DOI: 10.11817/j.ysxb.1004.0609.2020-36371
弱碱性溶液中硫砷铜矿的电化学氧化过程及表面相构成
俞 娟1, 2,孟必成1,黄文龙1,李林波1, 2
(1. 西安建筑科技大学 冶金工程学院,西安 710005;
2. 陕西省冶金工程技术研究中心,西安 710055)
摘 要:采用循环伏安(CV)和X射线光电子能谱(XPS)对硫砷铜矿在pH值为9.2溶液中的电化学氧化过程以及电位对硫砷铜矿表面氧化相的构成影响进行研究。结果表明:硫砷铜矿在电位0.17 V (氧化峰A1电位范围)氧化,主要发生Cu部分离开矿物表面进入溶液形成缺铜硫化物(Cu3-xAsS4)的初步氧化过程,表面不存在Cu(Ⅱ)的氧化相,S氧化形成少量的 ,As不发生氧化。当氧化电位提高到0.3 V(氧化峰A2电位范围),大量的Cu离开矿物表面进入溶液,表面仍然不存在Cu(Ⅱ)的氧化相,可能存在少量的CuSO4,但处于检测下限,表面存在一定量的
,As不发生氧化。当氧化电位提高到0.3 V(氧化峰A2电位范围),大量的Cu离开矿物表面进入溶液,表面仍然不存在Cu(Ⅱ)的氧化相,可能存在少量的CuSO4,但处于检测下限,表面存在一定量的 ,不存在As的氧化相。当电位提高到0.5 V(氧化峰A2电位范围),发生Cu和As的氧化沉积过程,分别在矿物表面形成Cu(Ⅱ)氧化相(Cu(OH)2,CuSO4)和As2O3氧化相。此外,表面还存在一定的
,不存在As的氧化相。当电位提高到0.5 V(氧化峰A2电位范围),发生Cu和As的氧化沉积过程,分别在矿物表面形成Cu(Ⅱ)氧化相(Cu(OH)2,CuSO4)和As2O3氧化相。此外,表面还存在一定的 相。当电位提高到0.8V(氧化峰A3电位范围),表面形成一定量的Cu(Ⅱ)氧化相(Cu(OH)2和CuSO4),As仍然以As2O3的形式存在,S除形成CuSO4外,部分仍以
相。当电位提高到0.8V(氧化峰A3电位范围),表面形成一定量的Cu(Ⅱ)氧化相(Cu(OH)2和CuSO4),As仍然以As2O3的形式存在,S除形成CuSO4外,部分仍以 的形式存在。
的形式存在。
关键词:电化学氧化;硫砷铜矿;亲水相;疏水相;XPS
文章编号:1004-0609(2020)-02-0467-12 中图分类号:TD91 文献标志码:A
硫砷铜矿(Cu3AsS4)是铜矿石中一种含砷硫化铜矿物,由于它的浮选性质与主要的含铜矿物黄铜矿(CuFeS2)十分接近,在常规泡沫浮选过程中很难将它与黄铜矿分离。它会进入产品铜精矿,并在一定程度上得到富集,造成铜精矿中砷含量超标[1-2]。铜精矿中砷含量超标不仅影响冶炼产品的质量,还会造成收尘、制酸、烟尘处理、废渣堆放、萃取、电解和电积等一系列的工艺和环境问题[3-4]。尽管铜精矿中的砷可以通过火法或湿法在一定程度上进行脱除,但随着环保要求的不断提高,可处理铜精矿中允许的含砷量逐渐降低。目前,国内铜冶炼厂冶炼的铜精矿含砷量不宜超过0.5%(质量分数),国外铜冶炼厂处理的铜精矿含砷量不能高于0.2%[5]。铜精矿中砷害的处理方法主要分为两大类,第一类是在铜的冶炼阶段处理,比如无害化造渣处理、冷凝固化含砷烟气、电解脱砷等;第二类是在火法冶炼之前进行预处理,如浸出除砷、水蒸气焙烧挥发除砷等。但是,无论从经济还是环境角度来说,在浮选阶段将铜精矿中的砷脱除是最理想的[6]。然而,采用传统泡沫浮选从铜精矿中分离Cu3AsS4较为困难,而作为矿物加工领域新兴的技术,电化学控制浮选在铜精矿浮选除砷方面具有良好的前景[7-11]。电化学调控浮选过程中,硫化物表面会发生一系列复杂的过程,比如氧化还原反应、化学反应、溶解、吸附和沉淀等。这些过程会导致矿物表面发生改变,形成非匀质成分的氧化相,从而影响矿物表面的亲水性和疏水性,进而影响硫化矿物的可浮性,达到不同矿物之间有效分离的目的[12-13]。因此,研究硫化矿物电化学氧化过程及表面氧化相的化学构成具有重要意义。
Cu3AsS4作为一种具有[AsmSn]x-基团的砷矿物,其化学态十分复杂。矿物晶体化学研究表明,其理论分子式为Cu2+Cu2+As3+ 。晶格中的As呈+3价,S呈-2价,Cu分别呈+2价和+1价,在溶解有氧的水溶液体系中它们具有不稳定性,很容易被氧化。国内外学者对主要含铜矿物CuFeS2在不同浮选体系中的电化学氧化过程及氧化相构成做了大量研究[14-20]。尽管Cu3AsS4与CuFeS2浮选性质相似,但它们具有不同的晶体结构,氧化过程中相界面上发生的电化学过程不相同,氧化后表面相的性质(成分、结构)也不同,进而亲疏水性也存在差异。
。晶格中的As呈+3价,S呈-2价,Cu分别呈+2价和+1价,在溶解有氧的水溶液体系中它们具有不稳定性,很容易被氧化。国内外学者对主要含铜矿物CuFeS2在不同浮选体系中的电化学氧化过程及氧化相构成做了大量研究[14-20]。尽管Cu3AsS4与CuFeS2浮选性质相似,但它们具有不同的晶体结构,氧化过程中相界面上发生的电化学过程不相同,氧化后表面相的性质(成分、结构)也不同,进而亲疏水性也存在差异。
