DOI:10.19476/j.ysxb.1004.0609.2017.10.21
离子膜电解法分离氯化浸出液中宏量铜镍
何 洋1,唐忠阳1,刘旭恒1,陈星宇1, 2
(1. 中南大学 冶金与环境学院,长沙 410083;
2. 中南大学 稀有金属冶金与材料制备湖南省重点实验室,长沙 410083)
摘 要:硫化镍矿中常伴有铜,在浸出时镍和铜往往同步进入浸出液,但由于铜镍化学性质相近而难以分离。针对这一难题,采用阴离子膜电解,通过控制槽电压,分离溶液中的铜镍,考察极距、电解液温度、槽电压、铜镍浓度等因素对铜镍分离效果的影响。结果表明:当溶液中Cu2+ 25 g/L、Ni2+ 37.5 g/L时,在极距4 cm、电解液温度40 ℃、槽电压0.53 V、电解10 h的条件下,阴极液中残余总铜浓度可降到0.24 g/L,ΡNi/Cu比为155.7,铜镍分离效果良好。
关键词:阴离子膜电解;铜镍分离;硫化镍矿;浸出液
文章编号:1004-0609(2017)-10-2128-08 中图分类号:TF803.27 文献标志码:A
镍是国民经济、社会发展、国防工业建设必不可少的基础材料和重要物资,因其具有耐腐蚀性和可塑性好、导磁性佳等优良特性,广泛应用于冶金、化工、建筑航空航天、军工制造等行业[1-4]。我国甘肃金昌素有“镍都”之称,铜镍硫化矿储量丰富,为世界在采的第三大铜镍硫化物矿床,保有储量约占全国的61.9%,是我国镍及其制品的重要产地[5-6]。传统的硫化镍矿冶炼工艺为“磨浮分离—硫化镍阳极电解”工艺,该工艺成熟可靠,生产稳定,但存在着金属直收率低、贵金属损失大、能耗高、“三废”问题突出等显著缺点。因此,国内外广大研究者开发了一系列湿法工艺以期解决这些问题,其中以氯化浸出工艺最为突出,近年来在国内外发展也很快,如国外MUKHERJEE[7]用氯化铜浸出镍铜硫化矿,镍的浸出率达96.8%,铜的浸出率达99.7%;张凤君等[8]、李金丽等[9]用三氯化铁浸出高冰镍,镍、钴的浸出率均大于90%,铜的浸出率达98%。这一过程实现了高冰镍中镍和铜的同步浸出。然而,由于化学性质相近,溶液中的铜镍难以分离。传统的镍湿法冶金过程中,铜镍分离的方法主要有硫化沉淀法、溶剂萃取法、离子交换法、置换法、电沉积法等,如卢建波等[10]、李江涛等[11]分别以非晶态硫化镍、硫化锰为沉淀剂从镍电解液中除铜;戴宽等[12]以AMPY-1为萃取剂从镍电解液中除铜;温俊杰等[13]用硅胶-聚合胺树脂从镍电解液中除铜;王春花等[14]、王瑞忠等[15]分别用铁粉和镍粉从铜镍混合溶液中置换除铜;SO等[16]、KUDELSKI等[17]、READ等[18]、曾振欧等[19]均曾以电积法从溶液中除铜。虽然以上方法均能实现镍电解液中铜镍的分离,但这些方法主要用于将镍电解液中的少量铜进行分离,其中的Cu2+浓度通常在0.4~0.8 g/L[19],对于同时含有宏量镍和铜的溶液,如何实现二者的有效分离成为了一个新的难题。
铜、镍离子的标准还原电位差较大[20],达到0.59 V,可利用这一性质来实现溶液中宏量铜、镍的分离。由于铜的还原电位比镍的还原电位更正,通过控制体系的电势,可以使溶液中的大部分铜优先沉积出来,而镍仍留在溶液中,得到含少量铜的镍溶液,如此一来,则可用传统的方法来进行铜镍分离以满足镍电解的要求。因此,本文作者针对含有大量镍和铜的溶液中的铜镍分离进行了探索,在此基础上开展了系统的实验研究,以期为溶液中宏量铜镍的分离开发出一条新的途径。
1 实验
1.1 阴极极化曲线测试
