Transformation of β to α phase of isotactic polypropylene nucleated with nano styrene butadiene rubber-based β-nucleating agent under microwave irradiation
来源期刊:中南大学学报(英文版)2018年第12期
论文作者:Nawadon PETCHWATTANA Phisut NAKNAEN Jakkid SANETUNTIKUL
文章页码:3098 - 3106
Key words:polypropylene; crystallization; mechanical properties; thermal properties
Abstract: This paper investigates the effect of microwave irradiation on the β to α phase transformation of the β-nucleated isotactic polypropylene (iPP). Ten microwave irradiation cycles was applied to the iPP and iPP modified with 0.3 wt% and 0.5 wt% β-NA, and the data at 2nd, 4th, 6th, 8th and 10th irradiation were reported. As expected, the sample temperature was found to increase with the irradiation time, by more than 130 °C, due to high frequency of microwave processing. This was the major factor that induced the β-phase transformation and structural change. Both the differential scanning calorimetry (DSC) and X-ray diffraction (XRD) results indicated that β-phase was mainly transformed to α-phase and partially converted to the amorphous section. It was reflected as 1) the reduction of the enthalpy of β-crystal melting (DHmβ), 2) the increased enthalpy of α-crystal melting (DHmα), 3) the decreased β-crystalline phase fraction (Kβ) and 4) the decrease of the overall degree of crystallinity (Xall). Under impact force, neat iPP showed a slight increase in the impact strength with the irradiation time, due to the increase of amorphous region. For the β-iPP, it decreased due to the reduction of the β-phase content.
Cite this article as: Nawadon PETCHWATTANA, Phisut NAKNAEN, Jakkid SANETUNTIKUL. Transformation of β to α phase of isotactic polypropylene nucleated with nano styrene butadiene rubber-based β-nucleating agent under microwave irradiation [J]. Journal of Central South University, 2018, 25(12): 3098–3106. DOI: https://doi.org/10.1007/ s11771-018-3977-3.

J. Cent. South Univ. (2018) 25: 3098-3106
DOI: https://doi.org/10.1007/s11771-018-3977-3
Nawadon PETCHWATTANA1, Phisut NAKNAEN2, Jakkid SANETUNTIKUL3
1. Division of Polymer Materials Technology, Faculty of Agricultural Product Innovation and Technology, Srinakharinwirot University, Ongkharak, Nakhon Nayok 26120, Thailand;
2. Division of Food Science and Nutrition, Faculty of Agricultural Product Innovation and Technology, Srinakharinwirot University, Ongkharak, Nakhon Nayok 26120, Thailand;
3. Faculty of Engineering and Technology, King Mongkut's University of Technology North Bangkok, Bankhai, Rayong 21120, Thailand
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Abstract: This paper investigates the effect of microwave irradiation on the β to α phase transformation of the β-nucleated isotactic polypropylene (iPP). Ten microwave irradiation cycles was applied to the iPP and iPP modified with 0.3 wt% and 0.5 wt% β-NA, and the data at 2nd, 4th, 6th, 8th and 10th irradiation were reported. As expected, the sample temperature was found to increase with the irradiation time, by more than 130 °C, due to high frequency of microwave processing. This was the major factor that induced the β-phase transformation and structural change. Both the differential scanning calorimetry (DSC) and X-ray diffraction (XRD) results indicated that β-phase was mainly transformed to α-phase and partially converted to the amorphous section. It was reflected as 1) the reduction of the enthalpy of β-crystal melting (DHmβ), 2) the increased enthalpy of α-crystal melting (DHmα), 3) the decreased β-crystalline phase fraction (Kβ) and 4) the decrease of the overall degree of crystallinity (Xall). Under impact force, neat iPP showed a slight increase in the impact strength with the irradiation time, due to the increase of amorphous region. For the β-iPP, it decreased due to the reduction of the β-phase content.
Key words: polypropylene; crystallization; mechanical properties; thermal properties
Cite this article as: Nawadon PETCHWATTANA, Phisut NAKNAEN, Jakkid SANETUNTIKUL. Transformation of β to α phase of isotactic polypropylene nucleated with nano styrene butadiene rubber-based β-nucleating agent under microwave irradiation [J]. Journal of Central South University, 2018, 25(12): 3098–3106. DOI: https://doi.org/10.1007/ s11771-018-3977-3.
