粗大,说明Nd含量对Mg-Nd二元合金微观组织有明显影响。图1(g)和(h)所示为Mg-10Nd5Ni三元合金SEM像,由图可见,合金由初生Mg、块状Mg12Nd(标记为B)及共晶组织组成,共晶组织由Mg、Mg12Nd和长条状Mg2Ni(标记为C)三相交替排布而成,且共晶组织的尺寸比Mg-Nd二元合金的尺寸要细,说明适当地添加Ni可有效细化Mg-Nd合金。为更清楚地观察三元合金的微观组织,采用透射电镜对铸态合金进行了组织观察,图2所示为Mg-10Nd5Ni三元合金共晶区域的TEM像及相应位置的选区衍射(SAED)图。由图2(a)可清楚地观察到长条状的Mg2Ni相分布于Mg和Mg12Nd相之间。
镁基储氢合金的颗粒尺寸会影响其吸/放氢性能,为定量表征球磨后合金的颗粒尺寸,首先通过扫描电子显微镜获得球磨颗粒在低倍下的分散形貌照片,然后采用Image Tool软件进行颗粒尺寸统计,每一合金均选择同一大小视场中的150个颗粒进行统计[27],得到颗粒尺寸分布直方图,结果如图3所示。对比统计结果可以发现,对于Mg-Nd二元合金,随着Nd含量的增加,球磨后合金平均颗粒尺寸逐渐减小,Mg-15Nd合金2 h球磨后的平均颗粒尺寸为30.1 μm,对于Mg-10Nd5Ni合金,其平均颗粒尺寸与Mg-15Nd合金接近,为29.7 μm,说明合金化元素的含量对球磨后颗粒尺寸影响较明显,而元素种类(Ni、Nd)对颗粒尺寸影响较小,通过合金化,首先在镁中形成Mg2Ni及Mg12Nd等相,并在合金内均匀分布,在球磨过程中,较硬的Mg2Ni与Mg12Nd均可帮助Mg颗粒的细化,同时有效减少颗粒之间的团聚,提高了球磨效率。此外,不同成分合金球磨后颗粒尺寸均集中分布于10~60 μm范围内。
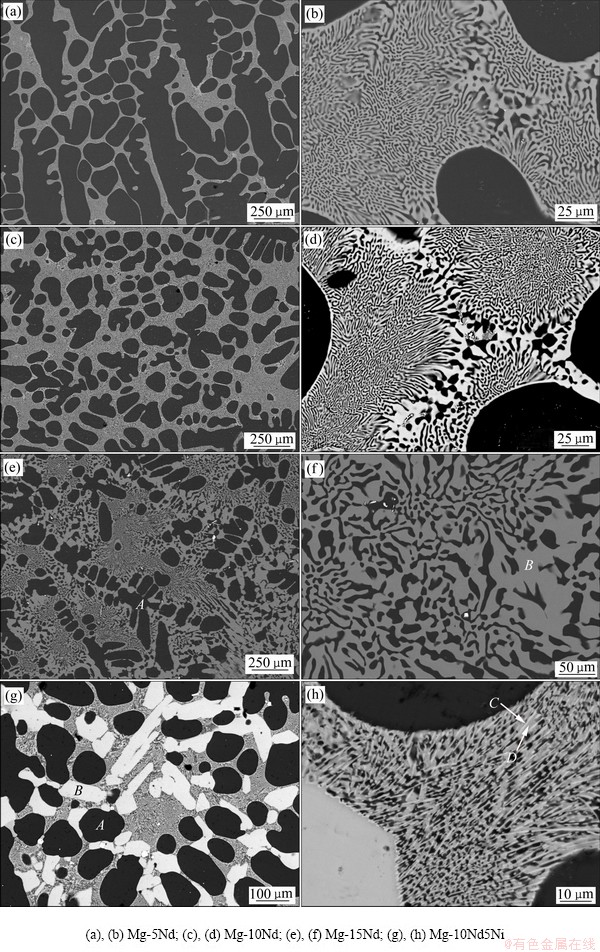
图1 铸态合金SEM像
Fig. 1 SEM/BSE images of as-cast alloys
表1 铸态合金EDS分析结果
Table 1 EDS results of as-cast alloys in Fig. 1
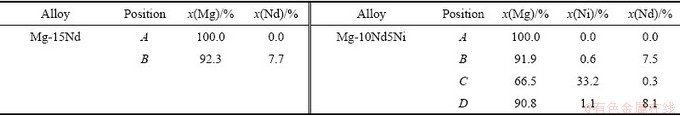
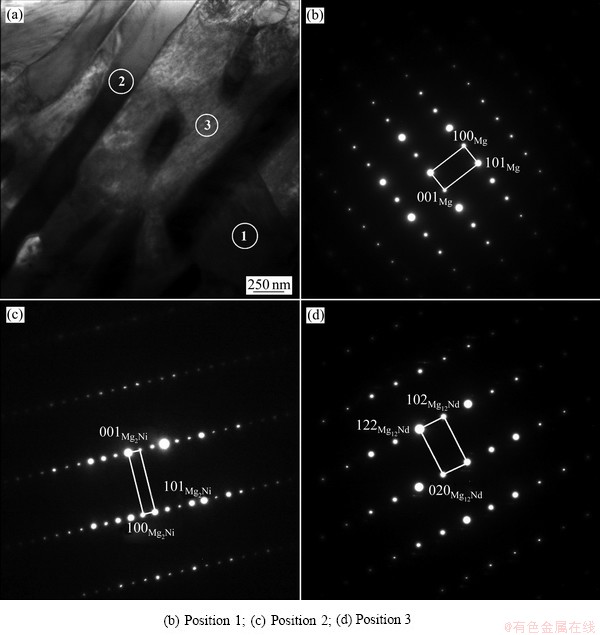
图2 Mg-10Nd5Ni合金TEM像和SAED谱
Fig. 2 TEM image of as-cast Mg-10Nd5Ni alloy (a) and corresponding SAED patterns ((b)-(d))
2.2 吸放氢性能