用普林斯顿电化学工作站(型号:VersaSTAT4)进行测试,采用三电极体系,工作电极和辅助电极均为铂电极,尺寸分别为1 cm×1 cm、4.5 cm×5.5 cm,参比电极为饱和甘汞电极;工作电极和辅助电极使用前用稀硝酸浸泡5 min,将表面抛光后用去离子水冲洗干净;电解液分别为CuCl2溶液和NiCl2溶液,其组成如表1所示;扫描范围为CuCl2溶液:-0.5~0.5 V(vs SCE),NiCl2溶液:-0.8~0.5 V(vs SCE);由正向负扫描,扫描速率1 mV/s。
表1 电解液的组分含量
Table 1 Components of electrolytes
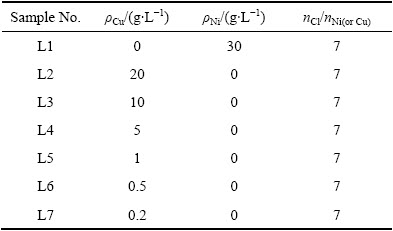
1.2 阴离子膜电解实验原料
实验采用自制聚四氟乙烯电解槽,阳阴极均采用铜作为电极(规格14.5 cm×14.5 cm),电极使用前处理方法与1.1节中相同,阴离子膜为国产均相阴离子膜,电解所需待分溶液为CuCl2和NiCl2混合溶液(Ni、Cu质量比为1.5,Cl、Cu摩尔比约为7,pH=0),支持电解质溶液为NaCl+HCl混合溶液(NaCl摩尔浓度2.5 mol/L,pH=0)。
1.3 阴极液中镍铜比的计算
电解结束后阴极液中镍铜比为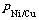 ,计算公式如式(1)所示:
,计算公式如式(1)所示:
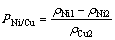 (1)
(1)
式中: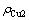 表示电解结束时阴极液中的铜离子浓度;
表示电解结束时阴极液中的铜离子浓度;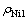 表示阴极液中镍离子的初始浓度;
表示阴极液中镍离子的初始浓度; 表示电解结束时扩散进入阳极室的镍离子的浓度。
表示电解结束时扩散进入阳极室的镍离子的浓度。
2 结果与讨论
2.1 阴极极化测试研究
CuCl2溶液和NiCl2溶液的循环伏安曲线如图1和2所示。
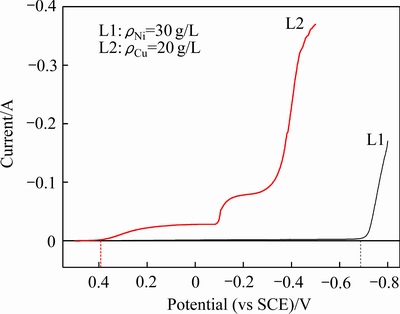
图1 NiCl2和CuCl2溶液阴极极化曲线
Fig. 1 Cathodic polarization curves of NiCl2 solution and CuCl2 solution

图2 不同Cu2+浓度阴极极化曲线
Fig. 2 Cathodic polarization curves at different mass concentrations of Cu2+
由图1可知,当镍溶液中Ni2+为30 g/L时,在约-0.69 V(vs SCE)时Ni2+开始被还原;当铜溶液中Cu2+浓度为20 g/L时,相同扫描速度下,在约0.39 V(vs SCE)处Cu2+即开始被还原,实验过程中能明显观察到电极表面有铜的沉积。Cu2+和Ni2+之间的还原电位差非常显著,达到将近1.1 V。由图2可以看出,随着溶液中Cu2+浓度的不断降低,其还原电位逐渐负移,但即便溶液中Cu2+浓度降低至0.2 g/L时,铜溶液中Cu2+的还原电位仍约0.19V(vs SCE),远大于Ni2+的还原电位(-0.69 V(vs SCE))。以上结果表明,无论在宏量镍、宏量铜还是在宏量镍、微量铜的情况下,完全可以通过电位的控制来实现溶液中铜镍的有效分离,这为后续的研究奠定了理论基础。
2.2 离子膜电解工艺
