文章编号:1004-0609(2008)04-0733-05
硫脲在碱性介质中的电化学行为
王云燕,柴立元
(中南大学 冶金科学与工程学院,长沙 410083)
摘 要:为表征硫脲在碱性介质中的电化学行为并为碱性硫脲浸金提供理论依据,采用稳态极化法和循环伏安法等电化学测试技术,系统研究碱性介质中硫脲的氧化分解电势、硫脲分解与浓度的关系。结果表明:硫脲的阳极氧化分解为不可逆反应,其氧化分解峰电流对应的电势约为0.5 V。硫脲浓度由0.05 mol/L增加至0.15 mol/L,其氧化分解的峰电流由1.559×10-4 A增大至9.068×10-4 A,峰电势由0.505 V负移至0.430 V,硫脲分解加剧。随pH值的升高,硫脲的氧化分解趋于严重,碱性介质中硫脲易于分解。
关键词:硫脲;碱性介质;分解;电化学行为
中图分类号:TF 801 文献标识码:A
Electrochemical behaviors of thiourea in alkaline medium
WANG Yun-yan, CHAI Li-yuan
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: In order to describe electrochemical behaviors of thiourea and provide fundamental for gold ore leached by alkaline thiourea system, the decomposed potential of thiourea oxidized in alkaline medium and the relationship between decomposition and concentration of thiourea were studied in detail by the methods of electrochemical techniques, such as stable polarization and cyclic voltammetry. The results indicate that the decomposition of thiourea is irreversible, and the corresponding decomposition potential is 0.5 V. With the addition of thiourea concentration from 0.05 mol/L up to 0.15 mol/L, the decomposition of thiourea is accelerated, the peak current increases from 1.559×10-4 A up to 9.068×10-4 A and the peak potential moves from 0.505 V down to 0.430 V. The decomposition of thiourea becomes serious with increasing pH value of system.
Key words: thiourea; alkaline medium; decomposition; electrochemical behavior
硫脲,又称硫代脲素,分子式为(NH2)2·CS,无色斜方晶体或结晶粉末,主要用于纺织、染料、造纸、照像、冶金、合成树脂涂料、生产阴离子交换树脂、电镀、金属酸洗缓蚀剂、制冷烫精、二氧化硫脲等。硫脲作为有机配合剂,可与许多金属离子形成配合物,已在化学和化工工业中获得广泛应用。在矿物加工及提取冶金领域,硫脲可作为金属硫化矿物的浮选药剂、金银的浸出剂及离子交换和溶剂萃取过程的洗提 剂[1-2]等。
自从1941年首次报道硫脲法提金以来,由于其无毒、高效、受其它元素干扰小等特点,进而成为该研究领域中倍受关注的热点,世界各国对硫脲提金的理论及工艺开展了广泛的研究。研究者们对酸性硫脲浸金的机理、工艺条件、影响因素、动力学等进行了大量研究,这些研究奠定了酸性硫脲浸金的工业应用基础。但是采用酸性硫脲法提金存在溶金过程中硫脲消耗过多、酸性溶液引起设备严重腐蚀、溶金不具有选择性、溶液的再生和净化工序复杂等一系列问题[3-4]。