将高能球磨后的合金粉末在PCT设备上进行了三次吸放氢活化。图4(a)所示为铸态Mg-15Nd和Mg-10Nd5Ni合金的XRD谱。由图4(a)可见,Mg-15Nd合金由Mg和Mg12Nd两相组成,Mg-10Nd5Ni合金由Mg、Mg2Ni及Mg12Nd三相组成,与图1中SEM像一致。图4(b)所示为活化后Mg-15Nd和Mg-10Nd5Ni合金的XRD谱。由图4(b)可见,Mg-15Nd合金吸氢后由MgH2和NdH3相组成,Mg-10Nd5Ni合金吸氢后由MgH2、NdH3、Mg2NiH0.3和Mg2NiH4组成,Mg-10Nd5Ni合金放氢后由Mg、Mg2Ni和NdH3三相组成。由于Nd与H之间有较强的亲和力,在随后的吸放氢过程中NdH3会稳定存在[28],而Mg2NiH0.3和Mg2NiH4相会释放氢生成Mg2Ni[29],再次吸氢时Mg2Ni会与H反应,生成Mg2NiH0.3或Mg2NiH4。
图5所示为活化后的样品在300 ℃的吸放氢动力学曲线。由图5(a)可见,Mg-Nd二元合金随着Nd含量的增加,合金的吸氢速率和吸氢量均逐渐增加。Mg-15Nd合金的吸氢速率最快,在5 min之内可吸收5.1%的H,总吸氢量可达5.9%,而Mg-5Nd合金20 min吸氢量仅为4.7%。对于Mg-10Nd5Ni合金,其吸氢速率和吸氢量与Mg-10Nd合金接近,且均低于Mg-15Nd合金,说明Ni对合金吸氢性能改善作用不明显。这是由于吸氢时Mg2Ni相会首先固溶少量氢形成Mg2NiH0.3相,Mg2NiH0.3在后续吸氢时会短时存在,直到所有Mg转化为MgH2后才缓慢吸氢变为Mg2NiH4[29],而稀土元素Nd与H有较强的结合力,吸氢时会加速H2分解为H原子,NdH3不断地将产生的H原子转移到Mg/NdH3界面处,起到“氢泵”的作用[20, 30]。Mg-5Nd、Mg-10Nd、Mg-15Nd和Mg-10Nd5Ni合金在300 ℃、3 MPa初始氢压下,最终吸氢量分别为5.2%、5.3%、5.9%和5.4%。但Mg2NiH4可以明显促进MgH2的放氢,从图5(b)可见,Mg-10Nd5Ni合金的放氢速率最快,在1 min之内放氢量可达4.9%,而不同Nd添加量的Mg-Nd二元合金的放氢速率相差不大,且明显慢于Mg-10Nd5Ni三元合金的,说明NdH3对镁基储氢合金放氢速率的改善作用不明显。这是由于Mg2NiH4/ Mg2NiH0.3相放氢温度低,合金放氢时Mg2NiH4/ Mg2NiH0.3相首先释放氢生成Mg2Ni,在MgH2与Mg2NiH4/Mg2NiH0.3界面处造成晶格收缩,对MgH2产生拉应力[31],诱发Mg—H键断裂,从而显著加快基体MgH2的放氢。对比吸放氢曲线可以发现,在较低氢压下,所有合金在300 ℃基本可实现完全放氢。