在实际的通过控制电位法来实现铜镍分离工艺过程设计中,不仅需要考虑Cu2+、Ni2+ 离子阴极析出电位的差别,阳极选择对工艺也至关重要。一方面,阳极的选择对整个电解分离体系的槽电压具有重大影响,阴阳极电极反应电位差越大,工艺的槽电压也越大,能耗则越高;另一方面,不同阳极的选择往往对应于不同的阳极反应,而阳极反应的类型对电解体系的稳定性具有决定性作用。如阳极为析氧反应时,溶液体系酸度会升高;阳极为析氯反应时,不仅使电解液酸度增加,还容易恶化操作环节和设备防腐难度。而且,镀覆在阴极的铜也还需要专门的工序脱镀。
在粗铜的电解精炼过程中,整个过程中的槽电压仅为0.2~0.3 V,溶液酸度基本保持不变。因此,能否利用铜电解精炼的操作方式来从镍溶液中除铜呢?需要注意的是,在铜的电解精炼过程中,电解液中的铜虽然不断在阴极沉积,但是同时阳极中的铜也在不断溶解进入到电解液中。因此,实现铜镍分离的关键在于如何避免阳极溶解的Cu2+进入到含镍溶液中。
基于这一思考,设想采用阴离子交换膜将电解槽分隔成阴极室和阳极室,电解过程中,阴极液中的Cu2+在阴极不断沉积,铜阳极逐渐溶解进入阳极液,由于阴离子交换膜阻止阳离子通过,两极室中的阳离子无法交换。据此设想,设计了如图3所示的铜镍分离的离子膜电解装置:阴阳极采用铜作为电极,阴阳极室用阴离子交换膜隔开;将含有大量镍和铜的溶液加入到阴极室中,阳极室中加入不含镍的支持电解质溶液;在电场作用下,阴极液中的Cu2+逐渐沉积得到阴极铜,阳极室中的铜阳极逐渐溶解进入到阳极液中;由于阴离子交换膜阻止阳离子的穿透,阴极室中的阴离子则可透过交换膜到达阳极室中,保持体系电荷的平衡; 电解一周期后,更换新的阴极液和阳极液,并将阴阳极板对调,继续进行电解,过程与上述相同。如此循环,可不断将阴极镍溶液中的铜提取去除而富集在阳极液中,实现阴极液中宏量镍铜的分离。
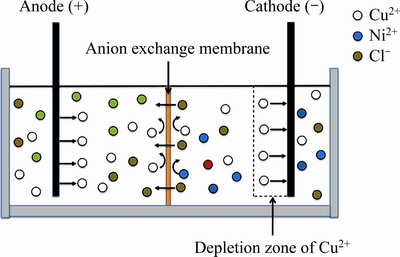
图3 电解槽结构及原理
Fig. 3 Electrolytic cell structure and electrolysis principle
2.2.1 极距的影响
极距(d)对铜镍分离效果及Ni2+渗透速率的影响如图4和5所示;电解结束后,阴极液中的ΡNi/Cu比见表2(其中ρCu1为阴极液中初始铜浓度,下同)。
表2 电解结束后阴极液中镍铜比
Table 2 ΡNi/Cu in catholyte after electrolysis
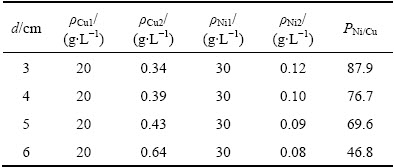

图4 极距对铜镍分离效果的影响
Fig. 4 Effect of polar distance on separation of copper from nickel
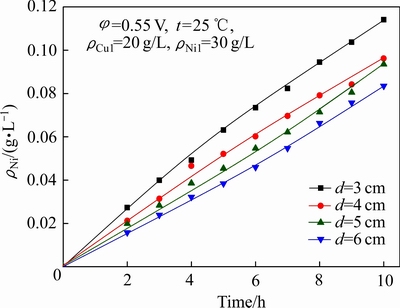
图5 极距对Ni2+渗透速率的影响
Fig. 5 Effect of polar distance on permeation rate of Ni2+
由图4、图5及表2可以看出,随着极距的减小,阴极室中Cu2+的电沉积速率和Ni2+的渗透速率均有所上升,当极距由6 cm减小至3 cm时,10 h后阴极液中残余铜(包括Cu2+和Cu+,下同)浓度由0.64 g/L降低至0.34 g/L,ΡNi/Cu比由46.8增大至87.9,而阳极液中Ni2+浓度由0.08 g/L升至0.12 g/L。缩小极距有利于溶液电阻的降低,从而有效改善阴极液中的铜镍分离效果,但同时由于极距缩小,体系电流密度增大,驱动力增强,使得阴极液中Ni2+的渗透速率加快,使其透过阴离子交换膜进入阳极室造成阴极液中Ni2+的损失。因此,在合理控制阴极液中Ni 2+渗透损失的前提下,减小极距有利于提高反应速率,改善铜镍分离效果。
