J. Cent. South Univ. (2012) 19: 85-92
DOI: 10.1007/s11771-012-0976-7
Macro kinetics for synthesis of dimethyl carbonate from urea and methanol on Zn-containing catalyst
ZHAO Wen-bo(赵文波)1, 2, HAN Bing(韩冰)1, ZHAO Ning(赵宁)2, XIAO Fu-kui(肖福魁)2, WEI Wei(魏伟)2
1. Faculty of Chemical Engineering, Kunming University of Science and Technology, Kunming 650224, China;
2. Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract: In a stainless steel autoclave, the synthesis kinetics of dimethyl carbonate (DMC) from urea and methanol was separately investigated without catalyst and with Zn-containing catalyst. Without catalyst, for the first reaction of DMC synthesis (the reaction of urea with methanol to methyl carbamate (MC)), the reaction kinetics can be described as the first order with respect to the concentrations of both methanol and urea. For the second reaction of DMC synthesis (the reaction of MC with methanol to DMC), the results exhibit characteristics of zero-order reaction. For Zn-containing catalyst, the first reaction is neglected in the kinetics model since its rate is much faster than the second reaction. The macro-kinetic parameters of the second reaction are obtained by fitting the experimental data to a pseudo-homogenous model, in which a side reaction in forming process of DMC is incorporated since it decreases the yield of DMC drastically at high temperature. The activation energy of the reaction from MC to DMC is 104 kJ/mol while that of the side reaction of DMC is 135 kJ/mol. The highest yield of DMC is 23%.
Key words: macro-kinetics; dimethyl carbonate; urea; methanol; methyl carbamate
1 Introduction
Dimethyl carbonate (DMC), as an important green chemical raw material, has attracted much attention in recent years [1-5]. Since its molecule includes CH3—, CH3O—, CH3O—CO— and —CO— groups, it could replace phosgene, dimethyl sulphate, chloromethane and methyl chloroformate as carbonylation, methylation, esterification or ester interchange reagent. It could also be used in many fields, such as medicine, pesticide, composite material, dyestuff, additive of gasoline, flavoring agent of foodstuff, and electronic chemical. The DMC synthesis techniques reported mainly consist of phosgenation of methanol, oxidative carbonylation of methanol, ester exchange, esterification of carbon dioxide methanol etc. But all of them suffer from corresponding shortcomings such as poisonous characteristics, easy explosion, complex reaction course and extremely low conversion.
A route of the DMC synthesis from urea and methanol was developed for low cost and facile separation of production. During this course of DMC synthesis, water was not produced to form methanol-water-DMC ternary azeotrope, therefore the subsequent purification of DMC was simplified greatly. In this synthesis approach, urea reacted with methanol to produce intermediate methyl carbamate (MC), which further converted to DMC by reaction with methanol. Besides, byproduct N-methyl methyl carbamate (NMMC) was produced via the reaction of DMC and MC, which was a main side reaction of this route. The reaction mechanism could be described by following expressions [6]:
NH2CONH2+CH3OH→NH2COOCH3+NH3 (1)
NH2COOCH3+CH3OH→CH3OCOOCH3+NH3 (2)
CH3OCOOCH3+NH2COOCH3→
CH3NHCOOCH3+ CH3OH (3)
The liberated ammonia during the first two reactions, which can be recycled for urea synthesis, must be removed from the reacting system on time, otherwise the DMC yield would be relatively low.
For the above DMC synthesis route, many compounds such as K2CO3, CH3ONa and CaO could be used as catalyst, but the DMC yield was far from satisfactory [7]. Among all catalysts, zinc compounds were considered as the best catalysts for their innocuity, high selectivity and conversion [8-9]. Furthermore, compared with acidity or basicity of metal oxides, they did not promote the thermal decomposition of DMC [6, 10]. In the reaction of urea and diol, zinc compounds also showed excellent catalytic performance [11-13]. Based on these previous researches [14-15], a new Zn-containing industrial heterogeneous catalyst has been developed by our group, which showed excellent catalytic performance [16].