图3 球磨后合金颗粒尺寸分布及SEM像
Fig. 3 Particle morphologies at low magnification and particle size distribution histograms of ball milled alloys
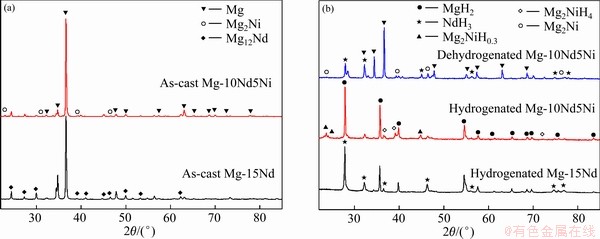
图4 铸态及活化后合金的XRD谱
Fig. 4 XRD patterns of as-cast(a) and as-activated(b) Mg-15Nd and Mg-10Nd5Ni alloys
图6(a)所示为在3 MPa氢压下活化Mg-15Nd合金放氢后样品的明场(BF)像。从图6(a)可以观察到大量不规则的NdH3第二相均匀地分布于基体Mg中,NdH3相的尺寸在50~200 nm之间。图6(b)所示为放氢后Mg-15Nd合金中NdH3的暗场(DF)像,清楚地展示了NdH3相的尺寸及分布。图6(c)所示为活化Mg-10Nd5Ni合金放氢后样品的BF像,与Mg-15Nd合金类似,大量不规则的第二相均匀分布于基体Mg中。图6(d)所示为放氢后Mg-10Nd5Ni合金中Mg2Ni相的暗场(DF)像。图6(d)中的SAED结果确定了放氢后样品中的Mg2Ni相,其在SAED谱中为明锐衍射环,说明Mg2Ni为纳米多晶相。由图6(d)可见,大量纳米Mg2Ni相均匀分布于基体内,Mg2Ni相的颗粒尺寸小于NdH3的。
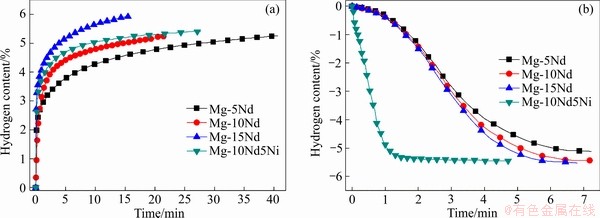
图5 活化合金等温吸放氢曲线
Fig. 5 Hydrogen absorption (a) and desorption (b) kinetics curves at 300 ℃ of as-activated alloys
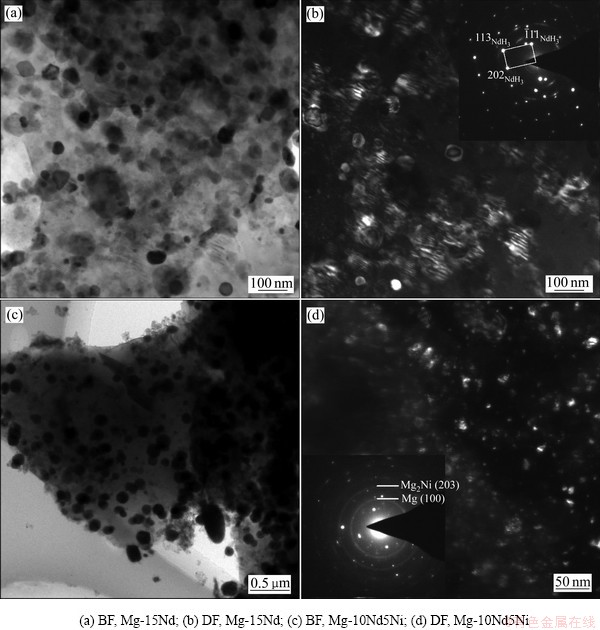
图6 活化合金放氢后样品的明场像、暗场像及相应SAED图
Fig. 6 BF and DF images of as-activated Mg-15Nd alloy after desorption and dehydrogenated Mg-10Nd5Ni alloy (Corresponding SAED patterns are also given)
2.3 高压活化细化合金