2.2.2 电解液温度的影响
电解液温度对铜镍分离效果及Ni2+渗透速率的影响如图6和7所示;电解结束后,阴极液中ΡNi/Cu见表3。
表3 电解结束后阴极液中的镍铜比
Table 3 ΡNi/Cu in catholyte after electrolysis


图6 电解液温度对铜镍分离效果的影响
Fig. 6 Effect of electrolyte temperature on separation of copper from nickel

图7 电解液温度对Ni2+渗透速率的影响
Fig. 7 Effect of electrolyte temperature on permeation rate of Ni2+
由图6、图7和表3可以看出,随着电解液温度的升高,阴极室中Cu2+的电沉积速率和Ni2+的渗透速率均有所上升,这是由于升高电解液温度有利于离子扩散,使得浓差极化现象减弱,从而推动溶液中的铜在阴极上沉积;当电解液温度由25 ℃升高至40 ℃时,10 h后阴极液中残余铜浓度由0.39 g/L降低至0.21 g/L,ΡNi/Cu比由76.7增大至142.2,进一步将温度提高到45 ℃时,其效果与40 ℃时相差不大。另外,由能斯特方程[21](式2)可知:电解液温度升高,使得Cu2+还原电位正移,有利于提高铜镍分离的深度。然而,升高电解液温度会使阴极液中Ni2+的渗透速率加快,使其透过阴离子交换膜进入阳极室造成阴极液中Ni2+的损失。因此,本研究选择温度为40 ℃。
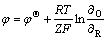 (2)
(2)
式中: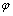 为平衡电压;
为平衡电压; 为标准电势;R为摩尔气体常数,8.3143 J/(K·mol);T为热力学温度,K;Z为电极反应中得失的电子数;F为法拉第常数;
为标准电势;R为摩尔气体常数,8.3143 J/(K·mol);T为热力学温度,K;Z为电极反应中得失的电子数;F为法拉第常数;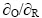 为氧化态和还原态的比值。
为氧化态和还原态的比值。
2.2.3 槽电压的影响
槽电压对铜镍分离效果及Ni2+渗透速率的影响如图8、9所示。电解结束后,阴极液中ΡNi/Cu见表4。
结合图8、图9和表4可以看出,随着槽电压的升高,阴极室中Cu2+的电沉积速率和Ni2+的渗透速率均有所上升,当槽电压由0.48 V升高至0.58 V时, 10 h后阴极液中残余铜浓度由3.30 g/L降低至0.21 g/L,ΡNi/Cu比由9.1增大至142.2,而阳极液中Ni2+浓度由0.11 g/L升至0.14 g/L。提高槽电压有利于铜在阴极上的沉积,提高阴极液中铜镍分离的深度。但增大槽电压会导致阴极液中Ni2+的渗透速率加快,使其透过阴离子交换膜进入阳极室造成阴极液中Ni2+的损失,同时槽电压过高还可能导致镍在阴极上沉积。因此,为了实现阴极液中铜镍分离的同时又减少阴极液中Ni2+的损失,本实验选择槽电压为0.53 V。
表4 电解结束后阴极液中的镍铜比
Table 4 ΡNi/Cu in catholyte after electrolysis
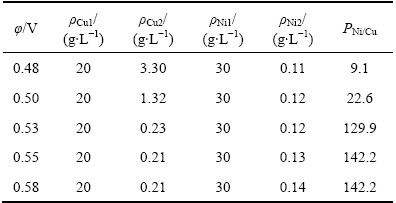
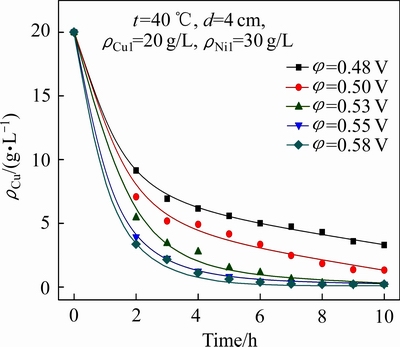
图8 槽电压对铜镍分离效果的影响