1 Introduction
Isotactic polypropylene (iPP) is the commodity plastic which has been applied as a food packaging or food utensil for many decades [1]. It is widely known as a dielectric material or electrical insulator. This kind of material has high ability to absorb microwaves and store energy [2, 3]. Most of iPP utensils are employed to store the high moisture and oil contents of foods. They were reported to absorb energy from the microwaves in a process called dielectric heating [4]. These plausibly increase the temperature and change some properties of foods as well as their container. BURDICK et al [5] reported that the poultry samples placed in the microwave oven reached a maximum temperature of 186 °C. This temperature is higher than that of the crystallization temperature (Tc) and the melting temperature (Tm) of iPP, which possibly transforms β to new α phase (α′) in iPP and affects both thermal and mechanical properties of the iPP packages.
iPP crystal is classified into four different forms such as monoclinic (α), trigonal (β), orthorhombic (g) and smectic [6, 7]. In accordance with the dissimilarities of the molecular structures, the different crystal forms of iPP display different mechanical performances. Excellent tensile modulus and tensile strength but poor toughness normally observes in the α-iPP. The β-iPP is easier to allow the plastic deformation, which enhanced the toughness and impact resistance [6, 7]. However, the β-iPP has a lower stability than the α-crystal form, especially under heating or under deformation processes [6]. The β-form can be obtained under a temperature gradient, under shearing or under a specific β-nucleating agent (β-NA) [6–8]. Among these methods, adding β-NA into iPP is the most appropriate and the easiest method for preparing the iPP with high β-phase fraction. Our previous research indicated that adding 0.1 wt% to 0.5 wt% β-NA remarkable increased the β-crystal content in iPP with the β-crystalline phase fraction (Kβ) around 0.7 to 0.85. Under reheating by the extrusion process, the transformation of β to α was clearly detected [6]. LI et al [9] found a transformation from β to α-phase during the tensile deformation. Higher transformation was found when the larger local strain was applied to the test specimen. This is in agreement with other works which β to α-phase transformation occurred under uniaxial compression [10], UV irradiation [11] and tension [12, 13].
The current research intended to extend the observation on the crystalline transformation from β to α-phase of the β-NA nucleated iPP under microwave irradiation. Ten irradiation times was applied to the iPP and iPP modified with 0.3 wt% and 0.5 wt% β-NA, and the data at 2nd, 4th, 6th, 8th and 10th irradiation were collected and reported. The mechanical, thermal and morphological properties were observed in order to explain the phenomenon occurred under microwave heating.
2 Experimental works
2.1 Materials and processing
An injection grade of iPP homopolymer (PP1100 NK, IRPC Public Company Limited, Thailand) was selected as a polymer matrix. Its melting temperature and the melt flow rate at 190 °C were 165 °C and 11 g/(10 min) respectively. Neat iPP was collected suddenly after polymerization process without further modification. It was then nucleated with a selective styrene butadiene based β-NA (Narpow VP101T, SINOPEC Beijing Research Institute of Chemical Industry, China). The selected β-NA composed of the styrene butadiene rubber, sodium salt and sodium benzoate, and its average particle size was 55 nm. β-NA contents of 0.3 wt% and 0.5 wt% were compounded and fabricated, by using a twin screw extruder and injection molding machine. To simulate the real customer use and observe the influence of the microwave irradiation on the properties and the β to α transformation, the test was conducted by placing the iPP and β-iPP in a 800 W microwave oven (Electrolux, EMM2398X) for 3 min in each test, for ten cycle times with the interval time of 3 min.
VP101T, SINOPEC Beijing Research Institute of Chemical Industry, China). The selected β-NA composed of the styrene butadiene rubber, sodium salt and sodium benzoate, and its average particle size was 55 nm. β-NA contents of 0.3 wt% and 0.5 wt% were compounded and fabricated, by using a twin screw extruder and injection molding machine. To simulate the real customer use and observe the influence of the microwave irradiation on the properties and the β to α transformation, the test was conducted by placing the iPP and β-iPP in a 800 W microwave oven (Electrolux, EMM2398X) for 3 min in each test, for ten cycle times with the interval time of 3 min.
2.2 Testing and characterizations
To study the thermal transition temperature and the transformation of β-iPP before and after microwave treat, a differential scanning calorimeter (DSC) (Perkin Elmer, DSC6000) was employed to determine the glass transition temperature (Tg), crystallization temperature (Tc), melting temperature (Tm) and area under Tm peak (DHm) of iPP and iPP/β-NA samples. The test condition was set following the procedure described in our previous work [6]. DHm was estimated by Eqs. (1) to (3) [14–18].