对于Cu3AsS4在浮选体系中的电化学氧化行为及氧化相构成,研究人员开展了相关研究[21-26]。其中,GUO等[27]和PLACKOWSKI等[21]的研究表明,在无捕收剂体系中,硫砷铜矿在低电位下氧化,表面主要发生Cu氧化形成Cu2+进入溶液的过程,但氧化后的Cu2+是否形成Cu(Ⅱ)相沉积于矿物表面存在争论。另外,由于硫砷铜矿在较高电位下氧化,表面主要发生As和S的氧化,As氧化形成 ,但S氧化形成S0还是
,但S氧化形成S0还是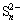 也存在争论。VELASQUEZ等[28]的研究也显示,硫砷铜矿在较高电位下氧化,表面形成CuO、 As2O5、As2O3、CuSO4及相应的Cu(OH)2氧化相。可见,关于Cu3AsS4在无捕收剂浮选体系中的氧化过程及氧化相的构成,研究结果还存在不一致性。因此,对于含砷硫化铜矿物Cu3AsS4的电化学氧化机制和表面相构成有待进一步研究。
也存在争论。VELASQUEZ等[28]的研究也显示,硫砷铜矿在较高电位下氧化,表面形成CuO、 As2O5、As2O3、CuSO4及相应的Cu(OH)2氧化相。可见,关于Cu3AsS4在无捕收剂浮选体系中的氧化过程及氧化相的构成,研究结果还存在不一致性。因此,对于含砷硫化铜矿物Cu3AsS4的电化学氧化机制和表面相构成有待进一步研究。
基于此,本文作者主要采用循环伏安和X射线光电子能谱研究了硫砷铜矿电化学氧化过程及表面氧化相的构成。旨在确定疏水氧化层能够稳定存在的电位范围,为电位调控分离浮选提供依据,也为下一步研究捕收剂体系中的硫砷铜矿电化学氧化过程及表面相构成提供基础。
1 实验
1.1 样品
采自天然硫砷铜矿样品,纯度大于98%(质量分数)。
1.2 溶液
采用蒸馏水中配制0.1 mol/L的KNO3溶液,然后加入0.05 mol/L Na2B4O7使得溶液的pH保持在9.2[29],作为测试缓冲电解液。KNO3和Na2B4O7均为分析纯。GUO等[27]提出缓冲溶液的脱氧对硫砷铜矿的循环伏安无影响,因此,本实验中所用的电解液均没有进行脱氧处理。
1.3 循环伏安测试
循环伏安测试采用传统的三电极体系。工作电极为硫砷铜矿块状样品,将其和铜导线连接在一起后,按照图1所示方式对电极进行密封,形成0.5 cm2的测试面。辅助电极和参比电极分别为铂片(纯度99.99%,尺寸2 cm×1 cm)和饱和甘汞电极(SCE)。文中所有的电位值均是相对于饱和甘汞电极(SCE)。每次实验前工作电极在均用砂纸(1500#)进行打磨以去除表面氧化还原产物并使电极保持光滑平整。
循环伏安测试采用采用恒电位仪(EG&G potentiostat Model 273A),扫描起始电位为-0.242 V,扫描速率为5 mV/s,扫描范围分别为-1.0~0.8 V。测试开始前延时600 s使得测试系统达到稳定。

图1 硫砷铜矿工作电极示意图
Fig. 1 Schematic diagram of working electrodes of enargite
1.4 X射线光电子能谱测定
XPS检测样品分别用1500号砂纸和1 μm的金刚石抛光膏进行打磨抛光,再将样品放在超声波中用蒸馏水清洗干净,并用冷风吹干。然后将样品放入测试液中,分别在选定的电位值下对样品进行恒电位极化,时间1800 s。之后,将样品从电解液中取出并用去氧蒸馏水冲洗,在氮气中风干,最后转入到XPS真空槽中。XPS分析仪器型号为ESCALAB 250 (Thermo VG),X射线源为Al Kα (1486.6 eV),电压和功率分别为15 kV和150 W。样品表面分析区域面积为2 mm×2 mm。文中所有XPS峰的结合能值都已校正为相对于标准结合能C1s(284.6 eV)。XPS数据分析采用XPSPEAK4.1软件。
2 结果与讨论
2.1 循环伏安曲线
图2所示为天然硫砷铜矿在pH=9.2的电解液中的循环伏安扫描曲线。从图2中可以看出,在阳极方向上出现了氧化峰A1、A2和A3。其中,阳极峰A1从0.1 V开始,0.17 V达到峰值;阳极峰A2从0.26 V开始,0.5 V达到峰值;阳极峰A3从0.7 V开始,0.8 V达到峰值。阴极方向上出现了3个还原峰C1、C2和C3。阳极峰与硫砷铜矿表面发生的电化学氧化过程有关,阴极峰与阳极氧化过程中形成表面产物的不完全可逆还原反应相关。该循环伏安曲线与VELASQUEZ等[28]的测定结果基本一致,但与PLACKOWSKI等[21]的测定结果存在一定的差别,这可能与所采用硫砷铜矿样品中杂质的类型及含量不同有关。

图2 硫砷铜矿在pH 9.2的电解液中的循环伏安曲线
Fig. 2 Cyclic voltammogram of enargite in electrolyte solution at pH=9.2
由于整个循环伏安扫描过程较短,扫描过程中在硫砷铜矿表面形成氧化相的量很少,不便于对氧化相的组成进行研究。为此,分别在氧化峰A1、A2和A3电位值范围内对硫砷铜矿进行极化(1800 s),使其表面形成一定量的氧化相后开展。选定的极化电位与A1峰、A2峰和A3峰相关,分别为0.17 V、0.3 V、0.5 V和0.8 V,已标示于图2中。
2.2 天然硫砷铜矿的化学组成分析