对镁基储氢材料而言,基体Mg和催化相越细小,晶界、相界越多,吸放氢过程中新相的形核位点就越多,H原子的扩散就越容易,相应地,吸放氢动力学也会得到明显改善[32]。镁基储氢材料在快速吸放氢之前都要在氢气氛围中进行高温活化,以破碎表面的氧化膜,为H原子的快速扩散提供通道。本实验以Mg-15Nd合金为研究对象,通过控制活化过程中的氢压,实现了超细第二相均匀分布于基体中。
图7(a)和(b)所示为球磨后Mg-15Nd合金分别在3 MPa和8 MPa氢压下活化后的SEM像,活化温度为350 ℃。从图7(a)和(b)可以发现,在较高的氢压下活化的Mg-15Nd合金,第二相尺寸明显变小;在8 MPa氢压下活化的样品,第二相颗粒尺寸甚至可以达到0.1 μm,而在3 MPa氢压下活化的样品,其第二相尺寸大于1 μm。图7(c)所示为在8 MPa氢压下活化后,Mg-15Nd合金样品中第二相NdH3的DF像,与图6(b)相比,NdH3明显细化,颗粒尺寸在10 nm左右。图7(d)所示为Mg-15Nd合金在8 MPa氢压下活化后的高分辨电子显微像(HRTEM),从图中可以清晰地观察到大量Mg和NdH3的纳米晶粒,晶粒尺寸为3~8 nm,说明镁基储氢合金在超高氢压下活化,可以获得超细的纳米晶粒。高能球磨后,Mg-15Nd合金由Mg和Mg12Nd两相组成,在首次吸氢时,Mg12Nd相会与气态氢发生不可逆的歧化反应[33-34],生成MgH2和NdH3;在后续吸放氢过程中NdH3会稳定存在,催化吸放氢过程,其尺寸及分布对合金吸放氢动力学有重要影响。Mg12Nd与H的反应属于固态相变,MgH2和NdH3的形成为形核和长大过程;系统中氢压越大,反应驱动力越大,致使颗粒内众多质点同时达到氢化物形核所需的临界氢浓度从而大量形核,首次吸氢时MgH2和NdH3的形核率明显增加;由于氢化物核心多、间距小,大量晶核未来得及充分长大便相互碰撞,形成大量超细纳米晶。

图7 在3 MPa和8 MPa氢压下活化后合金颗粒的SEM像、NdH3相的DF像以及HRTEM像
Fig. 7 SEM images of Mg-15Nd alloy activated under hydrogen pressures of 3 MPa (a) and 8 MPa (b), and DF(c) and HRTEM(d) images of NdH3 phase of Mg-15Nd alloy activated under 8 MPa hydrogen pressure
图8(a)所示为在不同氢压下活化后Mg-15Nd合金在300 ℃、3 MPa氢压下的吸氢动力学曲线。由图8(a)可以看出,在8 MPa氢压下活化的样品,其吸氢速率明显快于在3 MPa氢压下活化的样品。图8(b)所示为在不同氢压下活化后样品的放氢DSC曲线。由图8(b)可见,在8 MPa氢压下活化的样品,其开始放氢温度和放氢峰值温度均低于在3 MPa氢压下活化的样品,说明在较高氢压下活化可以细化合金中的NdH3催化相,从而显著改善合金吸放氢动力学。