为克服酸性硫脲提金过程存在的缺点,人们试图采用碱性硫脲溶液选择性提金。由于硫脲在碱性溶液中不稳定,易分解为硫化物和氨基氰,而且有研究报道硫脲溶金时pH值必须小于1.78,否则金不能溶解,因此许多人认为碱性硫脲溶液提金只能是一种设想。然而,有研究人员对碱性硫脲从含金废料中选择性溶金进行了研究,并从硫脲在碱性介质中的稳定性入手,进行了大量探索,发现某种化学物质(称之为稳定剂)在一定程度上能抑制碱性硫脲的不可逆分解,实现碱性硫脲溶液从废料中电化学选择性溶金[5-15]。
硫脲在碱性介质中的电化学行为、机理等基础研究可为碱性硫脲浸金新技术的成功工业化提供理论支撑。其中,硫脲本身的稳定性及其分解动力学又尤为关键。关于硫脲氧化反应的动力学研究可追溯到19世纪末,但是关于硫脲在碱性介质中的电化学行为研究几乎未见报道[16-24]。
本文作者采用各种电化学测试技术,如循环伏安法、稳态极化法等系统研究碱性介质中硫脲的氧化分解电势、硫脲分解与浓度的关系等电化学行为,旨在为高稳定性碱性硫脲浸金体系提供一定的参考。
1 实验
采用稳态极化曲线及循环伏安实验[25]进行硫脲在碱性溶液中分解的电化学行为研究。
稳态极化曲线的测量采用CHI660A电化学工作站。电极体系为三电极体系,电解槽为带有砂芯玻璃隔膜的H型电解槽,研究电极为铂丝电极(面积为0.442 mm2),辅助电极为大面积铂片,参比电极为 1 mol/L NaOH的Hg/HgO电极(-0.114 V vs SHE),采用带有Luggin毛细管的盐桥用以消除不同溶液间的液体接界电势及降低溶液的欧姆电阻。研究电极在每次使用前先用10 μm金相砂纸抛光至镜面光亮,然后用王水浸泡,二次蒸馏水洗涤。所研究的溶液用分析纯试剂及二次蒸馏水配制。实验时溶液温度由DL501型超级恒温器控制,每次实验前通氮气15 min以除去溶液中的溶解氧。本研究中的电势均相对于标准氢电极(SHE)。
工艺条件为:pH值12.5,温度25 ℃,稳态极化扫描速度取1 mV/s。
2 结果与讨论
2.1 碱性溶液中硫脲的分解电势
本研究采用铂电极为工作电极,研究碱性溶液中硫脲的氧化分解情况。碱性硫脲溶液的稳态极化曲线如图1所示。由图可以看出,硫脲氧化分解峰电流对应的电势为0.5 V,说明碱性硫脲溶液随电势正移硫脲本身会发生氧化分解,而电势太高则会出现氧气的析出。
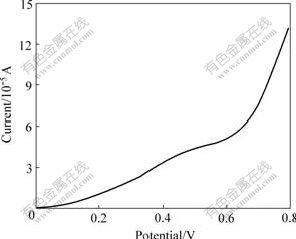
图1 碱性硫脲溶液的线性扫描伏安曲线
Fig.1 Linear sweep voltammetric curve of alkaline thiourea solution (Thiourea concentration: 0.10 mol/L)
2.2 硫脲分解与浓度的关系
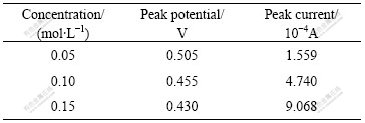
不同浓度硫脲溶液的稳态极化曲线如图2所示,相应的峰电流与峰电势见表1。由图2和表1可知,硫脲浓度由0.05 mol/L增加至0.15 mol/L,其氧化分解的峰电流由1.559×10-4 A增大至9.068×10-4 A,峰电势由0.505 V负移至0.430 V,硫脲分解加剧。因此,碱性硫脲溶液浸金时,硫脲浓度不宜太高。由图还可以看出,体系中氧气的析出基本上在0.8 V之后。因此,在实际浸出金矿物时,若对浸出体系施加外加电
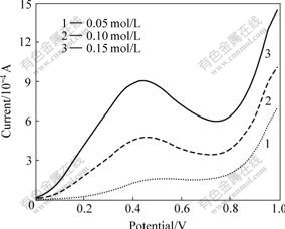
图2 不同浓度碱性硫脲溶液的稳态极化曲线
Fig.2 Stable polarization curves of alkaline thiourea solution with different concentrations
表1 不同浓度硫脲溶液稳态极化曲线的峰电流与峰电势
Table 1 Peak current and potential of stable polarization curves of thiourea with different concentrations
势,应使体系的电势控制在0.8 V以下,以避免氧气的大量析出。
不同浓度硫脲溶液的循环伏安曲线如图3所示。循环伏安曲线上可明显看出只有氧化峰而回扫时没发现有还原峰,这说明硫脲的阳极氧化分解(一般认为硫脲首先氧化分解为二硫甲脒,(SCN2H3)2+2H++2e= 2SC(NH2)2)为不可逆反应。硫脲浓度较低时,如0.05 mol/L,硫脲的氧化分解电流很小。随硫脲浓度的增 大,其起始分解电势逐渐变小,而分解峰电流显著增大,故浸出体系硫脲浓度不宜太高。其实,在实际浸出金矿物时,由于金矿中金的含量很低,即使采用低硫脲浓度的浸出液,溶液中硫脲的含量还是远远高于金矿中金完全浸出所需硫脲的量。
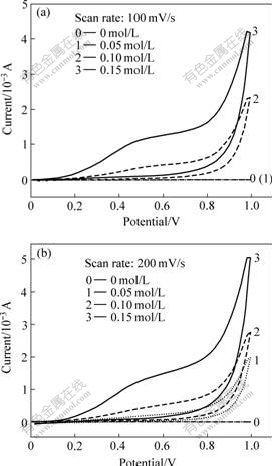
图3 不同浓度碱性硫脲溶液的循环伏安曲线