为了确定实验样品硫砷铜矿的矿物构成,采用XPS对矿物表面进行了成分检测,测定结果如图3所示。由图3可以看出,硫砷铜矿XPS谱中存在Cu、As、S、O、C的特征峰,Cu、As、S的相对摩尔分数分别为20.1%、7.9%、27.5%,即Cu3As1.2S4.1。与理论分子结构中Cu、As、S的含量有一定的偏差,硫砷铜矿样品中As和S的含量稍高于其化学计量结构中的理论含量。
图4所示为硫砷铜矿表面Cu 2p、S 2p、As 3d和O 1s的高分辨谱图,其中,Cu 2p、S 2p、As 3d谱图采用自旋-轨道劈裂峰进行拟合,表1所列为拟合后相应的结合能值。从图4中可以看出,Cu 2p谱中Cu 2p3/2谱峰位于结合能值932.2 eV,S 2p谱中代表S 2p3/2的谱峰位于161.9 eV,As 3d谱图中位于高结合能值的As 3d5/2峰位于43.3 eV,另一个位于低结合能值的As 3d5/2峰在42.3 eV。O 1s谱峰分别位于529.7 eV 和 532.6 eV。

图3 天然硫砷铜矿XPS全谱
Fig. 3 XPS survey spectrum of natural enargite
2.3 硫砷铜矿不同电位下氧化后表面相组成分析
2.3.1 0.17 V氧化后硫砷铜矿表面相构成
图5所示为硫砷铜矿在0.17 V氧化后表面的Cu 2p、S 2p、As 3d 和O 1s的高分辨谱图,其中,Cu 2p、S 2p、As 3d谱图采用自旋-轨道劈裂峰进行拟合,结合能值列于表2。
从图5可以看出,与未氧化硫砷铜矿表面谱图(见图4)相比,硫砷铜矿在0.17 V氧化后表面的Cu 2p,As 3d和O 1s谱均未发生明显变化,S 2p谱变化较为明显。Cu 2p 3/2谱峰结合能值位于932.3 eV,与未氧化前相比,没有发生明显的位移变化,但Cu的摩尔分数由20.1%降低到了12.42%,这说明硫砷铜矿表面发生了Cu离开晶格表面的初步氧化过程。CuS、Cu(OH)2和CuO等Cu(Ⅱ)的氧化相中Cu 2p3/2结合能值一般在934.6~935.1 eV,因此,该电位下矿物表面未形成Cu(Ⅱ)的氧化相,只可能形成Cu(Ⅰ)的氧化相。
与未氧化硫砷铜矿表面S 2p谱图相比,除了位于161.8 eV的S 2p3/2峰外,在163.2 eV新出现了一个相对小的S 2p3/2峰,此结合能值位于多硫化物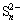 的结合能值范围162.0~163.7 eV,这表明该电位氧化后矿物表面形成了少量的
的结合能值范围162.0~163.7 eV,这表明该电位氧化后矿物表面形成了少量的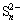 。
。
根据铜结合能值(932.3 eV)得到表面可能存在Cu(Ⅰ)氧化相,如Cu2S或Cu2O,但并无与之对应相的S 2p3/2和O 1s的结合能值出现。Cu2S中对应S 2p3/2的结合能值在162.6 eV,Cu2O中O的结合能值在530.2~530.6 eV范围内,但并未出现。这表明矿物表面的Cu(Ⅰ)不以Cu2S或Cu2O的形式存在。Cu3AsS4中Cu的结合能值在932.2~932.3 eV,S的结合能值在162 eV,这与测得的Cu、S结合能值接近。因此, Cu 2p 3/2谱峰932.3 eV结合能值为铜离开硫砷铜矿表面留下Cu3-xAsS4中的Cu所贡献。

图4 天然硫砷铜矿XPS高分辨图谱
Fig. 4 High resolution XPS spectra of natural enargite
表1 天然硫砷铜矿表面拟合后元素结合能值和摩尔分数
Table 1 Binding energy and mole fraction of elements identified on natural enargite surface

以上结果表明,硫砷铜矿在0.17 V氧化,主要发生Cu部分离开矿物表面进入溶液形成缺铜硫化物(Cu3-xAsS4)的初步氧化过程,表面不存在Cu(Ⅱ)的氧化相,S氧化形成少量的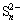 ,As不发生氧化。
,As不发生氧化。
2.3.2 0.3 V氧化后硫砷铜矿表面相构成
图6所示为硫砷铜矿在0.3 V氧化后表面Cu 2p、S 2p、As 3d和O 1s的高分辨谱图,表3所列为相应的结合能值。与硫砷铜矿在0.17 V氧化后的高分辨图谱对比,硫砷铜矿在0.3 V氧化后表面的Cu 2p、As 3d谱未发生明显变化,S 2p和O 1s谱变化明显。Cu 2p3/2谱峰的结合能值为932.3 eV,说明此电位下表面仍然没有形成Cu(Ⅱ)的氧化相,但是Cu的摩尔分数进一步降低,由0.17 V的12.42%降到了0.3 V的7.09%,这表明在此电位下氧化时,硫砷铜矿表面大量的Cu溶解进入了溶液。As 3d5/2的结合能值分别为42.9 eV和43.4 eV,与0.17 V氧化后As 3d5/2的结合能值(42.4 eV和43.2 eV)相比,分别提高了0.5 eV和0.2 eV,但并无As物相的变化。
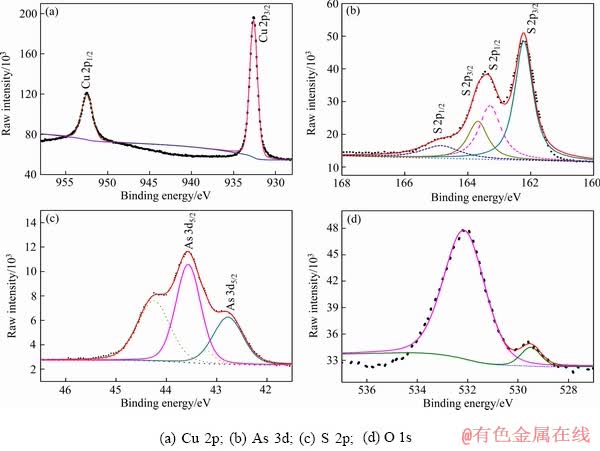