Up to now, the kinetics about this DMC synthesis route, which could provide useful information for further investigations of the simulation and design of DMC synthesis reactor, has been rarely reported. In a previous work, the activation energy of 110 kJ/mol for the reaction of urea to MC without catalyst was determined by using pseudo-first-order model [17]. Whereas, the further kinetics research for the reaction of MC to DMC without catalyst was not performed. There are a few contributions focused on the kinetics study of DMC synthesis by using a coupling reagent or homogenous catalyst [18-19]. By taking organotin as homogenous catalyst and high boil electron donor solvent as a co-catalyst, the activation energies for the reactions of urea to MC and MC to DMC were obtained by fitting the experimental data to a kinetics model using genetic algorithm, whose values were 98.1 kJ/mol and 107 kJ/mol, respectively [19]. However, the kinetics study with heterogeneous catalyst was scarce.
In the present work, the kinetics research of DMC synthesis from urea and methanol was carried out without catalyst, and then for the first time with the Zn-containing heterogeneous catalyst devised by our research team. Kinetics experiments without catalyst were performed in two independent steps, the reactions of urea to MC and MC to DMC. But kinetics experiments on the Zn-containing heterogeneous catalyst were carried out without being divided into two separated steps. All the experiments were carried out in a stainless steel stirring autoclave with autogenous pressure, i.e. the sum of vapor pressure of methanol, ammonia and MC at the reaction temperature.
2 Experimental
Urea, methanol, MC, DMC, isopropyl alcohol are all commercial reagents with the purities of the reagents greater than 99.9%. Catalyst was prepared according to the patent [16]. The method for preparing catalyst comprised the following steps: 1) Preparing an aqueous solution of soluble salt of Zn; 2) Adjusting pH value of the solution to 0-5; 3) Spraying and impregnating the aqueous solution on the carrier by equal-volume spraying and impregnating process; 4) Preparing an active component-supported carrier; 5) Drying the active component-supported carrier at a temperature from 373 to 423 K for 2-24 h; and at a temperature from 773 to 1 273 K for 2-12 h. The catalyst comprised 20%-50% (mass fraction) of an active component and 80%-50% (mass fraction) of a carrier component. The carrier component comprised at least one carrier selected from the group consisting of active carbon, alpha-alumina, lamda-alumina, silica, and molecular sieve.
DMC was synthesized in a 350 mL autoclave reactor with a reflux column under the assigned conditions. The temperature was controlled by a PID temperature controller with error less than 1 K. Reactant and catalyst were put into autoclave first, and then were rapidly heated to the desired temperature with stirring. Methanol in experiment not only was reactant, but also was solvent. After the reaction temperature was steady, sample was fetched at interval and the corresponding sampling time was recorded. Some residual liquid of last time sampling in the sample pipe was thrown out before the next sampling. The products were determined by gas chromatogram (Shanghai Haixin GC-920) configured with the GDX-203 column and thermal conductivity detector (TCD) using isobutyl alcohol as internal standard. Because urea cannot be analyzed by gas chromatogram due to the decomposition at high temperature, its concentration was got by the equilibrium of materials.
3 Results and discussion
3.1 Kinetic model and parameter estimation without catalyst
Because the yield of MC could reach 95% before the appearance of DMC in experiments, i.e., the reaction rate of MC synthesis was much faster than that of the following DMC synthesis. The kinetics experiments without catalyst were performed in two isolated steps for simplification.
In MC synthesis from urea and methanol, the concentration of MC over time was measured at several fixed temperatures (403-433 K). Sampling began when stable temperature was reached, and stopped once DMC appeared. The concentration curves of MC over time are shown in Fig. 1(a). It was not unexpected to observe that the concentration of MC was positively correlated with the reaction time, and increased faster with the increase of temperature. For instance, the MC concentration in solution after certain time at different temperatures ranked in this order: 433 K>423 K>413 K>403 K. At given temperature, the production rate of MC at the initial period of experiment was faster than that at the latter period. Thus, according to reaction expression (4), the reaction model equation is described as follows:
 (4)
(4)
where Cmc, Curea and Cme are the molar concentrations of MC, urea and methanol in solution at a given time, respectively; t is the reaction time; k is the reaction rate constant.

Fig. 1 MC concentration profile at different temperatures: (a) Cmc versus time; (b) 
 versus time
versus time
According to equilibrium of materials, Eq. (4) could be changed to
 (5)
(5)
where Curea0 and Cme0 are the initial molar concentrations of urea and methanol, respectively. Equation (6) was got by integrating Eq. (5):
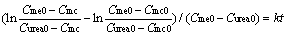 (6)
(6)
A plot of 
 versus reaction time gives a straight line with the slope of k (see Fig. 1(b)).
versus reaction time gives a straight line with the slope of k (see Fig. 1(b)).
