


Trans. Nonferrous Met. Soc. China 22(2012) 847-852
WC-Ni3Al-B composites prepared through Ni+Al elemental powder route
LONG Jian-zhan1, 2, ZHANG Zhong-jian1, 2, XU Tao1, 2, PENG Wen1, 2, WEI Xiu-yu1, 2, LU Bi-zhi1, 2, LI Ren-qiong1, 2
1. State Key Laboratory of Cemented Carbide, Zhuzhou 412000, China;
2. Zhuzhou Cemented Carbide Group Corp. Ltd., Zhuzhou 412000, China
Received 9 September 2011; accepted 5 January 2012
Abstract: In order to develop the liquid phase sintering process of WC-Ni3Al-B composites, the preparation process of WC+Ni3Al prealloyed powder by reaction synthesis of carbonyl Ni, analytical purity Al and coarse WC powders was investigated. DSC and XRD were adopted to study the procedure of phase transformation for the 3Ni+Al and 70%WC+(3Ni+Al) mixed powders in temperature ranges of 550-1200℃ and 25-1400℃, respectively. The results demonstrate that the formation mechanism of Ni3Al depends on the reaction temperature. Besides WC phase, there exist Ni2Al3, NiAl and Ni3Al intermetallics in the powder mixture after heat treatment at 200-660℃, while only NiAl and Ni3Al exist at 660-1100℃. Homogeneous WC+Ni3Al powder mixture can be obtained in the temperature range of 1100-1200℃. The WC-30%(Ni3Al-B) composites prepared from the mixed powders by conventional powder metallurgy technology show nearly full density and the shape of WC is round. WC-30%(Ni3Al-B) composites exhibit higher hardness of 9.7 GPa, inferior bending strength of 1800 MPa and similar fracture toughness of 18 MPa·m1/2 compared with commercial cemented carbides YGR45 (WC-30%(Co-Ni-Cr)).
Key words: WC-Ni3Al-B composites; Ni3Al intermetallic; liquid phase sintering; mechanical properties
1 Introduction
Cemented carbide, such as WC-Co, is generally considered an adequate combination of strength and hardness [1]. This is one of the important reasons for the selection of these materials for a large number of industrial applications, including cutting tools, wear parts, extrusion dies and mining equipment [2]. Metals, such as Fe, Ni and Co, have been used as ductile second phases to improve fracture toughness, but they have poor adaptation in aggressive environments [3], which limits the use of WC-based composites for high temperature critical applications. A lot of research about Ni3Al taken as alternative binder for cemented carbide has been investigated [3-5]. This is mainly due to the properties [6,7] that Ni3Al possess, such as increasing strength with temperature to a maximum at 700-800℃, good oxidation resistance and good resistance to aqueous acidic corrosion environments. Meanwhile, with the addition of small amounts of boron, the ductility of Ni3Al can be greatly improved [8]. At the same time, the wettability of Ni3Al on WC and TiC is sufficient and the possibility of forming compacts of WC/TiC-Ni3Al has been reported [9]. These properties make Ni3Al a potentially attractive replacement for cobalt in the fabrication of both titanium and tungsten carbide matrix composites [4].
Liquid phase sintering [10], hot-pressing [4] and melt infiltration [11] are the three common production techniques for the processing of WC-Ni3Al and TiC-Ni3Al composites with promising mechanical properties comparable to that of commercially available WC-Co and TiC-Ni cermets, but the WC-Ni3Al composites form a semi-continuous second phase with some remnant Ni3Al-rich areas owing to the relatively large size of the starting Ni3Al prealloyed powder, which may decrease the performances of the composites. Ni3Al prealloyed powder becomes an obstacle during the processing of these cermets by traditional liquid phase sintering techniques. However, little information has been published on the preparation of Ni3Al prealloyed powder by reaction synthesis from powder state specimens instead of compaction specimens [12]. Moreover, the effect of temperature on phase transition during the synthesis from powder state specimens is not very clear. Further studies on the processing of these composites by traditional liquid phase sintering are still necessary.