图5 天然硫砷铜矿在pH 9.2溶液中0.17 V氧化后表面XPS高分辨图谱
Fig. 5 High resolution XPS spectra of natural enargite surface after 0.17 V oxidation at pH=9.2:)
表2 天然硫砷铜矿在pH=9.2溶液中0.17 V氧化后表面拟合元素结合能值和摩尔分数
Table 2 Binding energy and mole fraction of elements identified on enargite surface after 0.17 V oxidation at pH=9.2

S 2p谱中除了位于162.0 eV和163.5 eV的S 2p3/2峰外,位于167.9 eV结合能值处出现了一个新的强度较小的S 2p- 峰,此结合能值在此处代表CuSO4相中的S。但可能由于CuSO4相含量较低,XPS分析结果中(见表3)并无与CuSO4对应的Cu 2p3/2和O 1s的结合能值出现(Cu 2p3/2 934.9 eV、O 1s 532.2~532.4 eV)。S 2p3/2峰的结合能达到163.5 eV,相比0.17 V氧化后的S 2p3/2峰的结合能值(163.2 eV),提高了0.3 eV,但此结合能值仍属于
峰,此结合能值在此处代表CuSO4相中的S。但可能由于CuSO4相含量较低,XPS分析结果中(见表3)并无与CuSO4对应的Cu 2p3/2和O 1s的结合能值出现(Cu 2p3/2 934.9 eV、O 1s 532.2~532.4 eV)。S 2p3/2峰的结合能达到163.5 eV,相比0.17 V氧化后的S 2p3/2峰的结合能值(163.2 eV),提高了0.3 eV,但此结合能值仍属于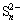 的结合能值范围。有报道称,S0的结合能值位于163.5~164.4 eV,但由于本研究体系为碱性体系,形成S0的可能性较小。故在0.3 V电位条件下S以
的结合能值范围。有报道称,S0的结合能值位于163.5~164.4 eV,但由于本研究体系为碱性体系,形成S0的可能性较小。故在0.3 V电位条件下S以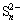 的形式存在于矿物表面。
的形式存在于矿物表面。
通过以上分析,硫砷铜矿在电位0.3 V氧化,大量的Cu离开矿物表面进入溶液,表面仍然不存在Cu(Ⅱ)的氧化相,可能存在少量的CuSO4,但处于检测下限,表面存在一定量的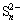 ,不存在As的氧化相。
,不存在As的氧化相。
2.3.3 0.5 V氧化后硫砷铜矿表面相构成
图7所示为硫砷铜矿在0.5 V氧化1800 s后的Cu 2p、S 2p、As 3d 和O 1s的高分辨谱图,拟合后相应的结合能值列于表4中。
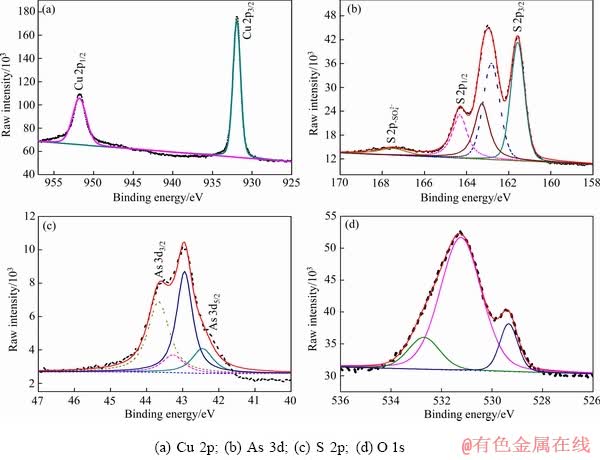
图6 天然硫砷铜矿在pH=9.2溶液中0.3 V氧化后表面XPS高分辨图谱
Fig. 6 High resolution XPS spectra of natural enargite surface after 0.3 V oxidation at pH=9.2
表3 天然硫砷铜矿在pH=9.2溶液中0.3 V氧化后表面拟合元素结合能值和摩尔分数
Table 3 Binding energy and mole fraction of elements identified on enargite surface after 0.3 V oxidation at pH=9.2

与0.3 V氧化后的高分辨XPS谱相比,硫砷铜矿在0.5 V氧化后,Cu 2p谱、As 3d谱出现了明显的变化,但S 2p谱无显著变化。Cu 2p谱中,除位于932.5 eV的Cu 2p 3/2峰外,在高结合能值934.7eV出现了另一个Cu 2p3/2峰,并且还出现了Cu 2p3/2和 Cu 2p1/2谱的多重分裂电子震激峰。结合能值位于932.5 eV的Cu 2p3/2理论上是由CuS相中的Cu贡献,但S 2p的结合能值中(见表4)没有出现代表CuS中S 2p的结合能值(162.6~162.7 eV)。因此,矿物表面不存在CuS相,Cu 2p3/2谱峰932.5 eV结合能值仍为Cu离开硫砷铜矿表面留下的Cu3-xAsS4中的Cu所贡献。新出现的结合能值为934.7eV的Cu 2p3/2峰代表Cu(Ⅱ)相的形成,对应的物质为Cu(OH)2。O1s图谱中,531.4 eV的结合能值为Cu(OH)2中的O贡献。因此,可判定此电位下硫砷铜矿表面氧化形成了Cu(Ⅱ)的氧化相Cu(OH)2。
S 2p谱中,163.6 eV的S 2p 3/2峰仍然位于 所在的结合能值范围,说明表面仍然存在
所在的结合能值范围,说明表面仍然存在 。结合能值168.1 eV的S 2p峰代表CuSO4相中的S;O 1s谱中,532.4 eV的结合能值为CuSO4中的O贡献;CuSO4相中的Cu 2p 3/2的结合能值在934.9 eV,与此处测定的Cu 2p 3/2峰934.7 eV结合能值无明显位移差别。因此,分析此电位下氧化表面形成了一定量CuSO4相。