Fig.3 Cyclic voltammetric curves of alkaline thiourea solutions with different concentrations
2.3 扫描速度对硫脲在碱性溶液中分解的影响
扫描速度对硫脲在碱性溶液中氧化分解的影响如图4所示。不同扫描速度时碱性硫脲溶液中Pt电极的循环伏安曲线上回扫时也没有出现硫脲氧化产物的还原峰,这亦可说明硫脲氧化的不可逆性。另外,随扫描速度的增大,硫脲氧化峰电流的增加先快后慢。
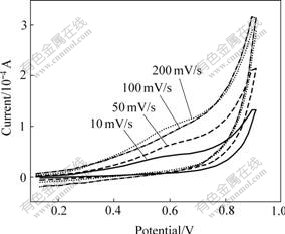
图4 不同扫描速度时碱性硫脲溶液的循环伏安曲线
Fig.4 Cycle voltammetric curves of alkaline thiourea solution at different scan rates (Thiourea concentration: 0.05 mol/L)
碱性介质中不同电势时硫脲氧化电流与扫描速度的关系如表2及图5所示。分析表中数据及图中曲线的走势可以看出,同一电势时,扫描速度低于100 mV/s时,随扫描速度的增大,阳极电流升高较快;超过100 mV/s后,随扫描速度的增大,硫脲氧化分解的电流增加变得缓慢。不同电势时,扫描速度对硫脲阳极氧化电流的影响类似。
表2 不同电势时碱性介质中硫脲氧化电流与扫描速度的关系
Table 2 Relationship between current of thiourea oxidation and scan rate at different potentials (10-5 A)
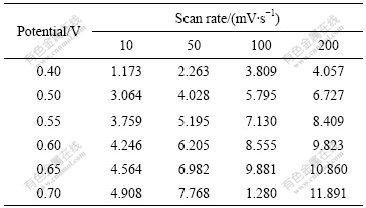
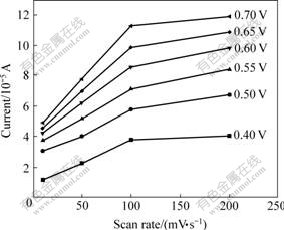
图5 不同电势时硫脲氧化电流与扫描速度的关系
Fig.5 Relationships between current of thiourea oxidation and scan rate at different potentials
2.4 pH值对硫脲在碱性溶液中分解的影响
pH值对硫脲在碱性硫脲溶液中氧化分解的影响如图6所示。由图可知,随体系pH值的升高,硫脲的氧化分解趋于严重。因此,碱性硫脲浸出液的pH值不宜太高,但是由前期研究中高稳定性碱性硫脲溶液浸出金矿物过程中体系pH值的变化可知,由于矿物中含有大量的酸性物质消耗大量的碱,因此,实际浸出金矿时,体系的pH值最好能稳定在某一数值,即体系具有pH缓冲性能,而课题组前期研究的碱性硫脲溶液的高效稳定剂Na2SiO3恰好具有此特殊性能。
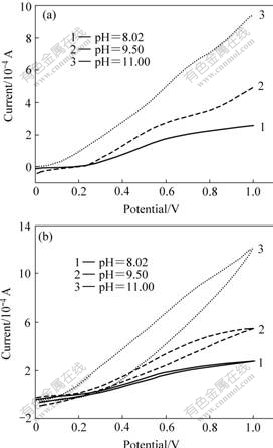
图6 不同pH值时碱性硫脲溶液的稳态极化曲线及循环伏安曲线
Fig.6 Stable polarization curves and cyclic voltammetric curves of alkaline thiourea solution with different pH values: (a) Stable polarization curve, scan rate 1 mV/s; (b) Cyclic voltammetric curve, scan rate 50 mV/s
3 结论
1) 硫脲的阳极氧化分解为不可逆反应,其氧化分解峰电流对应的电势约为0.5 V,体系中氧气的析出基本上在0.8 V之后。
2) 硫脲浓度增加时,峰电流增大,峰电势负 移,硫脲分解加剧,硫脲浓度由0.05 mol/L升高至0.15 mol/L,阳极电流则由1.559×10-4 A迅速升高至9.068×10-4 A。因此碱性硫脲浸金体系硫脲浓度不宜太高。在实际浸出金矿物时,由于金矿中金的含量很低,即使采用低硫脲浓度的浸出液,溶液中硫脲的含量还是远远高于金矿中金完全浸出所需硫脲的量。
3) 随体系pH值的升高,硫脲的氧化分解趋于严重。实际浸出金矿时,体系的pH值最好能稳定在某一数值,即体系具有pH缓冲性能,而前期研究的碱性硫脲溶液的高效稳定剂Na2SiO3恰好具有此特殊性能。
REFERENCES
[1] VEGLIO F. Factorial experiments in the development of a kaolin bleaching process using thiourea in sulphuric acid solutions[J]. Hydrometallurgy, 1997, 45(1/2): 181-197.