 (1)
(1)
 (2)
(2)
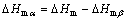 (3)
(3)
where △Hm is the enthalpy of melting obtained from whole melting endotherm. △Hmβ and DHmα are the enthalpies of melting under β and α curves respectively. △Hmβ* was calculated by using the calibration method as defined in Figure 1 and △Hmβ was calculated by multiplying with a calibration factor A (Eq. (2)). h1 and h2 are the heights from the baseline to the β-melting peak and minimum point respectively [14–18].
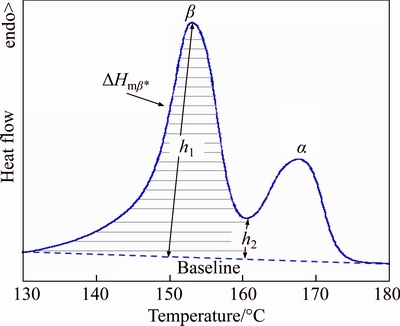
Figure 1 Identification of parameters used to calculate △Hmβ from DSC curve
An X-ray diffractometer (XRD) (Bruker AXS, D8 Advance) was used to confirm the crystal structure and the β to α phase transformation of the irradiated iPP and iPP/β-NA. The continuous scanning angle range applied in this study was run from 5° to 35° at 40 kV and 30 mA. The crystal structures of iPP were determined by using XRD profile, the principal reflections of the α-crystal are (110), (040) and (130), while the major reflection of the β-crystal is (300) [6, 19]. The β-crystalline phase fraction (Kβ), the overall degree of crystallinity (Xall), the degree of β-crystallinity (Xβ) and degree of α-crystallinity (Xα) were calculated by Eqs. (4) to (7) [6].
 (4)
(4)
where α(110), α(040) and α(130) are the integral intensities of the (110), (040) and (130) reflections of the α-PP respectively. β(300) is the integral intensity of (300) reflections of β-iPP.
 (5)
(5)
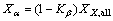 (6)
(6)
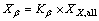 (7)
(7)
where Kβ is the relative amount of the β-crystal phase with respect to the α-crystal phase. Aamorphous represents the area of the amorphous peak. The Xall, Xβ and Xα are the overall degree of crystallinity, degree of β-crystallinity and degree of α-crystallinity respectively [6].
The time required to obtain full crystallinity (tc) was determined by the DSC isothermal test. The test samples were heated from room temperature to 190 °C at a heating rate 10 °C/min, held at 190 °C for 5 min. Then, they were cooled down to 120 °C at a cooling rate 10 °C/min and held isothermally until crystallization was completed. The notched Izod impact strength was evaluated in accord with ASTM D 256. The test was run by using an impact tester (Yasuda, 258PC). The average values from ten replicated samples were calculated and reported.
3 Results and discussion
3.1 Thermal and crystallization behaviors
Table 1 shows the heat gain during microwave irradiation in each cycle. As expected, the elevated temperatures were found at all sample and irradiation cycles. After the 8th irradiation, the specimen temperature was elevated to more than 130 °C. Maximum temperature of around 149 °C was observed in iPP with 0.5 wt% β-NA at the 10th irradiation. These were mainly due to the high frequency electromagnetic energy of microwave processing converted it to heat [2–4]. This increased the molecular internal friction [4], causing a volumetric heating and possibly changed the crystal structures of iPP [3, 4]. BAI et al [19] reported the instability of the β-crystal under various annealing temperatures. At the temperature of 90 °C to 130 °C, the degree of β-crystallinity was found to increase. Above this temperature, the Xβ reduced dramatically inversely with the drastic increment of the Xα. These affected all the thermal, mechanical and crystallization behaviors of the iPP.
Figure 2 illustrates the influence of microwave irradiation on the melting behavior of iPP and β-NA-nucleated iPP. Further peak identification and the area under peaks were estimated and listed in Table 2. The melting endotherms of the non- irradiated iPP, in Figure 2(a), exhibited single melting peak indicating only the α-crystal formed at around 165 °C, with the DHm of 97.35 J/g. After microwave treat, no remarkable shift of Tmα was observed, and the DHm reduced only slightly. However, broader melting peak with the irradiation time was evidenced. This implied that less perfect lamellae were formed [16], when the heat gained in iPP samples.