。结合能值168.1 eV的S 2p峰代表CuSO4相中的S;O 1s谱中,532.4 eV的结合能值为CuSO4中的O贡献;CuSO4相中的Cu 2p 3/2的结合能值在934.9 eV,与此处测定的Cu 2p 3/2峰934.7 eV结合能值无明显位移差别。因此,分析此电位下氧化表面形成了一定量CuSO4相。
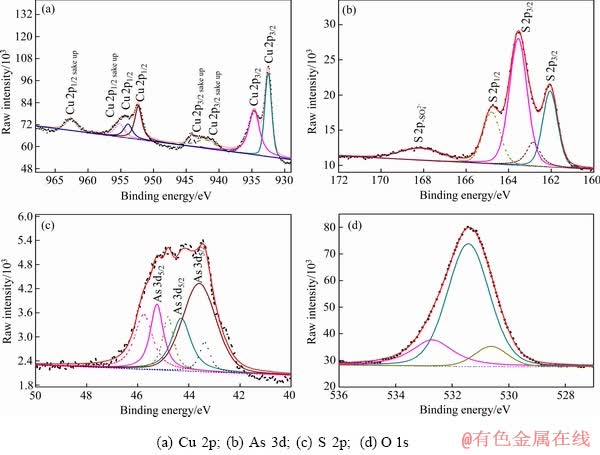
图7 天然硫砷铜矿在pH=9.2溶液中0.5 V氧化后表面XPS高分辨图谱
Fig. 7 High resolution XPS spectra of natural enargite surface after 0.5 V oxidation at pH=9.2
表4 天然硫砷铜矿在pH=9.2溶液中0.5 V氧化后表面拟合元素结合能值和摩尔分数
Table 4 Binding energy and mole fraction of elements identified on enargite surface after 0.5 V oxidation at pH=9.2
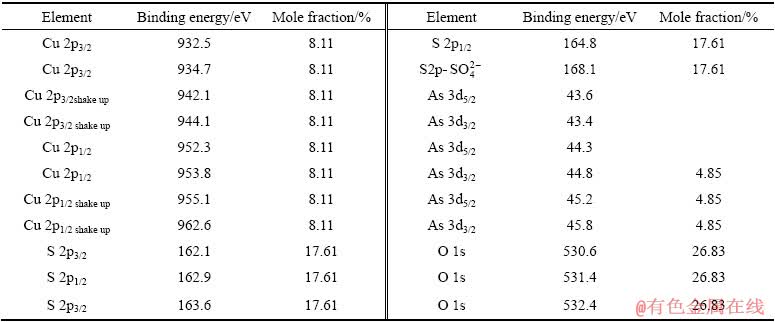
As 3d谱中,除位于43.6 eV和44.3 eV的As 3d5/2峰外,在高结合能值45.2 eV处出现了一个新的As 3d5/2峰。As2O5中As3d5/2的结合能值在45.9~46.5 eV范围内,因此,结合能值45.2 eV由As2O3中的As贡献。且据报道,As2O3中O 1s的结合能值在531.5 eV,故检测结果中O1s谱峰531.4 eV的结合能值应由As2O3中的O贡献。所以,证实表面不存在As2O5相,存在As2O3相。
通过以上分析,硫砷铜矿在电位0.5 V氧化,发生Cu和As的氧化沉积过程,分别在矿物表面形成Cu(Ⅱ)氧化相(Cu(OH)2,CuSO4)和As2O3氧化相,此外,表面还存在一定的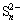 相。
相。
2.3.4 0.8 V氧化后硫砷铜矿表面相构成
图8所示为硫砷铜矿在0.8 V氧化1800 s后的Cu 2p、S 2p、As 3d 和O 1s的高分辨谱图,拟合后相应的结合能值列于表5中。与硫砷铜矿在0.5 V氧化后的高分辨图谱对比,硫砷铜矿在0.8 V氧化后表面的Cu 2p、S 2p、As 3d和O 1s谱图均没有发生明显的变化。
Cu 2p谱中代表Cu(Ⅰ)相的Cu 2p 3/2结合能值为932.5 eV,代表Cu(Ⅱ)相的Cu 2p 3/2结合能值为934.9 eV。同样,出现了Cu 2p3/2和Cu 2p1/2谱的多重分裂电子震激峰。硫砷铜矿表面形成了Cu(Ⅱ)氧化相,可能为Cu(OH)2、CuO和CuSO4等。CASTRO等[30]绘制了硫砷铜矿常温条件下的φ-pH图,并提出硫砷铜矿在溶液pH>4时,CuO是热力学稳定相,能够稳定存在,但有报道称溶液中的铜在向二价铜的氧化转变过程中,首先形成Cu(OH)2,然后随着氧化过程的进行,Cu(OH)2逐渐氧化形成CuO。因此,Cu(OH)2相对于CuO来说属于亚稳相。据报道CuO中的Cu2p3/2的结合能值应该在933.2~934.9 eV,虽然此电位下测得值934.9 eV,但并无与之对应的O 1s的结合能值出现(529.5~530.0 eV)。因此,分析此电位下氧化生成的主要是Cu(OH)2,即使存在CuO也不是主要相。

图8 天然硫砷铜矿在pH=9.2溶液中0.8 V氧化后表面XPS高分辨图谱
Fig. 8 High resolution XPS spectra of natural enargite surface after 0.8 V oxidation at pH=9.2
表5 天然硫砷铜矿在pH=9.2溶液中0.8 V氧化后表面拟合元素结合能值和摩尔分数
Table 5 Binding energy and mole fraction of elements identified on enargite surface after 0.8 V oxidation at pH=9.2

S 2p3/2谱中结合能值在163.5 eV的峰在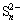 结合能值范围内,表明表面仍然存在
结合能值范围内,表明表面仍然存在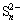 相。结合能值168.1 eV的S 2p峰代表CuSO4相中的S;O1s谱中532.4 eV的结合能值为CuSO4中的O贡献;CuSO4相中的Cu 2p3/2的结合能值在934.9 eV,这表明硫砷铜矿在0.8 V下氧化后表面相中存在一定量的CuSO4。
相。结合能值168.1 eV的S 2p峰代表CuSO4相中的S;O1s谱中532.4 eV的结合能值为CuSO4中的O贡献;CuSO4相中的Cu 2p3/2的结合能值在934.9 eV,这表明硫砷铜矿在0.8 V下氧化后表面相中存在一定量的CuSO4。