[2] OMER Y, RECEP Z. Recovery of gold and silver from copper anode slime[J]. Separation Science and Technology, 2000, 35(1): 133-141.
[3] PYKE P D, SWAMINATHAN C, JOHNSTON R F. An investigation into alternative eluants from activated carbon at atmospheric pressure[J]. Minerals Engineering, 1997, 10(8): 817-849.
[4] ARDIWILAGA S. Effects of cysteine and oxygen on recovery of cemented gold from leach liquors in a thiourea system[J]. Minerals Engineering, 1999, 12(6): 645-653.
[5] CHAI Li-yuan, OKIDO M. Dissolution theory of gold in alkaline thiourea solution (Ⅰ): Anodic behavior on gold in alkaline thiourea solution containing Na2SO3[J]. Transactions of Nonferrous Metals Society of China,1999, 9(1): 145-151.
[6] CHAI Li-yuan, OKIDO M. Dissolution theory of gold in alkaline thiourea solution (Ⅱ) electrochemical study on Au-Fe mix anode in alkaline thiourea solution containing Na2SO3[J]. Transactions of Nonferrous Metals Society of China, 1999, 9(2): 393-398.
[7] CHAI Li-yuan, OKIDO M. Dissolution theory of gold in alkaline thiourea solution (Ⅲ) thermodynamics on dissolution of gold in alkaline thiourea solution containing Na2SO3[J]. Transactions of Nonferrous Metals Society of China, 1999, 9(3): 646-650.
[8] 柴立元, 闵小波. 亚硫酸钠对碱性硫脲稳定作用的构效关系[J]. 中南工业大学学报: 自然科学版, 2002, 33(5): 473-476.
CHAI Li-yuan, MIN Xiao-bo. Structure-property relationship between the stability of alkaline thiourea and the structure of thiourea and sulfite ion[J]. Journal of Central South University of Technology: Natural Science Edition, 2002, 33(5): 473-476.
[9] OKIDO M, ISHIKAWA M, CHAI Li-yuan. Anodic dissolution of gold in alkaline solutions containing thiourea, thiosulfate and sulfite ions[J]. Transactions of Nonferrous Metals Society of China, 2002, 12(3): 519-563.
[10] CHAI Li-yuan, WANG Yun-yan. Electrochemical behaviors of gold and its associated elements in various complexing agents solutions[J]. Journal of Central South University of Technology, 2003, 10(4): 287-291.
[11] WANG Yun-yan, CHAI Li-yuan, MIN Xiao-bo, PENG Bing, HE De-wen. Optimization of highly efficient stable reagent of alkaline thiourea solution for gold lixiviation[J]. Journal of Central South University of Technology, 2003, 10(4): 292-296.
[12] 王云燕, 柴立元, 闵小波, 彭 兵, 何德文. Na2SiO3对碱性硫脲溶液选择性溶金的影响[J]. 中南工业大学学报: 自然科学版, 2003, 34(6): 611-614.
WANG Yun-yan, CHAI Li-yuan, MIN Xiao-bo, PENG Bing, HE De-wen. Effect of Na2SiO3 on gold dissolution selectively in alkaline thiourea solutions[J]. Journal of Central South University of Technology: Natural Science Edition, 2003, 34(6): 611-614.