In this study, the preparation process of WC+Ni3Al prealloyed powder by the addition of ceramic particles to the Ni+25%Al (molar fraction) powder mixture (abbreviated as 3Ni+Al in the text) was presented. Furthermore, the effect of temperature on phase transition in the 70%WC+(3Ni+Al) (mass fraction) mixed powders was investigated in detail, and the thermal effect of the 3Ni+Al powder mixture was studied. In addition, the mechanical properties of WC-30% (Ni3Al-B) (volume fraction) composites prepared by liquid phase sintering through WC+Ni3Al prealloyed powder were compared with commercial cemented carbides YGR45 under similar processing conditions.
2 Experimental
Commercially available pure carbonyl Ni powder (purity 99.80%), coarse WC powder (purity 99.50%) and analytical purity Al powder (purity 99.30%) with average particle sizes of 3, 10 and 15 μm, respectively, were used as starting materials. Figure 1 shows the SEM images of the powders. To obtain 3Ni+Al or 70%WC+(3Ni+Al) mixed powders, Ni and Al powders or Ni, Al and WC powders were ball-mixed in a stainless steel container for 4 h. Stainless steel balls with diameter of 20 mm were used. The mass ratio of ball-to-powder was 1:1.
Thermal analysis was performed on 3Ni+Al mixed powders on a differential scanning calorimeter (DSC, Netzsch STA 449C). The heating was conducted in argon at a constant rate of 10℃/min from 25 to 1400℃. The 70%WC+(3Ni+Al) mixed powders were heated in a vacuum furnace with a vacuum degree of 1 Pa at 550, 750, 900, 1000, 1100 and 1200℃, respectively, and dwelled for 2 h, followed by furnace cooling to room temperature. The heating rate was 2℃/min during the whole process in order to avoid possible SHS procedure. During the heat treatment, all the mixed powder specimens were degassed at 400℃ for 2 h in hydrogen atmosphere. After heat treatment, the morphologies of these powder mixtures were observed on the surface and the cross-section on an optical microscope. All these powder mixtures were ground and sieved to obtain fine powders. Phase identification was done by XRD with Cu Kα radiation.
WC-30% (Ni3Al-B) composites were prepared from WC+Ni3Al prealloyed powder and balanced coarse WC, Ni and B powders by conventional powder metallurgy technology. After sintering, the density was determined by the Archimedes' method. Vickers hardness was determined from polished sample by indentation hardness tester under load of 98 N. Fracture toughness was measured by indentation method [13]. The 3-point bending tests (span length of 15 mm) of the as-sintered samples (5.25 mm×6.5 mm×20 mm) were also done at a cross-head speed of 2 mm/min. The phase compositions were characterized by means of X-ray diffraction. The samples were cut, ground and polished with diamond paste, and examined on an optical microscope and scanning electron microscope (Model JSM-6701F, JEOL, Japan) with an X-ray energy dispersive spectroscopy (EDS) system.
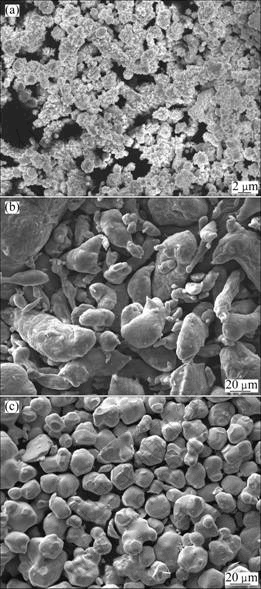
Fig.1 SEM images of powders used: (a) Carbonyl Ni; (b) Al; (c) WC
3 Results
3.1 Differential scanning calorimeter analysis
A simultaneous differential scanning calorimeter (DSC) was used to determine the procedure of the reactions. Since the starting exothermic or endothermic temperature is not well defined, the temperatures corresponding to the peak values in the curve are reported for the reactions. It can be noted that 4 endothermic peaks at about 659, 959, 1035 and 1172℃ while 2 exothermic peaks at about 434 and 848℃ exist, as shown in Fig. 2.