As的结合能值没有发生明显变化,仅是发生了0.1~0.2 eV的位移,这表明As仍然以As2O3存在于硫砷铜矿表面。
XPS结果证实,硫砷铜矿在电位0.8 V氧化,矿物表面形成了一定的Cu(Ⅱ)氧化相,Cu(OH)2和CuSO4;此外,As仍然以As2O3的形式存在,S除形成CuSO4外,部分仍以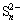 的形式存在。
的形式存在。
图9(a)所示为硫砷铜矿原矿及其分别在电位0.17 V、0.3 V、0.5 V和0.8 V下氧化1800 s后矿物表面Cu、As、S和O的摩尔分数。从图9(a)中可以看出,电位对硫砷铜矿表面相中各元素的摩尔分数有明显的影响。随着氧化电位的提高,Cu的摩尔分数先降低后缓慢升高,As的摩尔分数缓慢降低,O的摩尔分数急剧上升,而S的摩尔分数先缓慢升高后迅速降低。
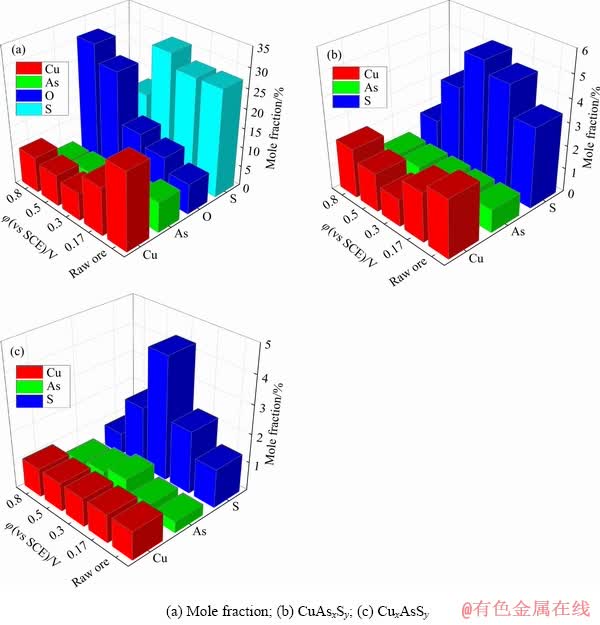
图9 天然硫砷铜矿不同电位下氧化后表面相中各个元素含量和摩尔比
Fig. 9 Mole fraction and mole ratio of natural enargite after polarization at various applied potentials in electrolyte solution
为了更直观地对比不同电位氧化后硫砷铜矿表面相各元素的相对摩尔分数,分别以As和Cu的摩尔分数为基准,计算出硫砷铜矿表面各原子的摩尔比,其结果如图9(b)和(c)所示。从图9(b)可以看出,随着电位的提高,硫砷铜矿表面Cu的原子比率从原矿的2.54减小到0.3 V的1.20,之后增加到0.8 V的2.14。结合XPS组成分析结果,进一步证实在低的氧化电位(0.17 V和0.3 V)下,硫砷铜矿表面发生Cu部分离开矿物表面进入溶液的氧化过程,导致硫砷铜矿表面呈现缺Cu的状态;而随着电位的提高(0.5V和0.8V)硫砷铜矿表面形成了大量的亲水相Cu(OH)2和CuSO4,并沉淀在硫砷铜矿表面使得表面Cu的摩尔比升高。此外,对于在不同电位下氧化后硫砷铜矿表面S相存在的相对含量可以用S的摩尔比来解释(见图9(b)和(c))。从图9(b)和(c)中可以看出,随着氧化电位的提高,表面S的摩尔比逐渐升高,到0.3 V达到最大,之后进一步提高电位到0.5 V和0.8 V,表面S的原子比率急剧下降。结合XPS组成分析结果,证实这是由于矿物表面氧化后形成大量的含有CuSO4的亲水相从电极表面脱落进入了溶液中引起的。
3 结论
1) 硫砷铜矿在弱碱性溶液中氧化表面发生A1、A2、A3 3个阳极氧化过程。其中,阳极峰A1从0.1 V开始,0.17 V时达到峰值;阳极峰A2从0.26 V开始,0.5 V时达到峰值;阳极峰A3从0.7 V开始,0.8 V时达到峰值。
2) 硫砷铜矿在阳极峰A1(0.17 V)范围内氧化,主要发生Cu部分离开矿物表面进入溶液形成缺铜硫化物(Cu3-xAsS4)的初步氧化过程,表面不存在Cu(Ⅱ)的氧化相,S氧化形成少量的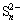 ,As不发生氧化。硫砷铜矿在阳极峰A2范围内的0.3 V氧化,大量的Cu离开矿物表面进入溶液,表面仍然不存在Cu(Ⅱ)的氧化相,可能存在少量的CuSO4,但处于检测下限,表面存在一定量的
,As不发生氧化。硫砷铜矿在阳极峰A2范围内的0.3 V氧化,大量的Cu离开矿物表面进入溶液,表面仍然不存在Cu(Ⅱ)的氧化相,可能存在少量的CuSO4,但处于检测下限,表面存在一定量的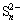 ,不存在As的氧化相。硫砷铜矿在阳极峰A2范围内的0.5V氧化,开始发生Cu和As的氧化沉积过程,分别在矿物表面形成Cu(Ⅱ)氧化相(Cu(OH)2、CuSO4)和As2O3氧化相,此外,表面还存在一定的
,不存在As的氧化相。硫砷铜矿在阳极峰A2范围内的0.5V氧化,开始发生Cu和As的氧化沉积过程,分别在矿物表面形成Cu(Ⅱ)氧化相(Cu(OH)2、CuSO4)和As2O3氧化相,此外,表面还存在一定的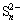 相。硫砷铜矿在阳极峰A3范围内(0.8 V)氧化,表面形成了一定的Cu(Ⅱ)氧化相,Cu(OH)2和CuSO4,As仍然以As2O3的形式存在,S除形成CuSO4外,部分仍以
相。硫砷铜矿在阳极峰A3范围内(0.8 V)氧化,表面形成了一定的Cu(Ⅱ)氧化相,Cu(OH)2和CuSO4,As仍然以As2O3的形式存在,S除形成CuSO4外,部分仍以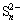 的形式存在。
的形式存在。
3) 硫砷铜矿在pH=9.2的电位调控浮选体系中,表面疏水相存在的电位范围为0.17~0.3 V,当电位高于0.3 V时,硫砷铜矿表面形成以亲水相为主的表面氧化相。
REFERENCES
[1] CASTRO S H. 硫砷铜矿的表面性质和可浮性[J]. 国外金属矿选矿, 2000(12): 25-27.