Fig. 8 Effect of cell voltage on separation of copper from nickel
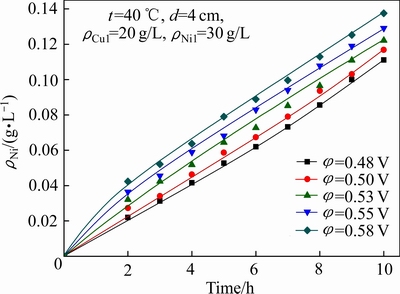
图9 槽电压对Ni2+渗透速率的影响
Fig. 9 Effect of cell voltage on permeation rate of Ni2+
表5 电解结后阴极液中的镍铜比
Table 5 ΡNi/Cu in catholyte after electrolysis
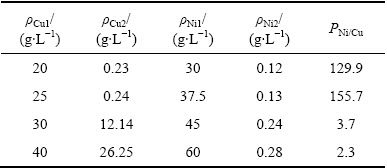

图10 起始Cu2+浓度对铜镍分离效果的影响
Fig. 10 Effect of initial Cu2+ concentration on separation of copper from nickel
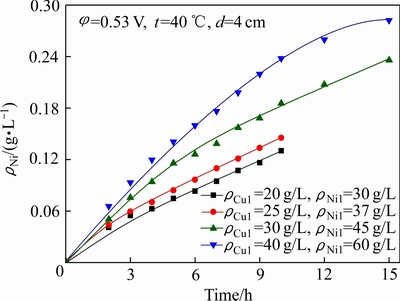
图11 起始Ni2+浓度对Ni2+渗透速率的影响
Fig. 11 Effect of initial Ni2+ concentration on permeation rate of Ni2+
2.2.4 初始铜镍浓度的影响
初始铜镍浓度对铜镍分离效果及Ni2+渗透速率的影响如图10和11所示;电解结束后,阴极液中ΡNi/Cu见表5。结合图10、图11和表5可以看出,当ρCu1为20和25 g/L时,铜电沉积速率较快,电解结束后,阴极液中残余铜浓度相差不大,分别为0.23和0.24 g/L,ΡNi/Cu分别为129.9和155.7,铜镍分离效果较好;当ρCu1为30和40 g/L时,铜电沉积速率较慢,经过15 h电解,阴极液中残留铜浓度仍然有12.14和26.26 g/L,ΡNi/Cu比分别只有3.7和2.3,分离效果较差,这主要是由于蚀刻反应所造成[24]。当阴极液中Cu2+浓度较高时,会与沉积在阴极上的铜发生如式(3)所示的蚀刻反应:
Cu2++Cu+2Cl-=2CuCl (3)
相当于用溶液中的Cu2+作为氧化剂浸出了已经沉积在阴极表面的铜。又由于本研究中所配置的阴极液中n(Cl)/n(Cu)始终保持在7左右,其中的Cl-大大过量,而过量的Cl-与CuCl络合生成可溶性的[CuCl3]2-,使得沉积在阴极上的铜发生反溶又进入到阴极液中,导致了铜镍分离效果变差。因此,在本研究固定了n(Cl)/n(Cu)的前提下,为了实现较好的铜镍分离效果,阴极液中铜离子浓度需要控制在25 g/L以下为宜。
3 结论
1) 针对硫化镍矿浸出液中铜镍浓度高、难以分离的难题,提出了采用阴离子交换膜电解法进行宏量铜镍分离,阴极极化曲线结果表明,无论在宏量镍、宏量铜还是宏量镍、微量铜的情况下,Ni2+和Cu2+均存在较大的还原电位差,通过电位调控可以实现溶液中铜镍的有效分离。
2) 进行了铜镍分离的工艺研究,当溶液中Cu2+ 25 g/L、Ni2+ 37.5 g/L时,在极距4 cm、电解液温度40 ℃、槽电压0.53 V的优化条件下,电解10 h,阴极液中铜离子浓度降低至0.24 g/L,完全满足现行的硫化沉淀法除铜对溶液中铜浓度的要求。
REFERENCES
[1] 张 亮, 杨卉芃, 冯安生, 谭秀民. 全球镍矿资源开发利用现状及供需分析[J]. 矿产保护与利用, 2016(1): 64-69.
ZHANG Liang, YANG Hui-peng, FENG An-sheng, TAN Xiu-min. Study on current situation and analysis of supply and demand of global nickel resource[J]. Conservation and Utilization of Mineral Resources, 2016(1): 64-69.