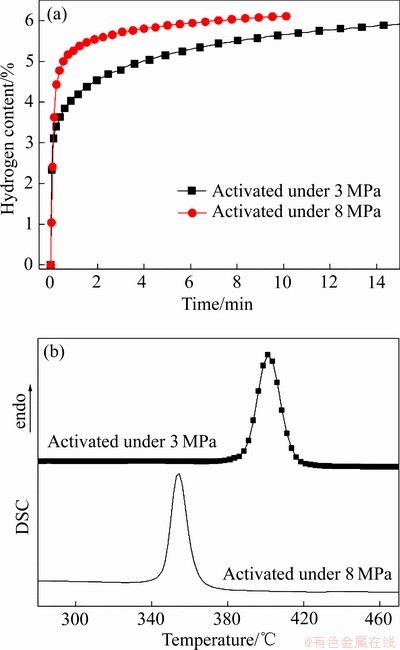
图8 不同氢压下活化样品的等温吸氢及放氢DSC曲线
Fig. 8 Absorption kinetics curves at 300℃ (a) and desorption DSC curves (b) of Mg-15Nd alloy activated under hydrogen pressures of 3 MPa and 8 MPa
3 结论
1) 采用熔剂保护法成功制备了Mg-Nd二元及Mg-Nd-Ni三元储氢合金,少量Ni、Nd元素添加可细化合金共晶组织。合金元素含量对高能球磨后颗粒尺寸有一定影响,随Ni、Nd含量增加,球磨后合金颗粒尺寸逐渐减小。
2) 合金活化后会生成Mg2Ni及NdH3第二相并均匀分布于基体中,催化吸/放氢过程。NdH3对镁基储氢合金吸氢速率改善较明显,而Mg2Ni显著催化镁基储氢合金的放氢过程。
3) 增加活化过程中氢压可改善合金微观组织,在超高氢压下活化,镁基储氢合金颗粒明显细化,获得超细纳米晶,在8 MPa氢压下活化的Mg-Nd合金颗粒,NdH3晶粒尺寸可达3~8 nm,吸/放氢动力学均得到明显改善。
REFERENCES
[1] OUYANG L Z, LIU F, WANG H, LIU J W, YANG X S, SUN L X, ZHU M. Magnesium-based hydrogen storage compounds: A review[J]. Journal of Alloys and Compounds, 2020, 832: 154865.
[2] YARTYS V A, LOTOTSKYY M V, AKIBA E, ALBERT R, ANTONOV V E, ARES J R, BARICCO M. Magnesium based materials for hydrogen based energy storage: Past, present and future[J]. International Journal of Hydrogen Energy, 2019, 44: 7809-7859.
[3] 黄显吞, 覃昌生, 卿培林, 谢政专, 李柳杰. 添加Si对Mg-Li合金储氢性能的影响[J]. 中国有色金属学报, 2017, 27(10): 2072-2078.