Figures 2(b) to (c) and Table 2 provided the graphical data of the irradiated iPP/β-NA samples. Double melting peaks were observed in all iPP/β-NA samples, which confirmed the β and α characteristics of the nucleated iPP. The less stable β-iPP was firstly melted and recrystallized as α-iPP with more stable crystalline structure. After that, the afresh formed α-iPP further melted at relatively high temperature [17]. The melting peak of the non-irradiated β-iPP was much greater than that of α-iPP, indicating that β phase was the major crystalline structure in iPP/β-NA composites. After microwave irradiation, these endotherms exhibited β phase transformation via recrystallization. The β-melting peak of iPP was found to weaken and Tmβ shifted to lower temperature by 2–3 °C at all β-NA contents, while the fusion endotherms of α-iPP were stronger. For Tmα, they shifted only 1 °C. The area under Tmβ (DHmβ) was found to decrease significantly together with the slight increased DHmα. These phenomena were clearly observed since the 8th irradiation cycle, where the specimen temperatures were higher than 130 °C. The overall area under melting endotherm or DHm of iPP was found to reduce only slightly at 0th to 6th irradiation. After 8th irradiation, the DHm tended to reduce significantly. These transformations converted β-phase to both the α and amorphous phases. Moreover, broader curves were also observed at all irradiated β-nucleated samples, which reflected the larger lamellar thickness distribution. The β melting peaks tended to shift slightly to lower positions, when the iPP/β-NA compositions were exposed to microwave.
Table 1 Elevated temperature of iPP and iPP/β-NA in each irradiation cycle
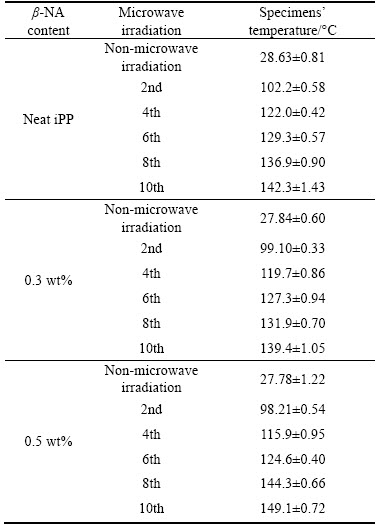
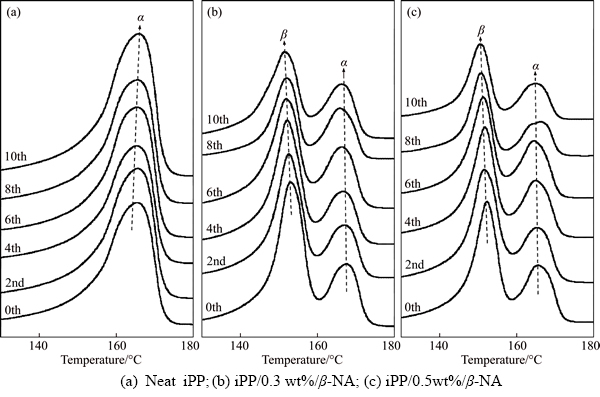
Figure 2 DSC melting thermogram of iPP and iPP/β-NA with various microwave irradiation time:
Table 2 DSC data of microwave irradiated neat iPP and iPP/β-NA composites
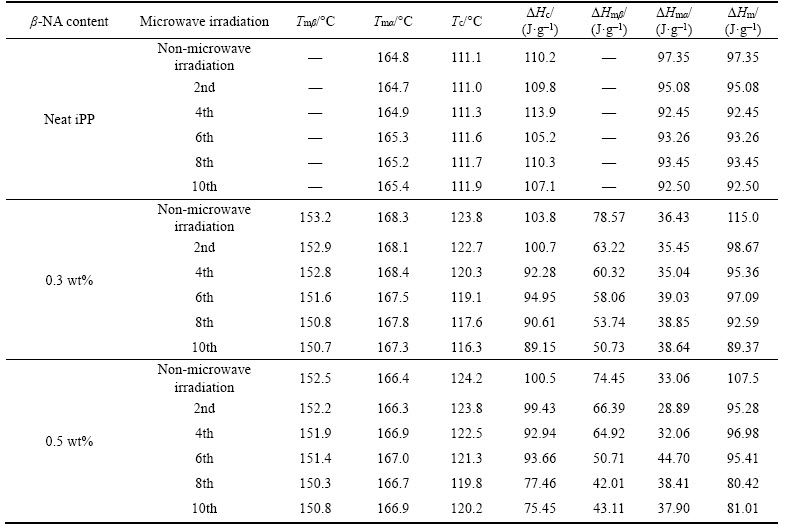
Figure 3 illustrates the crystallization curves of iPP and iPP/β-NA composites during non- isothermal cooling process, and their relative data were clearly listed in Table 2. In neat iPP, the irradiated samples did not show any notable difference in Tc compared with non-irradiated one. For the iPP/β-NA, the Tc dropped by 1–7 °C. Most of the irradiated iPP/β-NA compositions exhibited single crystallization peak except at the tenth irradiation. The peak seemed to overlap with two crystallization exotherms, indicating the imperfect form of β-crystal occurred under nonisothermal crystallization [18].
