Conversion and reaction kinetics of coke oven gas over a commercial Fe-Mo/Al2O3 catalyst
来源期刊:中南大学学报(英文版)2016年第2期
论文作者:屈一新 徐贺明 赵见峰 王志彦 王亚涛
文章页码:293 - 302
Key words:coke oven gas; conversion; Fe-Mo/Al2O3 catalyst; sulfur-containing compound; kinetics
Abstract: Producing methanol from coke oven gas (COG) is one of the important applications of COG. Removal of sulfur from COG is a key step of this process. conversion and reaction kinetics over a commercial Fe-Mo/Al2O3 catalyst (T-202) were studied in a continuous flow fixed bed reactor under pressures of 1.6-2.8 MPa, space time of 1.32-3.55 s and temperatures of 240-360 °C. Though the COG contains about 0.6 mol/mol H2, hydrogenation of CO and CO2 is not significant on this catalyst. The conversions of unsaturated hydrocarbons depend on their molecular structures. Diolefins and alkynes can be completely hydrogenated even at relatively low temperature and pressure. Olefins, in contrast, can only be progressively hydrogenated with increasing temperature and pressure. The hydrodesulfurization (HDS) of CS2 on this catalyst is easy. Complete conversion of CS2 was observed in the whole range of the conditions used in this work. The original COS in the COG can also be easily converted to a low level. however, its complete HDS is difficult due to the relatively high concentration of CO in the COG and due to the limitation of thermodynamics. H2S can react with unsaturated hydrocarbons to form ethyl mercaptan and thiophene, which are then progressively hydrodesulfurized with increasing temperature and pressure. Based on the experimental observations, reaction kinetic models for the conversion of ethylene and sulfur-containing compounds were proposed; the values of the parameters in the models were obtained by regression of the experimental data.
J. Cent. South Univ. (2016) 23: 293-302
DOI: 10.1007/s11771-016-3073-5

QU Yi-xin(屈一新)1, XU He-ming(徐贺明)1, 2, ZHAO Jian-feng(赵见峰)1,
WANG Zhi-yan(王志彦)2, WANG Ya-tao(王亚涛)2
1. College of Chemical Engineering, Beijing University of Chemical Technology, Beijing 100029, China;
2. Coal Chemical Research and Development Center of Kailuan Group, Tangshan 063611, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract: Producing methanol from coke oven gas (COG) is one of the important applications of COG. Removal of sulfur from COG is a key step of this process. conversion and reaction kinetics over a commercial Fe-Mo/Al2O3 catalyst (T-202) were studied in a continuous flow fixed bed reactor under pressures of 1.6-2.8 MPa, space time of 1.32-3.55 s and temperatures of 240-360 °C. Though the COG contains about 0.6 mol/mol H2, hydrogenation of CO and CO2 is not significant on this catalyst. The conversions of unsaturated hydrocarbons depend on their molecular structures. Diolefins and alkynes can be completely hydrogenated even at relatively low temperature and pressure. Olefins, in contrast, can only be progressively hydrogenated with increasing temperature and pressure. The hydrodesulfurization (HDS) of CS2 on this catalyst is easy. Complete conversion of CS2 was observed in the whole range of the conditions used in this work. The original COS in the COG can also be easily converted to a low level. however, its complete HDS is difficult due to the relatively high concentration of CO in the COG and due to the limitation of thermodynamics. H2S can react with unsaturated hydrocarbons to form ethyl mercaptan and thiophene, which are then progressively hydrodesulfurized with increasing temperature and pressure. Based on the experimental observations, reaction kinetic models for the conversion of ethylene and sulfur-containing compounds were proposed; the values of the parameters in the models were obtained by regression of the experimental data.
Key words: coke oven gas; conversion; Fe-Mo/Al2O3 catalyst; sulfur-containing compound; kinetics
1 Introduction
Coke oven gas (COG) is a by-product of coal carbonization process [1-3]. China has rich coal resources and its coke production ranks the first in the world. The COG production reached 1.9×1011 m3 in 2012 [2]. In the past, COG was used mainly as a fuel or even was simply burned off. With the advantage of technology for the application of COG, today most of COG is used as feedstock for production of valuable chemicals, such as, methanol. The annual capacity of methanol derived from COG in China is about 8.5×106 t in 2013 [3].
The composition of raw COG leaving a coke oven is complex, containing H2, CH4, CO, N2, hydrocarbons, tar, as well as nitrogen- and sulfur-containing compounds [2]. After a series of purification processes, most of the heavy hydrocarbons, tar, nitrogen- and sulfur-containing compounds in the raw COG are removed, and the purified COG contains mainly H2 (55%-60% in mole fraction), CO (5%-8% in mole fraction), N2 (3%-6% in mole fraction), CO2 (~2% in mole fraction), CH4 (23%-27% in mole fraction) and C2-C6 hydrocarbons (2%-3% in mole fraction). In addition, small amount of sulfur-containing compounds is still present [1-2]. If the COG is used for methanol synthesis, the small amount of sulfur-containing compounds has to be further removed via hydrodesulfurization (HDS) process since they are poisons to the methanol synthesis catalyst.