HUANG Xian-tun, QIN Chang-sheng, QING Pei-lin, XIE Zheng-zhuan, LI Liu-jie. Effect of Si addition on hydrogen storage performance of Mg-Li alloy[J]. The Chinese Journal of Nonferrous Metals, 2017, 25(10): 2072-2078.
[4] SONG W J, DONG H P, ZHANG G, LIU J, YANG G, LIU Y H. Enhanced hydrogen absorption kinetics by introducing fine eutectic and long-period stacking ordered structure in ternary eutectic Mg-Ni-Y alloy[J]. Journal of Alloys and Compounds, 2020, 820: 153187.
[5] CERMARK J, KRAL L, ROUPCOVA P. Significantly decreased stability of MgH2 in the Mg-In-C alloy system: Long-period-stacking-ordering as a new way how to improve performance of hydrogen storage alloys[J]. Renewable Energy, 2020, 150: 204-212.
[6] TIAN M, SHANG C. Mg-based composites for enhanced hydrogen storage performance[J]. International Journal of Hydrogen Energy, 2019, 44: 338-344.
[7] ZHANG X L, LIU Y F, ZHANG X, HU J J, GAO M X, PAN H G. Empowering hydrogen storage performance of MgH2 by nanoengineering and nanocatalysis[J]. Materials Today Nano, 2020, 9: 100064.
[8] PATELLI N, MIGLIORI A, MORANDI V, PASQUINI L. Interfaces within biphasic nanoparticles give a boost to magnesium-based hydrogen storage[J]. Nano Energy, 2020, 72: 104654.
[9] HU M, XIE X, CHEN M, ZHU C, LIU T. TiCX-decorated Mg nanoparticles confined in carbon shell: Preparation and catalytic mechanism for hydrogen storage[J]. Journal of Alloys and Compounds, 2020, 817: 152813.
[10] 张 健, 朱 璞, 毛 聪, 周惦武. 碳材料掺杂对镁基氢化物释氢性能的影响及其微观机理[J]. 中国有色金属学报, 2015, 25(9): 2464-2470.
ZHANG Jian, ZHU Pu, MAO Cong, ZHOU Dian-wu. Influence and micro-mechanism of carbon materials doping on dehydrogenation properties of magnesium based hydride[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(9): 2464-2470.
[11] LI W, LI C, MA H, CHEN J. Magnesium nanowires: Enhanced kinetics for hydrogen absorption and desorption[J]. Journal of the American Chemical Society, 2007, 129: 6710-6711.
[12] AGUEY K F, FERNANDEZ J R, KLASSEN T, BORMANN R. Using MgO to improve the (de)hydriding properties of magnesium[J]. Materials Research Bulletin, 2006, 41: 1118-1126.
[13] RANJBAR A, GUO Z P, YU X B, ATTARD D, CALKA A, LIU H K. Effects of SiC nanoparticles with and without Ni on the hydrogen storage properties of MgH2[J]. International Journal of Hydrogen Energy, 2009, 34: 7263-7268.
[14] LIN H J, HE M, PAN S P, GU L, LI H W, WANG H. Towards easily tunable hydrogen storage via a hydrogen- induced glass-to-glass transition in Mg-based metallic glasses[J]. Acta Materialia, 2016, 120: 68-74.
[15] ZHOU C, FANG Z Z, SUN P. An experimental survey of additives for improving dehydrogenation properties of magnesium hydride[J]. Journal of Power Sources, 2015, 278: 38-42.
[16] LIANG H, CHEN D, CHEN M, LI W, SNYDERS R. Study of the synthesis of PMMA-Mg nanocomposite for hydrogen storage application[J]. International Journal of Hydrogen Energy, 2020, 45: 4743-4753.
[17] WEBB C J. A review of catalyst-enhanced magnesium hydride as a hydrogen storage material[J]. Journal of Physics and Chemistry of Solids, 2015, 84: 96-106.