Although the DSC is generally employed to monitor the β-phase transformation in iPP but there is a possible erroneousness to analyze and determine the β and α phases [17]. Therefore, the XRD was conducted to ensure the phase transformation. The area under peaks was calculated and reported as the Xall, Xβ, Xα and Kβ in Table 3. Figure 4 shows the XRD patterns of iPP and iPP/β-NA before and after microwave irradiation. In Figure 4(a), the XRD profiles of the neat iPP show the typical characteristic of iPP with α-crystal. The diffractions were detected at (110), (040), (130) and (111) planes. After exposed to microwave ray, the XRD peaks still showed the α-characteristic but less intense, due to the slight decreased in α-crystallinity by the microwave heating as previously discussed in Tables 1 and 2. The Xall of iPP reduced from 44.51% to 38.12% or 14.35% reduction, when the iPP had been exposed to microwave ray for 10 times.
For iPP/β-NA samples (Figures 4(b) and (c)), the major peak at (300) and minor peak at (301) reflections represent the β-crystals. By using the Turner Jones equations, the fractions of β-crystalline phase of the iPP with 0.3 wt% and 0.5 wt% β-NA were around 0.85 and 0.89 respectively. This implied that they mainly composed of β-crystal (Table 3), which strongly accorded with the DSC results. After exposed to microwave ray, the Xall reduced slightly by 5%–6% together with the signification reduction of Xβ : Xα ratio or Kβ. This indicated that β-phase was mainly transformed to α phase and partially converted to the amorphous section. Compared to the non- irradiated samples, the peaks of α-crystal seemed to be more intense, due to the increasedα-crystallinity.
Figure 3 DSC crystallization thermogram of iPP and iPP/β-NA with various microwave irradiation times:
Table 3 XRD data of microwave irradiated iPP/β-NA composites
Microwave treatment caused an alteration of the structure of the β-nucleated iPP, and was caused by the increase of the matrix temperature (Table 1). The microwave heating was mainly caused by the ability of the iPP and β-iPP to absorb high frequency electromagnetic energy, and then converted it to heat [2–4]. The realignment took place more than a million times per second. This increased the molecular internal friction [4], causing a volumetric heating and possibly changed the crystal structures of β-iPP [3, 4]. In this case, the transformation of β-phase was clearly detected only when the β-iPP samples were exposed to microwave ray at least 6 or 8 times with the elevated temperature by more than 130 °C. Lower than these conditions, the β transformation was unclearly detected. BAI et al [19] concluded that at low annealing temperature (below 110 °C), the increment of β-phase was observed due to the transformation of mesophase to β. At moderate temperature (120 to 130 °C), the β fraction increased slightly. Above 130 °C, most of β-phase were transformed to α-iPP due to the melt- recrystallization.
Figure 5 illustrates the time required to obtain full crystallinity (tc) of iPP and β-iPP before and after microwave irradiation isothermally held at 120 °C. Neat iPP required more than 20 min to reach full crystallinity. Adding β-NA dramatically reduced the tc to less than 2 min. After microwave irradiation, neat iPP tended to crystallize at slower rate than the non-irradiated one. After irradiated for 8 times, the formation of nuclei was more difficult due to the reduced nucleation rate. For the β-NA samples, they required more tc due to the reduction of β phase together with the increment of the α phase. This is in agreement with the fact that the α-crystal grows much more rapidly than β-phase at the crystallization temperatures below 100 °C and above 140 °C. However, the β-phase generally grows quicker between 100–140 °C [20]. In this case, the samples were tested at 120 °C, which allowed β-phase to grow faster than α-crystal. Thus, the reduction of β-phase required more tc at this tested temperature. This possibly obstructed the recrystallization and converted β to amorphous phases.