The rate constant k for the MC synthesis computed by linear regression at every temperature is listed in Table 1. They are 1.15×10-6, 2.77×10-6, 4.15×10-6, 7.01×10-6 L·mol-1·s-1 for 403, 413, 423 and 433 K, respectively. The linear correlation coefficients r are all close to 1. The results showed that rate constants increased with increasing the temperature.
In Fig. 2, the negative logarithm of rate constant is plotted against the reciprocal temperature. Based on the four runs of the present study, the kinetics activation energy was determined to be 84.7 kJ/mol and the preexponential factor was evaluated as 1.20×106 L·mol-1·s-1 (see Table 1).
Table 1 Kinetics parameters and correlation coefficient for synthesis of MC
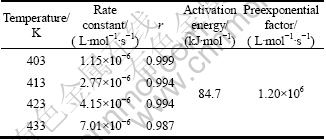

Fig. 2 Arrhenius plots for synthesis of MC
Figure 3 shows the distribution of residues of the calculation values predicted by Eq. (6). The relative residues of the experimental runs were below 8% and were stochastic. This meant that the above kinetic model equations were suitable.
In the second step (DMC synthesis directly from MC and methanol), kinetics studies were performed over a temperature range of 453-483 K. At high temperatures, urea could convert to MC completely in a short time. Thus, after urea has converted completely, we began to withdraw sample at intervals and corresponding sampling time was recorded. The DMC concentration profile versus time at different temperatures is illustrated in Fig. 4.

Fig. 3 Residue distribution for model predicting MC concentration

Fig. 4 DMC concentration profile at different temperatures
It was clear that the concentration of DMC was in a good linear correlation with time. This indicated that the synthesis of DMC from MC and methanol was a zero-order reaction, as shown in the model equation (7):
 (7)
(7)
The reaction rate constants for the DMC synthesis from MC at different temperatures obtained by linear fitting are 1.65×10-6, 2.97×10-6, 4.01×10-6, 5.93×10-6 (mol·L-1·s-1) for 453, 463, 473 and 483 K, respectively (Table 2).
Table 2 Kinetics parameters and correlation coefficient for synthesis of DMC
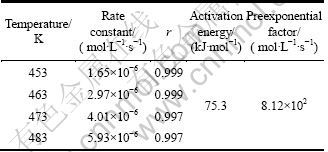
From the results of Fig. 5, we could deduce that the reaction activation energy was 75.3 kJ/mol and preexponential factor was 8.12×102 mol·L-1·s-1 (Table 2).
The comparison of all the experimental points and their corresponding values predicted by this model is plotted in Fig. 6, which indicated that the model fitted the experimental data reasonably well.

Fig.5 Arrhenius plots for synthesis of DMC
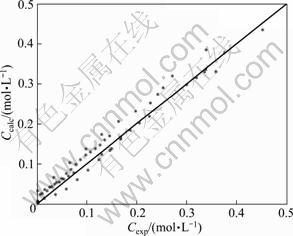
Fig. 6 Comparison between experimental and predicted concentrations for experimental points in DMC synthesis without catalyst
By comparing with the kinetics parameters of second step, it was found that the activation energy of the first step was a little higher (84.7 kJ/mol vs 75.3 kJ/mol), but its preexponential factor was much larger (1.20×106 L·mol-1·s-1 vs 8.12×102 mol·L-1·s-1), thus its reaction rate constant at the same temperature was about 10 times that of the second step. In other words, according to Eqs. (4) and (7), the rate of MC synthesis was about 200 times that of MC consume.
3.2 Kinetic model and parameter estimation over Zn-containing catalyst after optimization of reaction condition
3.2.1 Eliminating external and internal diffusion
The external diffusion resistance can be eliminated by accelerating stirring speed. The stirring speed of 1 000 r/min adopted in the experiment was fast enough to eliminate external diffusion resistance, i.e., the DMC yield did not change with the further increase of stirring speed. The inner diffusion resistance decreases with the reduction of catalyst diameter. Figure 7(a) showed that the DMC yield approximately kept stable by taking the catalyst with diameter less than 80 μm, that is to say, the inner diffusion was efficiently eliminated by this catalyst. Therefore, the catalyst with diameter less than 80 μm was chosen in the following experiment.