CASTRO S H. Surface properties and floatability of enargite[J]. Metallic Ore Dressing Abroad, 2000(12): 25-27.
[2] 梁铎强, 华一新, 蔡超君. 高砷锑铜精矿水蒸气脱砷锑工艺试验[J]. 云南冶金, 2005, 34(2): 38-40.
LIANG Duo-qiang, HUA Yi-xing, CAI Chao-jun. Technological experiment on removing antimony and arsenic form copper sulfide concentrate[J]. Yunnan Metallurgy, 2005, 34(2): 38-40.
[3] 李成秀, 王昌良. 铜砷浮选分离的进展[J]. 国外金属矿选矿, 2005(9): 9-12.
LI Xiu-cheng, WANG Chang-liang. Process on flotation separation of arsenopyrite and chalcopyrite[J]. Metallic Ore Dressing Abroad, 2005(9): 9-12.
[4] 罗小华. 含砷铜矿物的除砷研究[J]. 金属矿山, 2000(7): 56-58.
LUO Xiao-hua. Study on removal of arsenic from arsenic-bearing sulfide copper[J]. Metal Mine, 2000(7): 56-58.
[5] FORNASIERO D, FULLSTON D, LI C, RALSTO J. Separation of enargite and tennantite from non-arsenic copper sulfide minerals by selective oxidation or dissolution[J]. International Journal of Mineral Processing, 2001, 61: 109-119.
[6] 何晓川, 唐晓莲. 毒砂与铜铅锌多金属硫化矿分选试验研究[J]. 云南冶金, 1998, 27(4): 16-20.
HE Xiao-chuan, TANG Xiao-lian. An experiment upon separation of arsenopyrite from Cu/Pb/Zn polymetallic sulphide ore[J]. Yunnan Metallurgy, 1998, 27(4): 16-20.
[7] 汪镜意. 通过电化学控制浮选分离硫砷铜矿和黄铜矿[J]. 国外金属矿选矿, 2006(3): 25-30.
WANG Jing-yi. Separation of enargite from chalcopyrite by electrochemical control flotation[J]. Metallic Ore Dressing Abroad, 2006(3): 25-30.
[8] LONG G, PENG Y J, BRADSHAW D. A review of copper- arsenic mineral removal from copper concentrates[J]. Minerals Engineering, 2012, 36/38: 179-186.
[9] MIHAJLOVIC I, STRBAC N, ZIVKOVIC Z, KOVACEVIC R, STEHERNIK M. A potential method for arsenic removal from copper concentrates[J]. Minerals Engineering, 2007, 20: 26-33.
[10] GUO H, YEN W T. Selective flotation of enargite from chalcopyrite by electrochemical control[J]. Minerals Engineering, 2005, 18: 605-612.
[11] MAEDEH T K, MANLAPIGA E, FORBESB E, BRADSHAWC D, EDRAKID M. Selective flotation of enargite from copper sulphides in Tampakan deposit[J]. Minerals Engineering, 2017,112: 1-10.
[12] JACQUES S, GREET C J, BASTIN D. Oxidative weathering of a copper sulphide ore and its influence on pulp chemistry and flotation[J]. Minerals Engineering, 2016, 99: 52-59.
[13] PENG Yong-jun, ZHAO Sheng-li. The effect of surface oxidation of copper sulfide minerals on clay slime coating in flotation[J]. Minerals Engineering, 2011, 24: 1687-1693.
[14] LI Shuang-ke, GU Guo-hua, QIU Guan-zhou, CHEN Zhi-xiang. Flotation and electrochemical behaviors of chalcopyrite and pyrite in the presence of N-propyl-N′-ethoxycarbonyl thiourea[J]. Transactions of Nonferrous Metals Society of China, 2018, 28(6): 1241-1247.
[15] KALEGOWDA Y, CHAN Y L, WEI D H, HARMER S L. X-PEEM, XPS and ToF-SIMS characterisation of xanthate induced chalcopyrite flotation: Effect of pulp potential[J]. Surface Science, 2015, 635: 70-77.
[16] CHIMONYO W, CORIN K C, WIESE J G, O'CONNOR C T. Redox potential control during flotation of a sulphide mineral ore[J]. Minerals Engineering, 2017, 110: 57-64.
[17] FAIRTHORNE G, FORNASIERO D, RALSTON J. Effect of oxidation on the collectorless flotation of chalcopyrite[J]. International Journal of Mineral Processing, 1997, 49(1/2): 31-48.
[18] MANTON P, ZHENG X. A potential application of collectorless flotation in a copper/gold operation[J]. Minerals Engineering, 2010, 23(11/13): 895-902.
[19] YU Juan, YANG Hong-ying, FAN You-jing. Effect of potential on characteristics of surface film on natural chalcopyrite[J]. Transactions of Nonferrous Metals Society of China, 2011, 21(8): 1880-1886.
[20] 俞 娟, 杨洪英, 范有静, 陈燕杰. 电位对无捕收剂溶液中黄铜矿表面化学构成的影响[J]. 东北大学学报(自然科学版), 2011, 32(5): 700-707.
YU Juan, YANG Hong-ying, FAN You-jing, CHEN Yan-jie. Effect of electric potential on chemical composition of surface on chalcopyrite in collectorless solution[J]. Journal of Northeastern University(Natural Science), 2011, 21(8): 1880-1886.
[21] PLACKOWSKI C, HAMPTON M A, NGUYEN A V, BRUCKARD W J. An XPS investigation of surface species formed by electrochemically induced surface oxidation of enargite in the oxidative potential range[J]. Minerals Engineering, 2013, 45(8): 59-66.