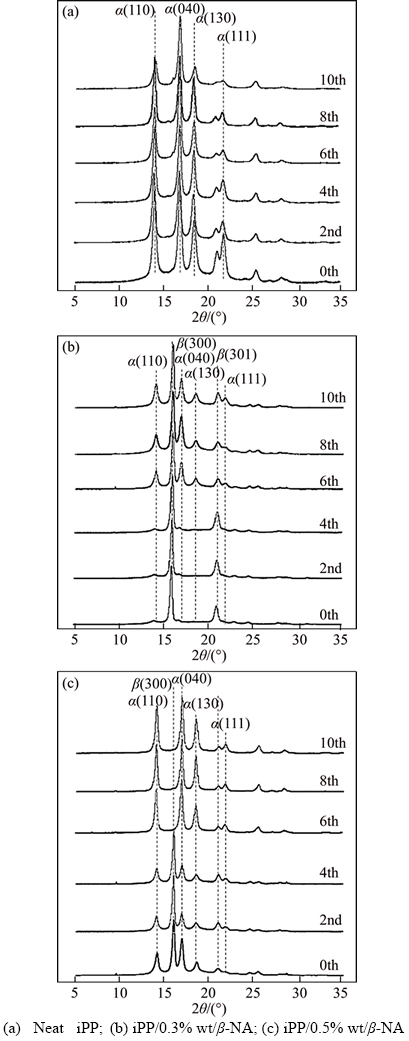
Figure 4 XRD patterns of iPP and iPP/β-NA with various microwave irradiation time:
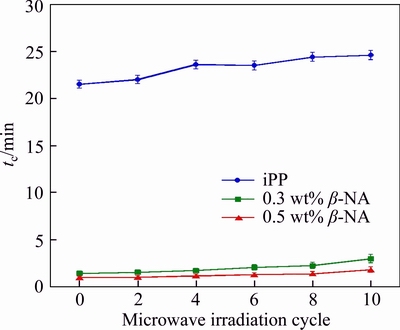
Figure 5 tc of iPP and iPP/β-NA with various microwave irradiation time
3.2 Impact strength
Figure 6 shows the impact strength of the iPP and β-iPP. Before microwave irradiation, adding β-NA to iPP greatly improved the impact resistance of iPP, which previously discussed in our previous work [6]. Under impact force, all iPP showed the slight increase in the impact strength with the irradiation time. In pure iPP, maximum increment by 22.5% was observed at the 10th irradiation. This was due to the increase of amorphous section as evidenced by the reduced Xall and DHm. For the β-iPP, it decreased slightly due to the reduction of the β-fraction. This is in good agreement with many papers; they indicated that the toughness of β-iPP is the combination effect of the microstructure, damping and β to α transformation during the mechanical test. The impact strength of β-iPP is greatly dependent on the β-phase content [19–21]. Moreover, TORDJEMAN et al [20] concluded that the tensile elongation at break of iPP was strongly correlated with the β-phase content as well. Another work by BAI et al [19] also confirmed the correlation between the β-fraction, sample temperature and the impact strength. They indicated that annealing the β-iPP above 130 °C, the impact strength was found to remarkably reduce together with the drastic reduction of Xβ.
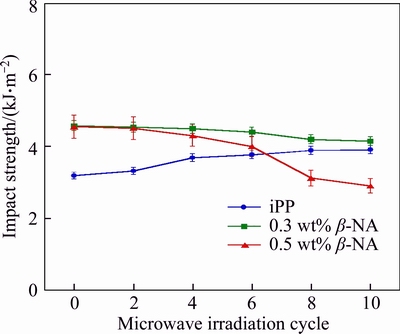
Figure 6 Impact strength of iPP and iPP/β-NA with various microwave irradiation time
4 Conclusions
The β to α phase transformation of iPP and β- iPP was investigated in the current paper. Under microwave treat, the sample temperature was found to increase with the irradiation time due to high frequency of microwave processing. Maximum elevated temperature of β-iPP was more than 140 °C at the 10th irradiation cycle. This was the major factor induced the phase transformation and structural change. After microwave treat, no remarkable shift of Tmα was observed, and the △Hm reduced only slightly. However, broader melting peak with the irradiation time was evidenced. Interestingly, the DHmβ was found to decrease significantly together with the increased DHmα. XRD results also confirm the β-phase transformation correlated to that observed by DSC. After exposed to microwave ray, the Xall reduced slightly by 5%–6% together with the signification reduction Kβ. This indicated that β-phase was mainly transformed to α phase and partially converted to the amorphous section. The transformation of β-phase was clearly detected, when the β-iPP samples were exposed to microwave ray at least 6 or 8 times with the elevated temperature by more than 130 °C. Lower than these conditions, the β transformation was unclearly detected. Moreover, neat iPP tended to crystallize at slower rate than the non-irradiated one. For the β-NA samples, they required more tc due to the reduction of β phase together with the increment of the α phase. Under impact force, neat iPP showed a slight increase in the impact strength with the irradiation time, due to the increase of amorphous region. For the β-iPP, it decreased slightly due to the reduction of the β-phase content.