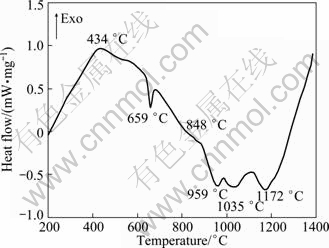
Fig. 2 DSC curve of 3Ni+Al mixed powders
3.2 X-ray diffraction analysis
For the X-ray diffraction analysis, a summary of the X-ray diffraction patterns obtained from the 70%WC+(3Ni+Al) mixed powders by reaction synthesis at different temperatures of 550, 750, 900, 1000, 1100 and 1200℃ is given in Fig. 3. As the temperature rises to 550℃, peaks related to the formation of Ni2Al3, NiAl and Ni3Al appear, but there are still Ni and Al peaks. According to the DSC analysis and the fact that the diffusion rate of Al is higher than that of Ni [14], aluminum-rich intermetallics, namely Ni2Al3, forms at the interface between Ni and Al powders by the solid state interdiffusion below the eutectic temperature of 640℃. When the temperature reaches 750℃, the Al and Ni2Al3 peaks are completely replaced by those corresponding to NiAl and Ni3Al, and the Ni peaks weaken. As the temperature is raised to 900, 1000 and 1100℃, the Ni and NiAl peaks become weak gradually. After being dwelt at 1200℃, the Ni and NiAl peaks almost disappear and Ni3Al becomes the major phase, showing that the reaction is completed and the specimens only consist of Ni3Al and WC.
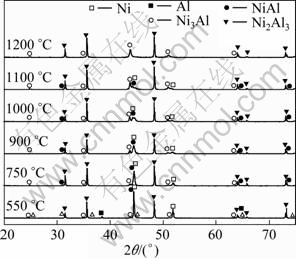
Fig. 3 XRD patterns of 70%WC+(3Ni+Al) mixed powders after reaction synthesis dwelt for 2 h
Figure 4 (a) represents the XRD pattern of the WC-30%(Ni3Al-B) composites. The Ni3Al peaks of WC-30%(Ni3Al-B) composites become left shift compared with the 70%WC+Ni3Al prealloyed powder (Fig. 4(b)) dwelt at 1200℃ for 2 h.
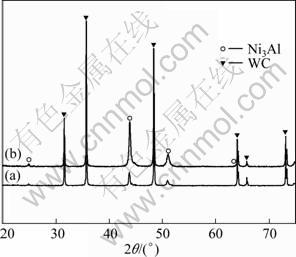
Fig. 4 X-ray diffraction patterns of WC-30%(Ni3Al-B) composites (a) and 70%WC+Ni3Al prealloyed powder (b)
3.3 Morphology of WC+Ni3Al mixture
Figure 5 illustrates the photos of 70%WC+ Ni3Al powder mixture dwelt for 2 h at 1200℃. The morphologies of the appearance and cross section are shown in Figs. 5(a) and (b), respectively, which exhibit uniformity.
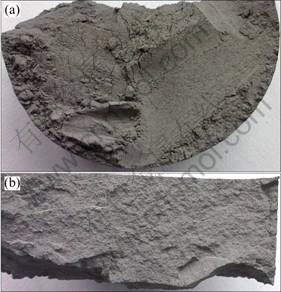
Fig. 5 Photos of 70%WC+Ni3Al powder mixture dwelt at 1200℃ for 2 h: (a) Appearance; (b) Cross-section
Figure 6 shows the back-scattered images of the cross-sections of 70%WC+Ni3Al powder mixture dwelt for 2 h at 1200℃. Different contrasts are seen from these back-scattered images, indicating different phases. EDS analysis was carried out to determine these phases and the results are demonstrated in Fig. 6(b). Glutinous Ni3Al slice appears on the large WC particles. The powder mixture is loose and large amount of pores (black regions in the micrograph) exist.
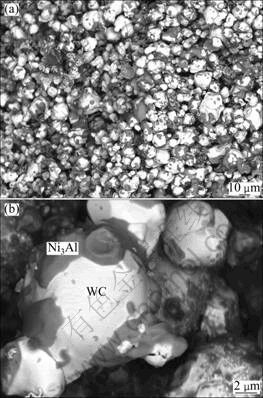
Fig. 6 SEM images of WC+Ni3Al mixture: (a) Lower magnification; (b) Higher magnification
The WC+Ni3Al powder mixture can be obtained between 1100 and 1200℃ and it can be easily crumbled in a grinding mill. The surface topography of the WC+Ni3Al prealloyed powder after grinding is illustrated in Fig. 7. Homogeneous and fine (≤40 μm) WC+Ni3Al prealloyed powder can be observed.
3.4 Microstructure and mechanical properties
The backscattered electron micrographs of WC-30%(Ni3Al-B) and WC–30%(Co-Ni-Cr) samples are shown in Fig. 8. The WC (gray phase) has a relatively uniform distribution in the binder phase Ni3Al (dark area in Fig. 8(a)).