[2] 路长远, 鲁雄刚, 邹星礼, 程红伟, 许 茜. 中国镍矿资源现状及技术进展[J]. 自然杂志, 2015, 37(4): 269-277.
LU Chang-yuan, LU Xiong-gang, ZOU Xing-li, CHENG Hong-li, XU Qian. Current situation and utilization technology of nickel ore in China[J]. Chinese Journal of Nature, 2015, 37(4): 269-277.
[3] 曾祥婷, 许 虹, 田 尤, 刘陟娜, 李梅梅. 中国镍资源产业现状及可持续发展策略[J]. 资源与产业, 2015, 17(4): 94-99.
ZENG Xiang-ting, XU Hong, TIAN You, LIU Zhi-na, LI Mei-mei. Situation and sustainable development strategy of China’s nickel resources industry[J]. Resources and Industries, 2015, 17(4): 94-99.
[4] REN Xiu-lian, WEI Qi-feng, LIU Zhe, LIU Jun. Electrodeposition conditions of metallic nickel in electrolytic membrane reactor[J]. Transactions of Nonferrous Metals Society of China, 2012, 22(2): 467-475.
[5] 杨志强, 王永前, 高 谦, 武拴军. 中国镍资源开发现状与可持续发展策略及其关键技术[J]. 矿产保护与利用, 2016(2): 58-69.
YANG Zhi-qiang, WANG Yong-qian, GAO Qian, WU Shuan-jun. Present situation and development strategy and key technologies of China’s nickel resources sustainable development[J]. Conservation and Utilization of Mineral Resources, 2016(2): 58-69.
[6] 曾认宇, 赖健清, 毛先成, 赵 莹, 刘 嫔, 朱佳玮, 岳 斌, 艾启兴. 金川铜镍硫化物矿床铂族元素地球化学差异及其演化意义[J]. 中国有色金属学报, 2016, 26(1): 149-163.
ZENG Ren-yu, LAI Jian-qing, MAO Xian-cheng, ZHAO Ying, LIU Pin, ZHU Jia-wei, YUE Bin, AI Qi-xing. Distinction of platinum group elements geochemistry in Jinchuan Cu-Ni sulfide deposit and its implication for magmatic evolution[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(2): 149-163.