[22] PLACKOWSKI C, NGUYEN A V, BRUCKARD W J. A critical review of surface properties and selective flotation of enargite in sulphide systems[J]. Minerals Engineering, 2012, 30: 1-11.
[23] PLACKOWSKI C, HAMPTON M A, NGUYEN A V, BRUCKARD W J. The effects of X-ray irradiation and temperature on the formation and stability of chemical species on enargite surfaces during XPS[J]. Minerals Engineering, 2013, 45: 59-66.
[24] PLACKOWSKI C, BRUCKARD W J, NGUYEN A V. Surface characterisation, collector adsorption and flotation response of enargite in a redox potential controlled environment[J]. Minerals Engineering, 2014, 65: 61-73.
[25] PINEDA D, PLACKOWSKI C, NGUYEN A V. Surface properties of enargite in MAA depressant solutions[J]. Minerals Engineering, 2015, 71: 180-187.
[26] GUO H, EN W T. Surface potential and wettability of enargite in potassium amyl xanthate solution[J]. Minerals Engineering, 2002, 15(6): 405-414.
[27] GUO H, YEN W T. Electrochemical study of synthetic and natural enargites[C]//Proceedings of the 24th International Mineral Processing Congress 1. Beijing: The Chinese Society of Non-ferrometals, 2008: 1138-1145.
[28] VELASQUEZ P, LEINEN D, PASCUAL J, RAMOS-BARRADO J R, CORDOVA R, GOMEZ H, SCHREBLER R. SEM, EDX and EIS study of an electrochemically modified electrode surface of natural enargite (Cu3AsS4)[J]. Journal of Electroanalytical Chemistry, 2000, 494: 87-95.
[29] GULER T, HICYILMAZ C, GOKAGAC G, EKMEKCI Z. Electrochemical behaviour of chalcopyrite in the absence and presence of dithiophosphate[J]. International Journal of Mineral Processing, 2005, 75(3/4): 217-228.
[30] CASTRO S H, BALTIERRA L. Study of the surface properties of enargite as a function of pH[J]. International Journal of Mineral Processing, 2005, 77(2): 104-115.
Electrochemical oxidation process and chemical composition of surface phase on enargite in weak alkaline solution
YU Juan1, 2, MENG Bi-chen1, HUANG Wen-long1, LI Lin-bo1, 2
(1. School of Metallurgical Engineering, Xi’an University of Architecture and Technology, Xi’an 710055, China;
2. Shaanxi Province Metallurgical Engineering and Technology Research Centre, Xi’an 710055, China)
Abstract: The electrochemical oxidation process of enargite in solution at pH=9.2, and influence of potential on the chemical composition of surface phase were studied by cyclic voltammetry (CV) and X-ray photoelectron spectroscopy (XPS). The results show that the primary oxidation process relates to the formation of copper-deficient(Cu3-xAsS4) sulfide induced by the dissolution of partial Cu into solution mainly occurs on enargite at the oxidation potential of 0.17 V (in the potential range of oxidation peak A1), and these is no Cu(Ⅱ) oxidation products existed in the surface film of enargite. A small amount of  forms through the S oxidation, and the As oxidation do not occur. When the oxidation potential increases to 0.3V(in the potential range of oxidation peak A2), plenty of Cu left the surface of sample and dissolves into solution. There are still no existence of the oxidation products of Cu(Ⅱ). A small amount of CuSO4 might exist, but its content is below low limit of detection. The Sn- phase is present in the surface film, but there are no existence of the oxidation products of As. The oxidation and deposition processes of Cu and As occur at the oxidation potential of 0.5 V (in the potential range of oxidation peak A2), and it results in the formation of the Cu(Ⅱ) oxidation products(Cu(OH)2 and CuSO4) and As2O3. Additionally, some Sn- phase is still present in the surface film. A certain amount of Cu(Ⅱ) oxidation product of Cu(OH)2 and CuSO4, are formed on sample when the oxidation potential increases to 0.8V(in the potential range of oxidation peak A3). The As element is still present in the form of As2O3 and parts of S element exists in the form of
forms through the S oxidation, and the As oxidation do not occur. When the oxidation potential increases to 0.3V(in the potential range of oxidation peak A2), plenty of Cu left the surface of sample and dissolves into solution. There are still no existence of the oxidation products of Cu(Ⅱ). A small amount of CuSO4 might exist, but its content is below low limit of detection. The Sn- phase is present in the surface film, but there are no existence of the oxidation products of As. The oxidation and deposition processes of Cu and As occur at the oxidation potential of 0.5 V (in the potential range of oxidation peak A2), and it results in the formation of the Cu(Ⅱ) oxidation products(Cu(OH)2 and CuSO4) and As2O3. Additionally, some Sn- phase is still present in the surface film. A certain amount of Cu(Ⅱ) oxidation product of Cu(OH)2 and CuSO4, are formed on sample when the oxidation potential increases to 0.8V(in the potential range of oxidation peak A3). The As element is still present in the form of As2O3 and parts of S element exists in the form of  besides some S element is oxidized to CuSO4.
besides some S element is oxidized to CuSO4.
Key words: electrochemical oxidation; enargite; hydrophilic phase; hydrophobic phase; XPS
Foundation item: Project(51304151) supported by the National Natural Science Foundation of China; Project (2018JK0474) supported by Shaanxi Provincial Education Department, China; Project (2018JM5135) supported by the Natural Science Basic Research Plan in Shaanxi Province, China
Received date: 2019-02-26; Accepted date: 2019-07-28
Corresponding author: LI Lin-bo; Tel: +86-15829273389; E-mail: yj-lilinbo@xauat.edu.cn
(编辑 李艳红)
基金项目:国家自然科学基金资助项目(51304151);陕西省教育厅资助项目(18JK0474);陕西省自然科学基础研究计划面上项目(2018JM5135)
收稿日期:2019-02-26;修订日期:2019-07-25
通信作者:李林波,教授,博士;电话:15829273389;E-mail:yj-lilinbo@xauat.edu.cn