[18] 张 琦, 李双寿, 董占民, 汤 彬, 李 睿, 林万明. Mg65Ni10RE25 (RE=La, Ce, Pr, Nd, Mm) 非晶储氢合金电极的性能[J]. 中国有色金属学报, 2015, 25(6): 1538-1545.
ZHANG Qi, LI Shuang-shou, DONG Zhan-min, TANG Bin, LI Rui, LIN Wan-ming. Properties of amorphous Mg65Ni10RE25 (RE=La, Ce, Pr, Nd, Mm) hydrogen storage alloys electrode[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(6): 1538-1545.
[19] 黄显吞, 卿培林, 覃昌生, 谢政专. 过渡金属V、Ti及Y的添加对Mg-Al合金储氢性能的影响[J]. 中国有色金属学报, 2020, 30(2): 333-340.
HUANG Xian-tun, QING Pei-lin, QIN Chang-sheng, XIE Zheng-zhuan. Influences of transition elements (V, Ti, Y) on hydrogen storage property of Mg-Al alloy[J]. The Chinese Journal of Nonferrous Metals, 2020, 30(2): 333-340.
[20] OUYANG L Z, YANG X S, ZHU M, LIU J W, DONG H W, SUN D L, ZOU J. Enhanced hydrogen storage kinetics and stability by synergistic effects of in situ formed CeH2.73 and Ni in CeH2.73-MgH2-Ni nanocomposites[J]. The Journal of Physical Chemistry C, 2014, 118: 7808-7820.
[21] YONG H, GUO S, YUAN Z, QI Y, ZHAO D, ZHANG Y. Improved hydrogen storage kinetics and thermodynamics of RE-Mg-based alloy by co-doping Ce-Y[J]. International Journal of Hydrogen Energy, 2019, 44: 16765-16776.
[22] YONG H, GUO S, YUAN Z, QI Y, ZHAO D, ZHANG Y. Phase transformation, thermodynamics and kinetics property of Mg90Ce5RE5 (RE=La, Ce, Nd) hydrogen storage alloys[J]. Journal of Materials Science & Technology, 2020, 51: 84-93.
[23] ZHANG Y, WANG H, ZHAI T, YANG T, QI Y, ZHAO D. Hydrogen storage characteristics of the nanocrystalline and amorphous Mg-Nd-Ni-Cu-based alloys prepared by melt spinning[J]. International Journal of Hydrogen Energy, 2014, 39: 3790-3798.
[24] ZOU J X, ZENG X Q, YING Y J, CHEN X, GUO H, ZHOU S, DING W J. Study on the hydrogen storage properties of core-shell structured Mg-RE (RE=Nd, Gd, Er) nano- composites synthesized through arc plasma method[J]. International Journal of Hydrogen Energy, 2013, 38: 2337-2346.
[25] LI Y, GU Q, LI Q, ZHANG T. In-situ synchrotron X-ray diffraction investigation on hydrogen-induced decomposition of long period stacking ordered structure in Mg-Ni-Y system[J]. Scripta Materialia, 2017, 127: 102-107.
[26] XIE L S, LI J S, ZHANG T B, SONG L, KOU H C. Microstructure and hydrogen storage properties of Mg-Ni-Ce alloys with a long-period stacking ordered phase[J]. Journal of Power Sources, 2017, 338: 91-102.
[27] HOU X J, HU R, ZHANG T B, KOU H C, LI J S. Hydrogenation thermodynamics of melt-spun magnesium rich Mg-Ni nanocrystalline alloys with the addition of multiwalled carbon nanotubes and TiF3[J]. Journal of Power Sources, 2016, 306: 437-447.
[28] CHEN L, HU C, LIU F. Microstructure and hydrogen storage kinetics of Mg89RE11 (RE=Pr, Nd, Sm) binary alloys[J]. RSC Advances, 2019, 9: 4445-4452.