[7] MUKHERJEE T K. copper-nickel sulfide concentrate leached by CuCl2-Oxygen[J]. Hydrometallurgy, 1986(15): 25-32.
[8] 张凤君, 马玖彤, 李滦宁, 王玉洁. 硫化矿中铜镍的浸取研 究[J]. 长春科技大学学报, 1998, 28(2): 231-234.
ZHANG Feng-jun, MA Jiu-tong, LI Luan-ning, WANG Yu-jie. Study on leaching technology of Cu and Ni from brassil[J]. Journal o f Changchun University of Science and Technology, 1998, 28(2): 231-234.
[9] 李金丽, 张明杰, 王洪宽. 三氯化铁浸出高冰镍[J]. 东北大学学报(自然科学版), 1998, 19(2): 152-154.
LI Jin-li, ZHANG Ming-jie, WANG Hong-kuan. Nis matte leached by ferric chloride[J]. Journal of Northeastern University (Natural Science), 1998, 19(2):152-154.
[10] 卢建波, 王得祥, 李亦婧, 赵 重, 郑军福, 陈自江, 郭 勇. 基于铜离子活化的非晶态硫化镍净化除铜研究[J]. 现代化工, 2014, 34(12): 104-107.
LU Jian-bo, WANG De-xiang, LI Yi-jing, ZHAO Chong, ZHENG Jun-fu, CHEN Zi-jiang, GUO Yong. Removal of copper by amorphous nickel sulfide based on copper ion activation[J]. Modern Chemical Industry, 2014, 34(12): 104-107.
[11] LI Jiang-tao, CHEN Ai-liang. Deep removal of copper from nickel electrolyte using manganese sulfide[J]. Transactions of Nonferrous Metals Society of China, 2015, 25(11): 3802-3807.
[12] 戴 宽, 刘云清, 胡慧萍, 程泽英, 刘士军, 陈启元. AMPY-1用于镍电解阳极液净化除铜的研究[J]. 有色金属(冶炼部分), 2015(10): 10-13.
DAI Kuan, LIU Yun-qing, HU Hui-ping, CHENG Ze-ying, LIU Shi-jun, CHEN Qi-yuan. Removal of copper from nickel anode electrolyte by AMPY-1[J]. Nonferrous Metals (Extractive Metallurgy), 2015(10): 10-13.
[13] WEN Jun-jie, ZHANG Qi-xiu, ZHANG Gui-qing, ZHANG Li-ping. Copper completely removal from artificial nickel sulfate electrolyte by using silica-polyamine resin[J]. Nonferrous Metals, 2009, 61(1): 50-55.
[14] 王春花, 曾佳娜, 林瑞玲. 电镀污泥中铜和镍的回收[J]. 化工环保, 2013, 33(6): 531-535.
WANG Chun-hua, ZENG Jia-na, LIN Rui-ling. Recovery of nickel and copper from electroplating sludge[J]. Environmental Protection of Chemical Industry, 2013, 33(6): 531-535.
[15] 王瑞忠, 王中祥, 尹继良. 在硝酸镍溶液中用“置换法”除铜工艺研究[J]. 电源技术, 2008, 32(10): 699-700.
WANG Rui-zhong, WANG Zhong-xiang, YIN Ji-liang. Technology study of removing copper in the nitric nickel solution by interchange method[J]. Chinese Journal of Power Sources, 2008, 32(10): 699-700.
[16] SO W W, CHOE S, CHUANG R, LEE C C. Effective diffusion barrier metallization process on copper[J]. Thin Solid Films, 2000, 376(1/2): 164-169.
[17] KUDELSKI A, JANIK-CZACHOR M, BUKOWSKA J, DOLATA M, SZUMMER A. Surface-enhanced Raman scattering (SERS) on copper electrodeposited under nonequilibrium conditions[J]. Journal of Molecular Structure, 1999, 482: 245-248.
[18] READ D T, CHENG Y W, GEISS R. Morphology, microstructure, and mechanical properties of a copper electrodeposit[J]. Microelectronic Engineering, 2004, 75(1): 63-70.