The mechanical properties of the sintered samples are listed in Table 1.
4 Discussion
4.1 Effect of temperature on phase transformation
According to the Ni-Al phase diagram in Fig. 9, the binary system exhibits ?ve stable intermetallic compounds, namely, NiAl3, Ni2Al3, NiAl, Ni5Al3 and Ni3Al. Three of them are detected in this study. The reaction mechanism of the 70%WC+(3Ni+Al) mixed powders can be determined.
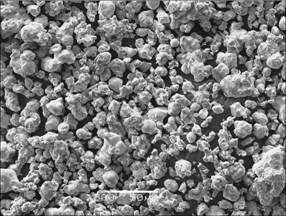
Fig. 7 SEM image of WC+Ni3Al prealloyed powder after grinding
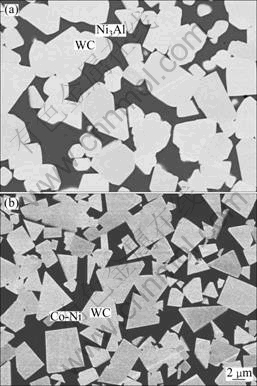
Fig. 8 Backscattered electron micrographs of liquid phase sintering processed WC-30%(Ni3Al-B) (a) and WC-30% (Co-Ni-Cr) samples (b)
Table 1 Mechanical properties of WC-Ni3Al-B composite and WC-Co-Ni-Cr cemented carbide
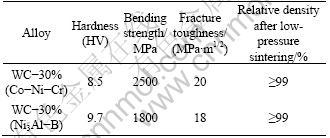
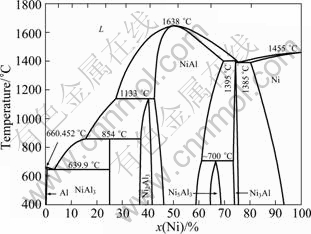
Fig. 9 Ni-Al phase diagram [15]
First, for 3Ni+Al mixed powders, two exothermic peaks are observed at about 434 and 848℃, respectively. A comparison of the temperature of the exothermic peaks and the XRD analysis results at these temperatures suggests that the exothermic peak at about 434℃ mostly results from the formation of Ni2Al3 and the gradient of the curve increases before the appearance of the exothermic peak at about 434℃ (Fig. 2), which indicates that the exothermic reaction (solid diffusion) between solid Ni and Al powders has happened. However, the exothermic peak at about 848℃ may be due to a small amount of melt aluminum-rich (NiAl3) phase and heat emission of further reaction. It is possible that there are so few aluminum-rich phases that can not be detected by instrument at 750℃. Four endothermic peaks appear at about 659, 959, 1035 and 1172℃. It is believed that 659℃ corresponds to the melting point of Al. There is no exothermic peak at around 640℃ in the DSC curve as mentioned in Ref. [16] due to the in?uences of particle size of Al powder and powder state specimens. The particle size of Al powder is bigger than that of Ni powder only when Al melts and begins to diffuse at a high rate, which promotes the reaction synthesis between Ni and Al in turn. The test specimens in the powder state instead of compaction ones can reduce the eutectic reaction degree. The endothermic peaks at about 959, 1035 and 1172℃ may be caused by further reaction in intermediate NiAl.
Second, for 70%WC+(3Ni+Al) mixed powders, WC acts as heat buffer and reduces the contact area between the elemental powders. Intermediates, such as Ni2Al3, NiAl and Ni3Al, are generated during the reaction synthesis depending on the temperature. According to the thermal analysis curve (Fig. 2), Al and Ni peaks disappear at temperature higher than 660 and 1100℃, respectively. As temperature increases, intermediates form at the interface between Ni and Al from 200 to 660℃. A certain amount of intermediates are produced with less amount of Al remaining and interstitial space formed due to the different solid diffusion coefficients of Ni and Al. When the temperature is close to 660℃, Ni3Al and NiAl grow at the expense of Ni2Al3, Ni and Al based on the XRD analysis. Upon further heating, Ni3Al and NiAl become the main phases when the temperature exceeds 660℃. In addition, from 660 to 1100℃, three-phase Ni, Ni3Al and NiAl co-exists and Ni almost disappears at 1100℃. When the temperature reaches 1200℃, the specimen contains uniform Ni3Al and WC, which indicates a complete reaction. Based on the above discussion, the formation sequence of the various phases of 70%WC+(3Ni+Al) mixed powders are described as follows:
Ni+Al→Ni2Al3 (200-660℃) (1)
Ni+Ni2Al3→NiAl+Ni3Al (about 660℃) (2)
Ni+NiAl→Ni3Al (660-1100℃) (3)
Finally, the main point here is that the results from the experiment agree with Ref. [16], which indicates the similar formation phenomenon.