3.2.2 Effect of catalyst loading
Under the condition of 463 K, 10 h, and methanol- to-urea molar ratio of 20, the effect of catalyst loading on DMC yield is shown in Fig. 7(b). It was found that the DMC yield increased sharply with the increase of catalyst amount till the maximum value of 11.3%, and then began to decrease when the catalyst amount was more than 3.00 g. This could be attributed to the side reaction of MC and DMC, which was also accelerated with the increase of catalyst amount. Hence, the catalyst amount of 3.00 g was selected for the following experiments.
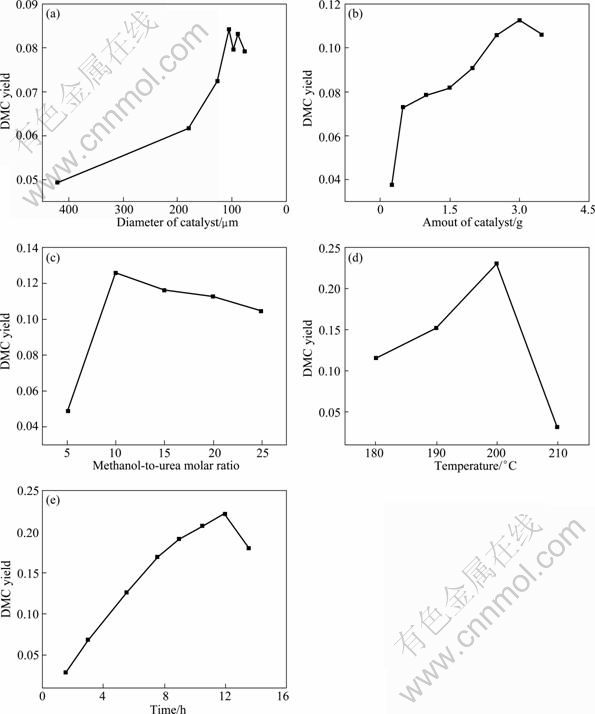
Fig. 7 Effects of reaction condition on DMC yield: (a) Particulate size of catalyst; (b) Amount of catalyst; (c) Methanol-to-urea molar ratio; (d) Reaction temperature; (e) Reaction time
3.2.3 Effect of methanol-to-urea molar ratio
The molar ratio of methanol to urea varied from 5 to 25, under the condition of 463 K, 10 h, and catalyst amount 3.00 g. With the increase of methanol-to-urea molar ratio, the yield of DMC passed through a maximum 12.5% at a methanol-to-urea molar ratio of 10, as seen in Fig. 7(c). When the molar ratio was less than 10, the urea concentration was so high that the decomposition of urea might be caused via unclear route, which resulted in low DMC yield. When the molar ratio was more than 10, the DMC yield fell slightly. This may be attributed to the slower reaction rate with the decline of urea concentration. Therefore, the methanol-to-urea initial molar ratio of 10:1 was selected in all the following experiments.
3.2.4 Effect of reaction temperature
Figure 7(d) demonstrates the effect of reaction temperature on DMC yield under the condition of 10 h, methanol-to-urea molar ratio 10, and catalyst amount 3.00 g. The DMC yield increased with the increase of temperature and decreased rapidly after 473 K. In theory, high temperature was benefit for the synthesis of DMC because this reaction was an endothermic reaction. However, the high temperature also accelerated the side reaction rate.
3.2.5 Effect of reaction time
Figure 7(e) illustrates the effect of reaction time on the yield of DMC, under the reaction condition of 473 K, methanol-to-urea molar ratio 10, and catalyst amount 3.00 g. The DMC yield increased before 12 h. At the same time, the side reaction increased gradually, which led to the consumption rate of DMC faster than the forming rate. At last, the DMC yield declined after 12 h.
