Trans. Nonferrous Met. Soc. China 23(2013) 2416-2421
Electrochemical study on adsorption behavior of surfactants at β-2CaO·SiO2/NaAlO2 interface
Hai-yan YU1, Bo WANG1,2, Xiao-lin PAN1, Ting-ting DING1, Shi-wen BI1
1. School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China;
2. School of Materials Science and Technology, Hebei University of Science and Technology, Shijiazhuang 050018, China
Received 26 July 2012; accepted 29 November 2012
Abstract: β-2CaO·SiO2 was obtained with analytical grade reagents. Polyethylene glycol (PEG), sodium polyacrylate (PAAS) and their mixture were used to inhibit the decomposition of β-2CaO·SiO2 in sodium aluminate solution. The potential of solid-liquid interface and the adsorption mechanism were studied by the methods of Zeta potential measurement and XPS. The results indicate that PEG and PAAS have synergistic effect on the inhibition of secondary reaction. The inhibitory effect is the best when the volume ratio of PAAS to PEG is 1:1 and the total concentration is 12.5 mg/L. PAAS adsorbs on the surface of β-2CaO·SiO2 by the formation of —COOCa coordinate bond, and the negative charge enters into Stern layer, which results in the decrease of particle potential and the obvious change of binding energy of Ca 2p, Si 2p and O 1s. PEG only physically adsorbs on the surface of β-2CaO·SiO2, and had little effect on particle potential and binding energy of Ca 2p, Si 2p and O 1s.
Key words: sodium aluminate solution; secondary reaction; surfactant; ζ-potential; interfacial adsorption; electrochemical performance
1 Introduction
During the alumina production of soda-lime sinter process, SiO2 of the clinker is mainly in the form of β-2CaO·SiO2 which is active and could easily be decomposed in sodium aluminate solution. The chief components of sodium aluminate solution are NaOH, Na2CO3 and NaAl(OH)4. The existence of the components mentioned above will decompose β-2CaO·SiO2, and the decomposition ability sequence is Na2CO3>NaAl(OH)4 >> NaOH [1-3].
A great deal of work has been done in order to inhibit the decomposition of β-2CaO·SiO2 and the occurrence of secondary reaction.
YUAN et al [4] found that low αK and proper Na2CO3 concentration could inhibit the secondary reaction and improve the alumina leaching rate. They also pointed out that the alumina leaching rate could reach 90.15% under the best conditions. CHEN and ZHOU [5] studied the effect of leaching condition on secondary reaction, and pointed out that the proper leaching conditions were as follows: leaching temperature of 75-85 °C, αK of about 1.20, Na2CO3 concentration of about 25 g/L. Using the optimized leaching conditions mentioned above, the degree of secondary reaction will decrease. The amount of alumina loss in secondary reaction was still high; optimizing leaching condition could not solve the problem of secondary reaction radically.
Therefore, inhibiting the decomposition of β-2CaO·SiO2 by adding surfactant has become a research focus recently in alumina production field.
LI [6] found that the surfactant could inhibit the decomposition of β-2CaO·SiO2 effectively. When the addition of YJG was 0.1% of dry red mud, the leaching rate could increase by 2%. Sodium humate whose addition amount was 0.43% of clinker could also inhibit the decomposition of β-2CaO·SiO2 and improve alumina leaching rate [7]. But its brown color and large addition amount determined that it could not be applied in industry.
ZHANG et al [8] found that the synergistic effect of polyethylene glycol (PEG) and sodium polyacrylate (PAAS) could reduce the addition amount. When the addition amount of the mixture was 0.005% of clinker, alumina leaching rate would increase by 1.21% in industrial test. The following research also indicated that 70% of additive would adsorb on the surface of solid phase and then be carried away from solution. The residual additive amount in sodium aluminate solution was less [9-11].
A great deal of researches have been done on the selection and the adsorption macrokinetics of additive, but the effect of the adsorption of additive on electrical property of liquid-solid interface has not been reported. Therefore, PEG and PAAS were selected as the additive in this work, and the electrical property of liquid-solid interface before and after adsorption were also studied by the methods of Zeta potential and XPS.
2 Experimental
2.1 Equipments
Gas shielded MoSi2 furnace, magnetic stirring constant temperature water bath, visible spectrophotometer (722 s), Zeta electric potential analyzer, and X-ray photoelectron spectroscopy (XPS, PHI5300 type) were used.