4.2 Effect of liquid phase sintering on microstructure and properties
As it can be seen from Fig. 8, the shape of WC grain in WC-30%(Ni3Al-B) composite becomes round, which is different from the facets shape of WC grain in WC-30%(Co-Ni-Cr). It may be related to the dissolution-precipitation in the sintering process. After liquid phase sintering, the XRD pattern of WC-30% (Ni3Al-B) composites in Fig. 4 presents the Ni3Al peaks are left shifted compared with 70%WC+Ni3Al prealloyed powder, which may be caused by the atom solid-solution in binder Ni3Al during sintering. As listed in Table 1, the density of the sample was measured above 99% of the theoretical density, which is consistent with the very little porosity observed in the microstructure. The sample of WC-30%(Ni3Al-B) has a remarkably high microhardness of 9.7 GPa, inferior bending strength of 1800 MPa and similar fracture toughness of 18 MPa·m1/2 in comparison to the commercial coarse grain cemented carbides YGR45 (WC-30%(Co-Ni-Cr), WC grain size of 2.4 μm).
5 Conclusions
1) The WC+Ni3Al powder mixture can be successfully obtained in the temperature range of 1100-1200℃ and it can be easily crumbled into fine powder due to the formation of pores during the reaction synthesis and powder state specimens instead of compaction specimens.
2) The formation mechanism of Ni3Al depends on the reaction temperature. Besides WC phase, there exist nickel aluminide phases of Ni2Al3, NiAl and Ni3Al in the powder mixture after heat treatment at 200-660℃, while only NiAl and Ni3Al exist at 660-1100℃.
3) WC-30%(Ni3Al-B) composites are prepared from WC+Ni3Al prealloyed powder and balanced coarse WC, Ni and B powders by conventional powder metallurgy technology and the composites can achieve nearly full density. WC-30%(Ni3Al-B) composites exhibit higher hardness, inferior 3-point bending strength and similar fracture toughness in comparison to the commercial coarse grain cemented carbides YGR45 samples.
Acknowledgements
The authors would thank Professors ZHANG Jing-men and KONG Wei-hong in Zhuzhou Cemented Carbide Group Corp. Ltd. for the review of this manuscript.
References
[1] ARISTIZABAL M, RODRIGUEZ N, IBARRETA F, MARTINEZ R, SANCHE Z J. Liquid phase sintering and oxidation resistance of WC–Ni–Co–Cr cemented carbides [J]. International Journal of Refractory Metals & Hard Materials, 2010, 28(4): 516-522.
[2] AHMADIAN M, WEXLER D, CHANDRA T, CALKA A. Abrasive wear of WC–FeAl–B and WC–Ni3Al–B composites [J]. International Journal of Refractory Metals & Hard Materials, 2005, 23(3): 155-159.
[3] PLUCKNETT K P, TIEGS T N, BECHER P F, WATER S B, MENCHHOFER P A. Ductile intermetallic toughened carbide matrix composites [C]// Victor Greenhut. Proceedings of the 20th Annual Conference on Composites: Advanced Ceramics, Materials, and Structures A: Ceramic Engineering and Science Proceedings. Cocoa Beach, Fla, USA: American Ceramic Society, 1996, 17(3): 314-321.
[4] TIEGS T N, ALEXANDER K B, PLUCKNETT K P, MENCHHOFER P A, BECHER P F, WATER S B. Ceramic composites with a ductile Ni3Al binder phase [J]. Materials Science and Engineering A, 1996, 209(1-2): 243-247.
[5] BECHER P F, PLUCKNETT K P. Properties of Ni3Al-bonded titanium carbide ceramics [J]. Journal of the Eurpean Ceramic Society, 1997, 18: 395-400.