[19] 曾振欧, 曾颖如, 吴鸿儒. 金川镍电解阳极液净化除铜的电沉积法研究[J]. 湖南冶金, 1994(4): 11-14.
ZENG Zhen-ou, ZENG Ying-ru, WU Hong-ru. Study of removing copper from nickel anode electrolyte by electrodeposition method[J]. Hunan Metallurgy, 1994(4): 11-14.
[20] 吴维昌. 标准电极电位数据手册[M]. 北京: 科学出版社, 1991: 80, 154.
WU Wei-chang. Standard electrode potential datasheet[M]. Beijing: Science Press, 1991: 80, 154.
[21] BARD A J, FAULKNER L R. Electrochemical methods: fundamentals and applications[M]. Austin: Department of Chemistry and Biochemistry University of Texas, 2001: 52.
[22] 杨 星, 杨声海, 郭 欢, 陈永明, 刘 青. 从蚀刻废液中隔膜电沉积金属铜板新工艺研究[J]. 有色金属(冶炼部分), 2011(9): 40-43.
YANG Xing, YANG Sheng-hai, GUO Huan, CHEN Yong-ming, LIU Qing. Study on electrowinning sheet-form copper from acidic etchant waste in diaphragm cell[J]. Nonferrous Metals (Extractive Metallurgy), 2011(9): 40-43.
[23] 陈双扣, 郭莉萍, 朱建芳, 李 丹, 王 月. 酸性氯化铜蚀刻液原理及影响因素分析[J]. 科技信息, 2006(10S): 8.
CHEN Shuang-kou, GUO Li-ping, ZHU Jian-fang. Brief principles to acid chlorination copper etching solution and factors analysis[J]. Science Information, 2006(10S): 8.
[24] 魏 静, 徐金来, 吴成宝. 印刷线路板动态蚀刻研究[J]. 电镀与涂饰, 2009, 28(7): 28-30.
WEI Jing, XU Jin-lai, WU Cheng-bao. Study on dynamic etching of printed circuit board[J]. Electropalting and Finishing, 2009, 28(7): 28-30.
Separation of macro amounts copper from nickel in chloride leaching solution by anion membrane electrolysis
HE Yang1, TANG Zhong-yang1, LIU Xu-heng1, CHEN Xing-yu1, 2
(1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Hunan Key Laboratory for Metallurgy and Material Processing of Rare Metals, Central South University, Changsha 410083, China)
Abstract: Nickel sulfide ore always accompanies by a large amount of copper, and copper can be often leached simultaneously into the nickel leaching solution. However, because of their similar chemical properties, it is difficult to separate. Here, anion exchange membrane electrolysis was provided to separate copper from solution by controlling the cell voltage. The effects of polar distance, electrolyte temperature, cell voltage and concentrations of copper and nickel on separation of copper-nickel were studied and the optimal operation conditions were obtained. The results show that, for solution containing 25 g/L Cu2+ and 37.5 g/L Ni2+,the total residual Cu concentration in catholyte is depressed to 0.24 g/L and the ratio of ΡNi/Cu reaches 155.7 under the optimal conditions of polar distance 4 cm, electrolyte temperature 40 ℃, cell voltage 0.53 V, electrolysis time 10 h. A satisfied effect of separation of copper from nickel is obtained.
Key words: anion membrane electrolysis; separation of copper from nickel; nickel sulfide ore; leaching solution
Foundation item: Project(2014CB643405) supported by the National Basic Research Development Program of China
Received date: 2016-10-12; Accepted date: 2017-03-27
Corresponding author: LIU Xu-heng; Tel: +86-731-88830476; E-mail: liuxuheng@csu.edu.cn
(编辑 何学锋)
基金项目:国家重点基础研究发展计划资助项目(2014CB643405)
收稿日期:2016-10-12;修订日期:2017-03-27
通信作者:刘旭恒,副教授,博士;电话:0731-88830476;E-mail: liuxuheng@csu.edu.cn