2.2 Materials
The chemical reagents CaCO3, Na2CO3, Al2O3, NaOH and SiO2 used in the experiments were all analytically pure.
2.3 Synthesis of β-2CaO·SiO2
Sample with C/S=2 (mole ratio of CaO to SiO2) was prepared, and 0.5% B2O3 was added as stabilizer of β-2CaO·SiO2. The raw material was mixed in ball mill for 12 h and sintered in a corundum crucible at 1440 °C for 2 h. Then the sample was naturally cooled in furnace to 350 °C. The sample was rod-milled to 74 μm and dried at 120 °C for 4 h. Figure 1 shows the XRD pattern of β-2CaO·SiO2, which indicates that the only phase of the sample is β-2CaO·SiO2.
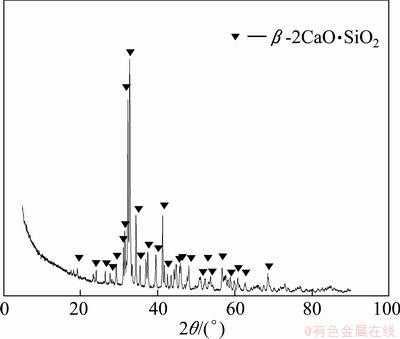
Fig. 1 XRD pattern of β-2CaO·SiO2
2.4 Adsorption experiments of surfactant
The additive was added into solution before leaching, because the decomposition of β-2CaO·SiO2 occurred at the beginning of leaching process. The feasible conditions for adsorption experiments are as follows: L/S ratio of 13.3 (the ratio of volume to mass), caustic alkali concentration of 36 g/L, alumina concentration of 45 g/L and sodium carbonate concentration of 15 g/L.
β-2CaO·SiO2 (7.5 g) was weighed and added into sodium aluminate solution (100 mL) which contained the additive with a certain concentration. The slurry was stirred for 5 min at room temperature. After 5 min standing, the supernatant was carried out to determine ζ-potential. Every measuring point was repeated three times, and an average was applied. The filter residue was washed and dried for XRD and XPS analysis.
3 Results and discussion
3.1 Inhibition of decomposition of β-2CaO·SiO2
In order to study the effect of surfactants on the decomposition of β-2CaO·SiO2, the SiO2 concentration of filtrate with different surfactant additions was determined. The results are shown in Fig. 2. The alumina concentration of filtrate with different surfactant additions is in the error range.
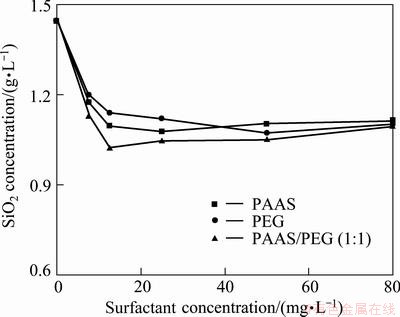
Fig. 2 SiO2 concentration in solution with different concentrations of surfactants
Figure 2 shows that the trend of SiO2 concentration with different surfactants is similar. The SiO2 concentration decreases obviously with the addition of surfactants, but the decrease becomes slow when the addition is over 12.5 mg/L. On the whole, when the surfactant is PAAS/PEG (1:1), the SiO2 concentration is lower than that of other surfactants. In other words, the concentration of SiO2 in the solution is the lowest when PAAS/PEG (1:1) concentration is 12.5 mg/L.
The solid compositions of the decomposition product were analyzed by X-ray fluorescence (XRF). The results are shown in Table 1.
Table 1 Solid compositions of decomposition product
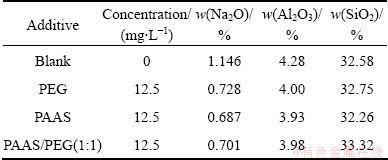
The results of XRF show that the Na2O and Al2O3 content of decomposition production with surfactants are lower than that without surfactants. However, because the main phase of the production is β-2CaO·SiO2 and the amount of sodium-silicon residue and calcium silica slag is less, the difference of Na2O and Al2O3 content of decomposition production with surfactants is not obvious. Therefore, the absolute value of solid composition could not reflect the effect of surfactant on inhibition of secondary reaction.
Based on this point, SiO2 which was the main component of residue was used as internal standard to calculate the inhibition degree (ηAO). The formula is shown as follows:
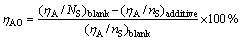 (1)
(1)
where (ηA/nS)blank is the molar ratio of Al2O3 to SiO2 in residue without additive; (ηA/nS)additive is the molar ratio of Al2O3 to SiO2 in residue with additive.