Acknowledgement
The authors acknowledge the support of iPP resins at IRPC Public Company limited. Thanks are extended to the Siam Extek Company Limited and SINOPEC Beijing Research Institute of Chemical Industry (BRICI) for providing the β-NA.
References
[1] LI J W, YANG K, SONG L, HAN L, FENG Y K. Non-isothermal crystallisation of polypropylene/hollow glass microspheres composites [J]. Plastics, Rubber and Composites, 2015, 44: 68–73. DOI: 10.1179/1743289814Y. 0000000121.
[2] CHANDRASEKARAN S, RAMANATHAN S, BASAK T. Microwave material processing-a review [J]. AIChE Journal, 2012, 58: 330–363. DOI: 10.1002/aic.12766.
[3] CLARK D E, FOLZ D C, WEST J K. Processing materials with microwave energy [J]. Materials Science and Engineering A, 2000, 287: 153–158. DOI: 10.1016/S0921- 5093(00)00768-1.
[4] VENKATESH M S, RAGHAVAN G S V. An overview of microwave processing and dielectric properties of agri-food materials [J]. Biosystems Engineering, 2004, 88: 1–18. DOI: 10.1016/j.biosystemseng.2004.01.007.
[5] BURDICK D, COX N A, THOMPSON J E, BAILEY J S. Heating by microwave, hot air, and flowing steam to eliminate inoculated Salmonella from poultry feed [J]. Poultry Science, 1983, 62: 1780–1785. DOI: 10.3382/ ps.0621780.
[6] PETCHWATTANA N, COVAVISARUCH S, SRIPANYA P. Effect of nano-scaled styrene butadiene rubber based nucleating agent on the thermal, crystallization and physical properties of isotactic polypropylene [J]. Journal of Alloys and Compounds, 2014, 582: 190–195. DOI: 10.1016/ j.jallcom.2013.08.019.
[7] MAO H, LIU Y, LIU W, NIE M, WANG Q. Investigation of crystallisation and interfacial nature of polyhedral oligomeric silsesquioxane/polypropylene composites in the presence of β-nucleating agent [J]. Plastics, Rubber and Composites, 2017, 46: 322–328. DOI: 10.1080/14658011.2017.1350793.
[8] CHEN Z, KANG W, KANG J, CHEN J, YANG F, CAO Y, XIANG M. Non-isothermal crystallization behavior and melting behavior of Ziegler–Natta isotactic polypropylene with different stereo-defect distribution nucleated with bi-component β-nucleation agent [J]. Polymer Bulletin, 2015, 72: 3283–3303. DOI: 10.1007/s00289-015-1466-5.
[9] LI X, WU H, WANG Y, BAI H, LIU L, HUANG T. Study on the β to α transformation of PP/POE blends with β-phase nucleating agent during the tensile deformation process [J]. Materials Science and Engineering A, 2010, 527: 531–538. DOI: 10.1016/j.msea.2009.08.007.
[10] XU W, MARTINA D C, ARRUDA E M. Finite strain response, microstructural evolution and β/α phase transformation of crystalline isotactic polypropylene [J]. Polymer, 2005, 46: 455–470. DOI: 10.1016/j.polymer. 2004.10.084.
[11] KOTEK J, KELNAR I, BALDRIAN J, RAAB M. Structural transformations of isotactic polypropylene induced by heating and UV light [J]. European Polymer Journal, 2004, 40: 2731–2738. DOI: 10.1016/j.eurpolymj.2004.07.017.