3.2.6 Kinetic model and parameter estimation
In the presence of Zn-containing catalyst, just the reaction kinetics of MC to DMC was taken into account, since it was the rate-control step. The amount of solvent methanol was large in reaction, thus it can be considered as a constant. MC cannot evaporate for the high autogenous pressure in reaction vessel. It was a liquid-solid catalytic reaction for the synthesis of DMC and the volume of reaction solution did not change with the reaction process. Most parts of the small molecular byproduct ammonia and carbon dioxide were considered to be separated from liquid for the distillation effect of reflux column. Thus, the reverse reactions in the synthesis of DMC could be neglected. By taking pseudo- homogeneous kinetics model, the reaction equations could be described as follows:
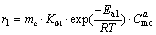 (8)
(8)
 (9)
(9)
 (10)
(10)
 (11)
(11)
 (12)
(12)
In these function, r1 and r2 are reaction rates; mc is the amount of catalyst; K01 and K02 are preexponential factors; Ea is activation energy; R is universal gas constant; T is reaction temperature; a, b, c are power exponents.
Defining

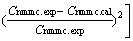 (13)
(13)
where M is the number of experimental run, with concentrations measured at N reaction times. The fourth- order Runge-Kutta method was used to solve Eqs. (10)- (12), and the simplex method was used in the nonlinear optimizations. By minimizing the objective function (13) with Matlab Optimization Toolbox, the parameters of kinetic model were obtained, as listed in Table 3.
Table 3 Kinetic parameters for synthesis of DMC on Zn-containing catalyst

By comparing with the reaction of MC and methanol without catalyst, it was found that the activation energy of the reaction with Zn-containing catalyst was higher than the former (104 kJ/mol vs 84.7 kJ/mol), but its preexponential factor was much larger (2.35×106 s-1 vs 8.12×102 mol·L-1·s-1). This unusual phenomenon could be considered as kinetics compensation effect. According to the collision theory, the activation energy was related with the threshold value of molecular energy for reaction, and preexponential factor was related with the frequency of molecular collision. Thus, the experimental results indicated that the main effect of catalyst was to increase the collision frequency, but was not to reduce the required energy of bond breaking.
The model-predicted reactant concentrations profile and the experimental data are plotted in Fig. 8. Furthermore, the comparison of all the experimental points and their corresponding predicted values is also provided in Fig. 9. As can be seen from these figures, the proposed model fitted the experimental data reasonably well. The highest residual was no more than 15%. The high prediction accuracy indicated that this model can be used for the DMC synthesis reactor analysis and design.

Fig. 8 Experimental and predicted DMC (a), MC (b) and NMMC (c) concentration profiles at different temperatures

Fig. 9 Comparison between experimental and predicted concentrations for all experimental points in DMC synthesis with Zn-containing catalyst: (a) DMC; (b) MC; (c) NMMC
4 Conclusions
1) The kinetics investigations of the synthesis of DMC from urea and methanol without catalyst and with Zn-containing catalyst are individually carried out in a stainless steel batch.
2) Without catalyst, the activation energies for the reaction of urea to MC and MC to DMC are 84.7 kJ/mol and 75.3 kJ/mol, respectively.
3) On Zn-containing catalyst, the experimental results are properly described with a pseudo- homogenous model. The activation energies for the DMC synthesis and its consecutive reaction are 104 kJ/mol and 135 kJ/mol, respectively. The highest yield of DMC reaches 23%. The residual analysis further proves that the model is adequate to provide support information for the further investigation of DMC synthesis reactor simulation.
References
[1] SHAIKH A A, SIVARAM S. Organic carbonates [J]. Chemical Reviews, 1996, 96(3): 951-976.
[2] TUNDO P. New developments in dimethyl carbonate chemistry [J]. Pure and Applied Chemistry, 2001, 73(7): 1117-1124.
[3] DELLEDONNE D, RIVETTI F, ROMANO U. Developments in the production and application of dimethylcarbonate [J]. Applied Catalysis A: General, 2001, 221(1/2): 241-251.
[4] PACHECO M A, MARSHALL C L. Review of dimethyl carbonate (DMC) manufacture and its characteristics as a fuel additive [J]. Energy & Fuels, 1997, 11(1): 2-29.
[5] ONO Y. Catalysis in the production and reactions of dimethyl carbonate, an environmentally benign building block [J]. Applied Catalysis A: General, 1997, 155(2): 133-166.
[6] ANDERSON S A, MANTHATA S, ROOT T W. The decomposition of dimethyl carbonate over copper zeolite catalysts [J]. Applied Catalysis A: General, 2005, 280(2): 117-124.
[7] WANG Mou-hua, WANG Hui, ZHAO Ning, WEI Wei, SUN Yu-han. Synthesis of dimethyl carbonate from urea and methanol over solid base catalysts [J]. Catalysis Communications, 2006, 7(1): 6-10.