The relative inhibition degree of different surfactants was calculated by the formula mentioned above, and the results are shown in Fig. 3.
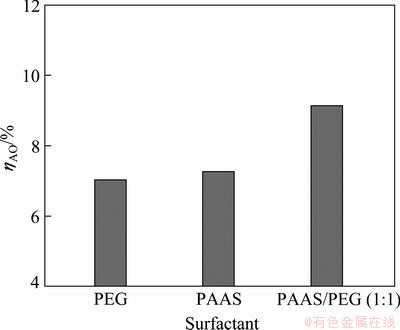
Fig. 3 Relative inhibition degree of different surfactants
The results indicate that the relative inhibition degree is higher when the additive is PAAS/PEG(1:1). The degree is similar when the additive is PEG and PAAS.
All the analyses show that PAAS/PEG(1:1) could not only decrease the SiO2 concentration of solution but also reduce the loss of alumina and sodium oxide in residue. In other words, PAAS/PEG(1:1) has better effect on inhibiting β-2CaO·SiO2 decomposition and reducing alumina loss.
3.2 ζ-potential of β-2CaO·SiO2 surface
Figure 4 shows the relationship between ζ-potential of β-2CaO·SiO2 surface and the surfactant concentration. The ζ-potential is obtained with 60 s magnetic stirring and 60 s standing at 25 °C.
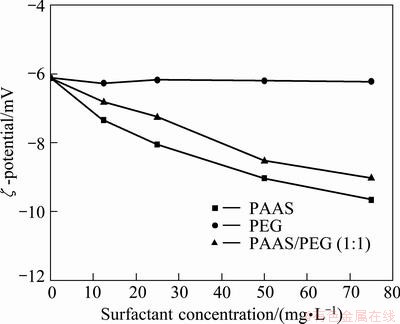
Fig. 4 Influence of surfactants on surface potential (ζ) of β-2CaO·SiO2
The trend of surface potential (ζ) of β-2CaO·SiO2 is not similar with different surfactants. When the additive is PEG, the potential changes little with the increase of PEG concentration, and the ζ-potential maintains the level of -6.12 mV. When the additive is PAAS, the potential decreases with the increase of PAAS concentration. The change of ζ-potential of PEG/PAAS (1:1) is between that of PEG and PAAS.
Electric double layer would be formed quickly when mineral particles meet with solution, but the adsorption of PEG on β-2CaO·SiO2 surface needs a period of time and does not affect the formation of electric double layer. Therefore, PEG has no effect on the proton translocation of the hydroxyl which has occurred on the surface of mineral particles. Comparing the PEG concentration of the solution before and after adsorption, it is found that most of the PEG adsorb on the surface of β-2CaO·SiO2 particles. This shows that the hydrogen- bonding adsorption is formed between the hydroxyl of β-2CaO·SiO2 surface and PEG first, and then the multilayer physical adsorption is formed through the hydrophobic interaction of PEG molecular.
When PAAS is added, it has no effect on the proton translocation of the hydroxyl of particle surface. Because the addition of PAAS is low, it has no effect on the electric double layer of particle surface. In addition, because of the ionization of PAAS, the active group which carries negative charge has repulsion effect on β-2CaO·SiO2 particles. All the theoretical analyses show that the effect of PAAS on ζ-potential is not obvious, but the actual value shows that the effect is obvious. This indicates that the adsorption exists with other forms.
In order to study the surface electrical behavior of particles after the addition of PAAS, the change of the surface potential with different pH was studied. The results are shown in Fig. 5.
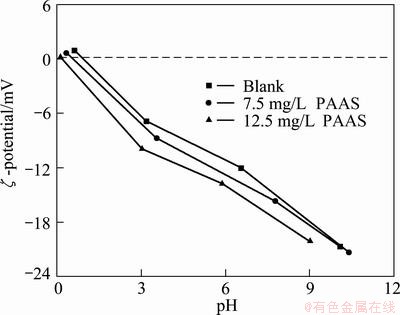
Fig. 5 pH—ζ potential curves of mud adsorbed with or without PAAS
It can be seen that the pH decreases with the increase of PAAS when the ζ-potential is fixed. The coordinate bond is formed between —COO and Ca—OH. This makes the negative electricity enter the Stern layer of particle. Therefore, the amount of H+ which was adsorbed increases and the pH—ζ curve shifts to the left.