[12] GREGORIOU V G, KANDILIOTI G, BOLLAS S T. Chain conformational transformations in syndiotactic polypropylene/layered silicate nanocomposites during mechanical elongation and thermal treatment [J]. Polymer, 2005, 46: 11340–11350. DOI: 10.1016/j.polymer.2005. 10.026.
[13] ZHANG R H, SHI D, TJONG S C, LI R K Y. Study on the β to α transformation of polypropylene crystals in compatibilized blend of polypropylene/polyamide-6 [J]. Journal of Polymer Science: Polymer Physics Edition, 2007, 45: 2674–2681. DOI: 10.1002/polb.21287.
[14] SHANGGUAN Y G, SONG Y H, PENG M, LI B P, ZHENG Q. Formation of β-crystal from nonisothermal crystallization of compression-molded isotactic polypropylene melt [J]. European Polymer Journal, 2005, 41: 1766–1771. DOI: 10.1016/j.eurpolymj.2005.02.033.
[15] LI J X, CHEUNG W L, JIA D M. A study on the heat of fusion of β-polypropylene [J]. Polymer, 1999, 40: 1219–1222. DOI: 10.1016/S0032-3861(98)00345-0.
[16] JAIN S, GOOSSENS H, VAN DUIN M, LEMSTRA P. Effect of in situ prepared silica nano-particles on non- isothermal crystallization of polypropylene [J]. Polymer, 2005, 46: 8805–8818. DOI: 10.1016/j.polymer.2004.12.062.
[17] LI X, WU H, HUANG T, SHI Y, WANG Y, XIANG F, ZHOU Z. β/α transformation of β-polypropylene during tensile deformation: effect of crystalline morphology [J]. Colloid and Polymer Science, 2010, 288: 1539–1549. DOI: 10.1007/s00396-010-2275-x.
[18] RADHAKRISHNAN J, ICHIKAWA K, YAMADA K, TODA A, HIKOSAKA M. Nearly pure α2 form crystals obtained by melt crystallization of high tacticity isotactic polypropylene [J]. Polymer, 1998, 39: 2995–2997. DOI: 10.1016/S0032-3861(97)00617-4.
[19] BAI H, WANG Y, ZHANG Z, HAN L, LI Y, LIU L, ZHOU Z, MEN Y. Influence of annealing on microstructure and mechanical properties of isotactic polypropylene with β-phase nucleating agent [J]. Macromolecules, 2009, 42: 6647–6655. DOI: 10.1021/ma9001269.
[20] TORDJEMAN P, ROBERT C, MARIN G, GERARD P. The effect of α, β crystalline structure on the mechanical properties of polypropylene [J]. The European Physical Journal E, 2001, 4: 459–465. DOI: 10.1007/s101890170101.
[21] BAI H, WANG Y, SONG B, LI Y, LIU L. Detecting crystallization structure evolution of polypropylene injection-molded bar induced by nucleating agent [J]. Polymer Engineering and Science, 2008, 48: 1532–1541. DOI: 10.1002/pen.21125.
(Edited by HE Yun-bin)
中文导读
微波辐照条件下基于纳米丁苯橡胶β相形核剂的全同立构聚丙烯的β–α相转变
摘要:本文研究了微波辐射对β-形核全同立构聚丙烯(iPP)β–α相转变的影响。对iPP及β-NA修饰的iPP样品进行10个周期的微波辐照,并采集第2、4、6、8和10周的数据。经过高频微波辐照,样品的温度随着辐照时间的延长而升高,最高超过130℃,这也是导致β相转变和结构变化的主要因素。差示扫描量热法(DSC)和X射线衍射(XRD)结果表明,β相主要转化为α相,部分转化为非晶态相,具体表现为:1)β相的熔化焓(DHmβ)降低;2)α相的熔化焓(DHmα)升高;3)β相含量(Kβ)减少;4)总体结晶度(Xall)降低。在冲击力作用下,纯iPP的冲击强度随辐照时间的延长略有提高,这是由于非晶态成分的增加。而对于β-iPP, 由于β相含量减少,其冲击强度降低。
关键词:聚丙烯;结晶;机械性能;热性能
Received date: 2017-07-04; Accepted date: 2018-01-02
Corresponding author: Nawadon PETCHWATTANA, Assistant Professor; Tel: +66-2649-5000; E-mail: nawadon@g.swu.ac.th; ORCID: 00000-0001-6713-8547