[6] DONG Hong-xing, HE Yue-hui. Progress in research on Ni3Al intermetallic alloys [J]. Materials Science and Engineering of Powder Metallurgy, 2009, 14(2): 83-88. (in Chinese)
[7] CHEN Jin-lu, ZHU Ding-yi, LIN Deng-yi. Advances in Ni3Al-based alloys research and application [J]. Materials Review, 2006, 20(1): 35-38.
[8] AOKI K, IZUMI O. Improvement in room temperature ductility of L12 type intermetallic compound Ni3Al by boron addition [J]. J Jpn Met, 1979, 43(12): 1190-1196.
[9] TUMANOV A V, GOSTEV Y V, PANOV V S, KOTS Y F. Wetting of TiC-WC system carbides with molten Ni3Al [J]. Soviet Powders Metall Metal Ceram, 1986, 25(5): 428-430.
[10] AHMADIAN M, WEXLER D, CALKA A. Liquid phase sintering of WC-FeAl and WC-Ni3Al composites with and without boron [C]// Tetsuo S, Torralba J M. THERMEC'2003: International Conference on Processing & Manufacturing of Advanced Materials. University of Virginia, USA: Trans Tech Publications, 2003: 1951-1956.
[11] SUBRAMANIAN R, SCHNEIBEL J H. Intermetallic bonded WC-based cermets by pressureless melt infiltration [J]. Intermetallics, 1997, 5(5): 401-408.
[12] MORSI K, WANG N. Combustion synthesis of microstructurally designed green powders compacts [J]. Materials Science and Engineering A, 2008, 478(1): 208-213.
[13] SHETTY D K, WRIGHT I G, MINCER P N, CLAUER A H. Indentation fracture of WC-Co cermets [J]. J Mater Sci, 1985, 20(5): 1873-1882.
[14] MORSI K. Review: Reaction synthesis processing of Ni-Al intermetallic materials [J]. Materials Science and Engineering A, 2001, 299: 1-15.
[15] FERGUS J W. High temperature corrosion of intermetallic alloys [J]. Shreir's Corrosion, 2010, 1: 646-667.
[16] DONG H X, JIANG Y, HE Y H, SONG Min, ZOU J, XU N P, HUANG B Y, LIU C T, LIAW P K. Formation of porous Ni-Al intermetallics through pressureless reaction synthesis [J]. Journal of Alloys and Compounds, 2009, 484(1): 907-913.
Ni+Al元素粉末法制备WC-Ni3Al-B复合材料
龙坚战1, 2, 张忠健1, 2, 徐 涛1, 2, 彭 文1, 2, 魏修宇1, 2, 陆必志1, 2, 李仁琼1, 2
1. 硬质合金国家重点实验室,株洲 412000;2. 株洲硬质合金集团有限公司,株洲 412000
摘 要:为发展WC-Ni3Al-B复合材料的液相烧结制备技术,研究由羰基Ni粉、分析纯Al粉和粗WC粉的混合粉末反应合成制备的WC+Ni3Al预合金粉末。采用DSC和XRD分别研究3Ni+Al和70%WC+(3Ni+Al)混合粉末在550~1200℃和25~1400℃温度范围的相变过程。结果表明:Ni3Al相的形成取决于反应温度。在200~660℃热处理温度范围内,除了WC相外,还存在Ni2Al3、NiAl和Ni3Al相;而在660~1100℃温度范围内,仅存在NiAl和 Ni3Al相;在1100~1200℃温度范围可以获得均匀的WC+Ni3Al预合金粉末混合物。采用该预合金粉末制备的WC-30%(Ni3Al-B)复合材料具有很高的致密度,且WC晶粒呈圆形。与普通商用YGR45(WC-30% (Co-Ni-Cr))相比,WC-30%(Ni3Al-B)复合材料具有更高的硬度(9.7 GPa),低的抗弯强度(1800 MPa)和相近的断裂韧性(18 MPa·m1/2 )。
关键词:WC-Ni3Al-B复合材料;Ni3Al金属间化合物;液相烧结;力学性能
(Edited by FANG Jing-hua)
Foundation item: Project (2012CB723906) supported by the National Basic Research Program of China
Corresponding author: LONG Jian-zhan; Tel: +86-731-28265634; E-mail: longjianzhan@yahoo.com.cn
DOI: 10.1016/S1003-6326(11)61255-7