[13] 郑 粟, 王云燕, 柴立元, 张晓飞. 高稳定性碱性硫脲体系对不同类型金矿的适应性研究[J]. 过程工程学报, 2005, 5(3): 289-294.
ZHENG Su, WANG Yun-yan, CHAI Li-yuan, ZHANG Xiao-fei. Adaptability of gold leaching from different ores in highly stable alkaline thiourea solutions[J]. The Chinese Journal of Process Engineering, 2005, 5(3): 289-294.
[14] 郑 粟, 王云燕, 柴立元. 基于配位理论的碱性硫脲选择性溶金机理[J]. 中国有色金属学报, 2005, 15(10): 1629-1635.
ZHENG Su, WANG Yun-yan, CHAI Li-yuan. Mechanism of gold dissolution selectively in alkaline thiourea solutions based on complex theory[J]. The Chinese Journal of Nonferrous Metals, 2005, 15(10): 1629-1635.
[15] ZHENG Su, WANG Yun-yan, CHAI Li-yuan. Research status and prospect of gold leaching in alkaline thiourea solution[J]. Minerals Engineering, 2006, 19(3): 1301-1306.
[16] 吴慧敏, 冯祥明, 李卫东, 左正忠. 碳酸钾溶液中硫脲在银电极上的电化学反应的研究[J]. 分析科学学报, 2003, 19(1): 66-69.
WU Hui-min, FENG Xiang-ming, LI Wei-dong, ZUO Zheng-zhong. Study on the electrochemical behaviour of thiourea in the processes of silver electroplating[J]. Journal of Analytical Science, 2003, 19(1): 66-69.
[17] 郑建周, 任 斌, 吴德印, 田中群. 硫脲在铂电极表面吸附行为的表面增强拉曼光谱研究[J]. 光散射学报, 2004, 16(1): 7-10.
ZHENG Jian-zhou, REN Bin, WU De-yin, TIAN Zhong-qun. Surface enhanced Raman spectroscopic study on adsorption of thiourea at platinum surface[J]. Chinese Journal of Light Scattering, 2004, 16(1): 7-10.
[18] WANG S, GAO Q Y, WANG J C. Thermodynamic analysis of decomposition of thiourea and thiourea oxides[J]. J Phys Chem B, 2005, 109(12): 17281-17289.
[19] XU L Q, GAO Q Y, FENG J M. Oscillations and period-doubling bifureations in the electrochemical oxidation of thiourea[J]. Chem Phys Lett, 2004, 397(3): 265-275.
[20] REDDY S J J, KRISHNAM V N. Electrochemical oxidation of thiourea[J]. J Electroanal Chem, 1970, 27(2): 473-476.
[21] ZHANG H G, RITCHIE I M, LA BROOY S R. Electrochemical oxidation of gold and thiourea in acidic thiourea solutions[J]. J Electrochem Soc, 2001, 148(2): D146-153.
[22] BOLZAN A E, PIATTI R C V, ARVIA A J. Electrochemical processed at gold|thiourea-containing aqueous acidic solution interfaces[J]. J Electroanal Chem, 2003, 552(1): 19-34.
[23] KIRCHNEROVA J, PURDY W C. The mechanism of the electrochemical oxidation of thiourea[J]. Anal Chim Acta, 1981, 123(1): 83-95.
[24] 刘 兵, 高庆宇, 冯加民, 陆永超. 硫脲氧化反应动力学研究进展[J]. 化学研究与应用, 2006, 18(7): 758-764.
LIU Bing, GAO Qing-yu, FENG Jia-min, LU Yong-chao. Reaction dynamics in the oxidation of thiourea[J]. Chemical Research and Application, 2006, 18(7): 758-764.
[25] BARD A J, FAULKNER L R. 电化学方法原理及应用[M]. 谷林瑛, 吕鸣祥, 宋诗哲, 译. 北京: 化学工业出版社, 1986: 615.
BARD A J, FAULKNER L R. Electrochemistry Principles and Application[M]. GU Lin-ying, L? Ming-xiang, SONG Shi-zhe, trans. Beijing: Chemical Industry Press, 1986: 615.
基金项目:国家自然科学基金资助项目(50004009)
收稿日期:2007-08-10;修订日期:2007-12-14
通讯作者:柴立元,教授,博士;电话:0731-8836921;传真:0731-8836921;E-mail: lychai@mail.csu.edu.cn
(编辑 何学锋)