[29] DENYS R V, POLETAEV A A, MAEHLEN J P, SOLBERG J K, TARASOV B P, YARTYS V A. Nanostructured rapidly solidified LaMg11Ni alloy II. In situ synchrotron X-ray diffraction studies of hydrogen absorption–desorption behaviours[J]. International Journal of Hydrogen Energy, 2012, 37: 5710-5722.
[30] LIN H J, TANG J J, YU Q, WANG H, OUYANG L Z, ZHAO Y J. Symbiotic CeH2.73/CeO2 catalyst: A novel hydrogen pump[J]. Nano Energy, 2014, 9: 80-87.
[31] OUYANG L Z, YE S Y, DONG H W, ZHU M. Effect of interfacial free energy on hydriding reaction of Mg-Ni thin films[J]. Applied Physics Letters, 2007, 90: 021917.
[32] SADHASIVAM T, KIM H T, JUNG S, ROH S H, PARK J H, JUNG H Y. Dimensional effects of nanostructured Mg/MgH2 for hydrogen storage applications: A review[J]. Renewable and Sustainable Energy Reviews, 2017, 72: 523-534.
[33] POLETAEV A A, DENYS R V, MAEHLEN J P, SOLBERG J K, TARASOV B P, YARTYS V A. Nanostructured rapidly solidified LaMg11Ni alloy: Microstructure, crystal structure and hydrogenation properties[J]. International Journal of Hydrogen Energy, 2012, 37: 3548-3557.
[34] LI Z Y, LI S L, YUAN Z M, ZHANG Y H, QI Y. Microstructure, hydrogen storage thermodynamics and kinetics of La5Mg95–xNi (x=5, 10, 15) alloys[J]. Transactions of Nonferrous Metals Society of China, 2019, 29(5): 1057-1066.
Microstructure and de-/absorption kinetics of Mg-Nd/Ni alloys
XIE Li-shuai1, 2, XU Man1, ZHANG Xiao-bo1, 2
(1. School of Material Science and Engineering, Nanjing Institute of Technology, Nanjing 211167, China;
2. Jiangsu Key Laboratory of Advanced Structural Materials and Application Technology, Nanjing Institute of Technology, Nanjing 211167, China)
Abstract: Mg-Nd binary and Mg-Nd-Ni ternary alloys with different Nd contents were prepared in graphite crucible under the protection of covering agent. High energy ball-milling was performed to obtain Mg-based nanocomposite hydrogen storage materials. The phase components, microstructure and hydrogen storage properties were systematically investigated by XRD, SEM, TEM, PCT and DSC. The results show that the contents of Ni and Nd influence the particle size of ball milled samples. Mg2Ni significantly favors desorption process, while NdH3 is more conducive to absorption. Ultrahigh hydrogen pressure during activation process can refine the microstructure of Mg alloys. The particle size of NdH3 is in the range of 50-200 nm for Mg-15Nd alloy activated under 3 MPa hydrogen pressure, while it is about 10 nm for Mg-15Nd alloy activated under 8 MPa hydrogen pressure. The sample activated under 8 MPa hydrogen pressure shows superior absorption and desorption kinetics than that activated under 3 MPa hydrogen pressure.
Key words: Mg alloys; hydrogen storage materials; high-energy ball milling; absorption and desorption kinetics
Foundation item: Project(BK20191020) supported by the Natural Science Foundation of Jiangsu Province, China; Project(YKJ201804) supported by the Scientific Research Foundation of Nanjing Institute of Technology, China
Received date: 2019-12-05; Accepted date: 2020-06-19
Corresponding author: ZHANG Xiao-bo; Tel: +86-15951722675; E-mail: xbxbzhang2003@163.com
(编辑 何学锋)
基金项目:江苏省自然科学基金资助项目(BK20191020);南京工程学院科研基金资助项目(YKJ201804)
收稿日期:2019-12-05;修订日期:2020-06-19
通信作者:章晓波,教授,博士;电话:15951722675;E-mail:xbxbzhang2003@163.com