[8] WANG Mou-hua, ZHAO Ning, WEI Wei, SUN Yu-han. Synthesis of dimethyl carbonate from urea and methanol over ZnO [J]. Industrial & Engineering Chemistry Research, 2005, 44(19): 7596-7599.
[9] WANG Mou-hua, WANG Hui, ZHAO Ning, WEI Wei, SUN Yu-han. High-yield synthesis of dimethyl carbonate from urea and methanol using a catalytic distillation process [J]. Industrial & Engineering Chemistry Research, 2007, 46(9): 2683-2687.
[10] FU Yu-chuan, ZHU Hai-yan, SHEN Jian-yi. Thermal decomposition of dimethoxymethane and dimethyl carbonate catalyzed by solid acids and bases [J]. Thermochimica Acta, 2005, 434(1/2): 88-92.
[11] BHANAGE B M, FUJITA S, IKUSHIMA Y, ARAI M. Transesterification of urea and ethylene glycol to ethylene carbonate as an important step for urea based dimethyl carbonate synthesis [J]. Green Chemistry, 2003, 5(4): 429-432.
[12] LI Qi-biao, ZHANG Wen-yu, ZHAO Ning, WEI Wei, SUN Yu-han. Synthesis of cyclic carbonates from urea and diols over metal oxides [J]. Catalysis Today, 2006, 115(1/4): 111-116.
[13] ZHAO Xin-qiang, ZHANG Yan, WANG Yan-ji. Synthesis of propylene carbonate from urea and 1,2-propylene glycol over a zinc acetate catalyst [J]. Industrial & Engineering Chemistry Research, 2004, 43(15): 4038-4042.
[14] ZHAO Wen-bo, WANG Feng, PENG Wei-cai, ZHAO Ning, LI Jun-ping, XIAO Fu-kui, WEI Wei, SUN Yu-han. Synthesis of dimethyl carbonate from methyl carbamate and methanol with zinc compounds as catalysts [J]. Industrial & Engineering Chemistry Research, 2008, 47(16): 5913-5917.
[15] ZHAO Wen-bo, PENG Wei-cai, WANG Deng-feng, ZHAO Ning, LI Jun-ping, XIAO Fu-kui, WEI Wei, SUN Yu-han. Zinc oxide as the precursor of homogenous catalyst for synthesis of dialkyl carbonate from urea and alcohols [J]. Catalysis Communications, 2009, 10(5): 655-658.
[16] SUN Yu-han, WEI Wei, ZHAO Ning. Catalyst for the synthesis of dimethyl carbonate from urea and methanol, preparation and use. US Patent 2006047136 [P]. 2006.
[17] SUN Jian-jun, YANG Bo-lun, LIN Hong-ye. A semicontinuous process for the synthesis of methyl carbamate from urea and methanol [J]. Chemical Engineering & Technology, 2004, 27(4): 435-439.
[18] SUN Jian-jun, YANG Bo-lun, WANG Xiao-ping, WANG Dong-peng, LIN Hong-ye. Synthesis of dimethyl carbonate from urea and methanol using polyphosphoric acid as catalyst [J]. Journal of Molecular Catalysis A: Chemical, 2005, 239(1/2): 82-86.
[19] LIN Hong-ye, YANG Bo-lun, SUN Jian-jun, WANG Xiao-ping, WANG Dong-peng. Kinetics studies for the synthesis of dimethyl carbonate from urea and methanol [J]. Chemical Engineering Journal, 2004, 103(1/3): 21-27.
(Edited by YANG Bing)
Foundation item: Project(2010ZC034) supported by the Science Foundation of Yunnan Province, China; Project(20105314120005) supported by the Research Fund for Doctor Program of Higher Education of China; Project(11-12-609) supported by the Open Foundation of State Key Laboratory of Coal Conversion, China; Project(KKJD201051012) supported by the Scientific Research Fund of Yunnan Provincial Education Department, China; Project(2009-096) supported by the Analysis and Measure Foundation of Kunming University of Science and Technology, China
Received date: 2010-11-18; Accepted date: 2011-05-02
Corresponding author: ZHAO Wen-bo, PhD; Tel: +86-871-3801114; E-mail: zhaowenbo@yahoo.cn