Figure 5 also presents that the ζ-potential changes into positive value when pH is low. The zero point of ζ-potential in the curve is called isoelectric point (IEP). Surface coordination of strong adsorbed ions will make the pHIEP of particle change to a new value. Therefore, the characteristic adsorption of PAAS on the surface of 2CaO·SiO2 leads to the IEP of adsorbed particle shifted towards the left.
The pH—ζ curves of adsorbed mud with or without PEG is shown in Fig. 6. The curves have the same trend. Although the intersection with ζ=0 is not coincidence completely, considering the error caused by the stability of instrument, it can be concluded that the IEP of particle is not changed after the adsorption of PEG. This proves that PEG cannot change the surface properties of 2CaO·SiO2 and the adsorption between them is physical adsorption.
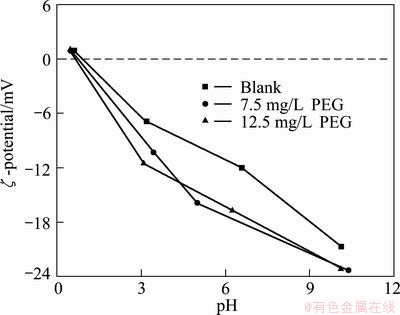
Fig. 6 pH—ζ potential curves of adsorbed mud with or without PEG
3.3 XPS characterization of adsorption of surfactants
The XPS analysis of resolving mud with or without surfactants was carried out to study the binding law between the functional groups of PAAS (PEG) and that of 2CaO·SiO2. Based on the XPS results the inhibition mechanism of additive on secondary reaction was discussed.
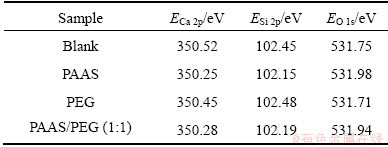
The peaks of the atom of Ca 2p, Si 2p and O 1s were obtained by the XPS scanning. The results are shown in Figs. 7-9. The binding energies of each atom are listed in Table 2.
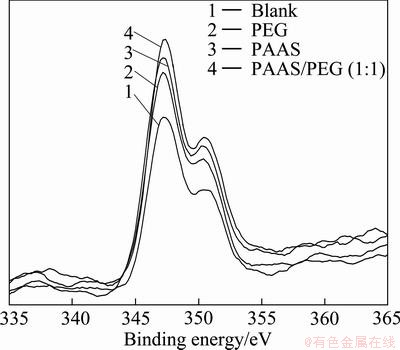
Fig. 7 Relationship of Ca 2p on surface of mud resolved with or without surfactants
Table 2 shows that the changes of binding energy of Ca 2p, Si 2p and O 1s are different, because the adsorption mode was different between surfactants and β-2CaO·SiO2. The binding energies of Ca 2p, Si 2p and O 1s of blank sample are 350.52, 102.45 and 531.75 eV; but those of PAAS sample are 350.25, 102.15 and 531.98 eV. The difference value of the samples mentioned above is between 0.2 and 0.3 (the error range is ±0.2 eV). The binding energies of Ca 2p, Si 2p and O 1s of PEG sample are similar to that of blank sample. When the additive is the mixture of PAAS and PEG (1:1), its binding energy is similar to that of PAAS sample.
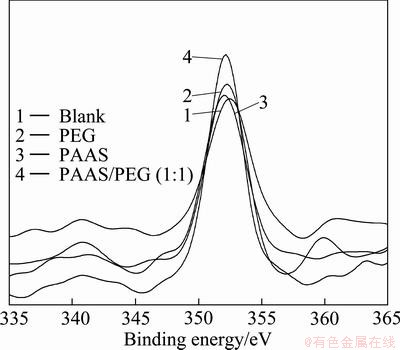
Fig. 8 Relationship of Si 2p on surface of mud resolved with or without surfactants
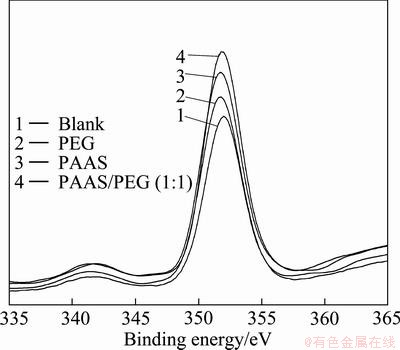
Fig. 9 Relationship of O 1s on surface of breakdown product with or without surfactants
Table 2 XPS data of mud resolved with or without surfactants
The change binding energy of Ca 2p is obvious, and it is larger than the measurement error. Therefore, the chemical bond is formed between PAAS and particle surface, and the adsorption mode is chemisorption. On the other hand, the binding energy of Ca 2p changes little, and it could be considered that the position of atom does not change. So the adsorption mode of PEG is not chemisorption. According to the research by WEI and WU [12], the edge atom of silicate could compensate the unbalance atomic force by the formation of Ca—OH, then hydroxylation surface is formed to adsorb with other ions or molecules.
Therefore, the formation of coordinate bond is carried out between the carbonyl oxygen and Ca atoms of 2CaO·SiO2 particle surface, and the Ca—O—Si bond affects the atom binding energy of Si atoms and O atoms. The formation of ionic bond leads to the existence of unoccupied orbit of Ca2+. The lone pair electrons of carbonyl oxygen could fill the unoccupied orbit. So, the charge density of Ca ions increases and the binding energy decreases. Meanwhile, the increase of electrostatic repulsion between Ca and O makes the electron of O atom redistribute towards Si atom. Therefore, the orbital binding energy of Si 2p decreases and that of O 1s increases.
The formula of PEG is H—(O—CH2—CH2)n—OH. It has ether bond and hydroxyl group, and its molecular chain is S-shaped in aqueous solution [13,14]. The formation of hydrogen bond between ether bond of PEG chain and —OH of 2CaO·SiO2 surface coats on particle surface. This macromolecule protective film hinders the contact between mineral particle and solution, so as to inhibit the decomposition of 2CaO·SiO2 and decrease the effect of secondary reaction.
For PAAS/PEG (1:1) mud, the changes of binding energy are similar to PAAS mud. And this also proves the reliability of the adsorption mechanism of surfactants. XPS analysis indicates that PAAS reacts with Ca2+ of 2CaO·SiO2, then the stable coordinate bond of —COOCa is formed. The adsorption mode is chemisorption, and the result is consistent with thermodynamics analysis [15]: the adsorption mode of PAAS is single molecule chemisorption, but that of PEG is multi-molecule physisorption.
4 Conclusions
1) When the addition of PAAS/PEG (1:1) is 12.5 mg/L, the concentration of SiO2 in solution is the lowest, and that of sodium-silicon residue in mud is the lowest.
2) The coordinate bond of —COOCa is formed between PAAS and 2CaO·SiO2. The negative electricity enters the Stern layer of particle, and leads to the decrease of particle potential. PEG which is a neutral surfactant has no effect on the particle potential.
3) The binding energy of Ca 2p, Si 2p and O 1s changes obviously when PAAS is added, which proves the formation of —COOCa bond. The binding energy of Ca 2p, Si 2p and O 1s changes little when PEG is added, which proves that the adsorption mode is physisorption.
References
[1] CHEN Bin, LI Xiao-bin, XU Hua-jun, PENG Zhi-hong, LIU Gui-hua, ZHOU Qiu-sheng. Thermodynamic analysis of secondary reactions in the clinker leaching process [J]. Journal of Beijing University of Chemical Technology, 2007, 34(2): 189-192. (in Chinese)
[2] LIU Gui-hua, LIU Yun-feng, LI Xiao-bin, PENG Zhi-hong, ZHOU Qiu-sheng, XU Hua-jun. Reducing loss of soda in red mud in process of Bayer digestion [J]. The Chinese Journal of Nonferrous Metals, 2006, 16(3): 355-359. (in Chinese)
[3] CHEN Bin, LI Xiao-bin, LIU Gui-hua. Behavior of SiO2 in the leaching process of alumina clinker with high concentration [J]. Journal of University of Science and Technology Beijing (Mineral, Metallurgy, Material), 2008, 15(5): 538-542.
[4] YUAN Yi, XIANG Yang, HUANG Fang, YUAN Hua-jun. An elementary analysis on the secondary of leaching sintering mixture of aluminium [J]. Light Metals, 1999(12): 18-21. (in Chinese)
[5] CHEN Hong-wu, ZHOU Zong-ke. Effect of sintering condition on secondary reaction of sintering process [J]. Light Metals, 2001(8): 14-16. (in Chinese)
[6] LI Tai-chang. Technical research of secondary reaction inhibitor and it’s sweetening process [J]. Nonferrous Metals, 2002(1): 26-28. (in Chinese)
[7] ZHANG Cheng-zhong, YU Hai-yan, ZHANG Li-qiang, LIU Xin, BI Shi-wen. Mechanism for humic acid sodium to inhibit secondary reaction in clinker extraction process [J]. Journal of Chemical Industry and Engineering, 2008, 59(2): 526-530. (in Chinese)
[8] ZHANG Cheng-zhong, YU Hai-yan, ZHANG Zhan-ming, ZHANG Xian-qi, BI Shi-wen. Adsorption and distribution of polyethylene glycol in clinker-sodium aluminate solution system [J]. Journal of Materials and Metallurgy, 2008, 7(3): 14-16. (in Chinese)
[9] AN Bai-chao, JI Gui-juan, WANG Wen-ying, GAN Shu-cai, XU Ji-jing, GAO Gui-mei, LI Guang-huan. Azeotropic distillation- assisted preparation of nanoscale gamma-alumina powder from waste oil shale ash [J]. Chemical Engineering Journal, 2010, 157(1): 67-72.
[10] KORETSKY C. The significance of surface complexation reactions in hydrologic systems: A geochemist’s perspective [J]. Journal of Hydrology, 2000, 230(3-4): 127-171.
[11] ZHANG Cheng-zhong, YU Hai-yan, DONG Bao-cai, BI Shi-wen. Adsorption and distribution of polymer AY in 2CaO·SiO2-sodium aluminate solution system [J]. Light Metals, 2008(2): 9-11. (in Chinese)
[12] WEI Jun-feng, WU Da-qing. Surface ionization and surface complexation models at mineral/water interface [J]. Advance in Earth Sciences, 2000, 15(1): 90-96. (in Chinese)
[13] XU Ming-xia, FANG Dong-pu, YANG Zheng-fang. Effects of macromolecular surfactant in preparation of ZrO2 (Y2O3) powders (Part Two) [J]. Journal of Inorganic Materials, 1991, 6(1): 39-44. (in Chinese)
[14] YU Hai-yan, PAN Xiao-lin, DING Ting-ting, ZHANG Wu, LIU Han, BI Shi-wen. Adsorption of sodium polyacrylate at interface of dicalcium silicate–sodium aluminate solution [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(10): 2323-2326.
[15] YU Hai-yan, PAN Xiao-lin, LU Zhong-ke, DING Ting-ting. Adsorption of polyethylene glycol at the interface of dicalcium silicate-sodium aluminate solution [C]//Light Metals 2010. San Diego: Minerals, Metals & Materials Society, 2011: 251-254.
表面活性剂在β-2CaO·SiO2/NaAlO2溶液界面吸附行为的电化学研究
于海燕1,王 波1,2,潘晓林1,丁婷婷1,毕诗文1
1. 东北大学 材料与冶金学院,沈阳 110004;
2. 河北科技大学 材料科学与工程学院,石家庄 050018
摘 要:利用分析纯试剂合成β-2CaO·SiO2,研究表面活性剂聚乙二醇(PEG)和聚丙烯酸钠(PAAS)及二者混合物对铝酸钠溶液中β-2CaO·SiO2分解的影响,并通过Zeta电位测量、XPS等手段对吸附前后固-液界面电化学性能以及吸附机理进行研究。结果表明:PEG与PAAS对抑制二次反应具有协同作用,在PAAS与PEG体积比为1:1的混合添加模式下,总浓度为12.5 mg/L时抑制二次反应效果最好;PAAS在β-2CaO·SiO2上通过形成—COOCa配位键发生吸附,负电荷进入Stern层,导致吸附颗粒电位下降,渣中Ca 2p、Si 2p和O 1s结合能均发生较大变化;PEG发生物理吸附,吸附对界面电位不产生影响,且渣中Ca 2p、Si 2p和O 1s结合能变化不明显。
关键词:铅酸钠溶液;二次反应;表面活性剂;ζ-电位;界面吸附;电化学性能
(Edited by Xiang-qun LI)
Foundation item: Projects (50974036, 51104041) supported by the National Natural Science Foundation of China; Project (2012M510821) supported by the Postdoctoral Science Foundation of China
Corresponding author: Hai-yan YU; Tel: +86-24-83686460; E-mail: yuhy@smm.neu.edu.cn
DOI: 10.1016/S1003-6326(13)62749-1