In the HDS process, hydrogenation of the unsaturated hydrocarbons releases large amount of heat, which imposes difficulty to carry out the HDS of COG by using a single reactor. Therefore, in industrial plants two reactors are usually employed in the HDS unit. The first reactor, containing a Fe-Mo/Al2O3 catalyst (T-202) and operated at temperatures of 300-360 °C and pressures of 2-2.4 MPa, is mainly for the saturation of ethylene and other unsaturated hydrocarbons and for the conversion of most of the sulfur-containing compounds to H2S. The second reactor, containing a Fe-Mo/Al2O3 catalyst (JT-8) and operated at temperatures of 360- 380 °C and pressures of 2.0-2.3 MPa, is mainly for the conversion of the remaining sulfur-containing compounds to H2S.Though the HDS units in methanol synthesis plants using COG as feedstock have been used for more than a decade in China, the operation of the reactors mainly relies on experience since no detailed report concerning the conversion and reaction kinetics of COG under the HDS conditions can be found in the open literature. In this work, the conversion and reaction kinetics of COG coming from an industrial plant on the T-202 catalyst are studied in a continuous flow fixed bed reactor in order to provide detailed information that is required for the optimization of the HDS reactor operation.
2 Experimental
2.1 Catalyst
The T-202 catalyst is a commercial product, which contains about 3% Fe2O3 (mass fraction) and 9% MoO3 (mass fraction) as the active components and Al2O3 as a support. The catalyst has a BET specific surface area of 296 m2/g, an average pore diameter of 3.44 nm and a pore volume of 0.314 ml/g (BJH method) measured by N2 adsorption.
2.2 Feed
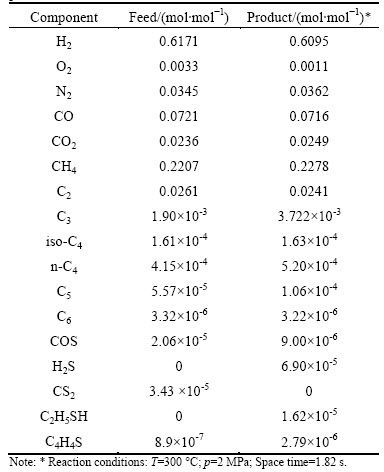
The COG feed is directly from an industrial plant, of which composition is given in Table 1. It contains H2 (~62% in mole fraction), CH4 (~22% in mole fraction), N2 (~3.5% in mole fraction), CO (~7.2% in mole fraction), CO2 (~2.4% in mole fraction), O2 (~0.3% in mole fraction) and C2-C6 hydrocarbons (~2.9% in mole fraction). The sulfur-containing compounds are COS (2.06×10-5 mol/mol), CS2 (3.43×10-5 mol/mol) and thiophene (C4H4S, 8.9×10-7 mol/mol).
2.3 HDS apparatus
The experiments were carried out in a continuous flow fixed bed reactor. The COG was pressurized with a booster pump, dried with a silica gel column, heated in a preheater to about 200 °C and then introduced into the reactor. The flow rate of the COG was controlled by a mass flow controller. The reactor was made of stainless steel with an internal diameter of 12 mm and a length of 600 mm. A thermocouple well with an outer diameter of 3 mm was inserted into the reactor to measure the temperature of the catalysts bed. The reactor was heated by an electric resistance furnace containing three heating blocks to maintain the catalyst bed isothermal. Typically, 3.85 g catalyst with sizes of 0.18-0.25 mm was packed in the middle section of the reactor. The pressure of the reaction system was controlled using a backpressure regulator. The reaction products were first cooled by a water cooler and then introduced into a gas-liquid separator. The composition of the product gas was determined on line by gas chromatographs (GCs).
Table 1 Typical compositions of COG feed and HDS reaction product
2.4 Presulfidation of catalyst
The catalyst was presulfided at pressure of 1.0 MPa using COG and CS2 which was diluted with n-hexane. The concentration of CS2 in n-hexane was 1% in mass fraction. The flow rate of COG was 2000 ml/h. The catalyst was first dried and presulfided using COG at 200 °C for 2 h. Then, the mixture of CS2 and n-hexane was injected into the reactor at a rate of 6 ml/h, meanwhile the temperature of the catalyst was raised to 370 °C at a rate of 85 °C/h. After 5 h, injection of the mixture of CS2 and n-hexane was stopped; the pressure decreased to 0.1 MPa; the temperature was raised to 400 °C in 1 h and kept at 400 °C for 2 h to remove the physically adsorbed sulfur-containing compounds.
2.5 Analysis
The sulfur-containing compounds were analyzed using an Agilent-7890A GC equipped with a GS-GasPro column (30 m×0.32 mm) and a flame photometric detector. Nitrogen with a purity of 99.99% was used as a carrier gas. The sulfur-containing compounds were identified by comparison of their retention time with that of the sulfur-containing compounds of a standard calibration gas and quantified using external standard method.
H2, O2, N2, CO, CO2 and CH4 were analyzed on a FuLi-GC-9790ⅡGC equipped with a thermal conductivity detector using helium (99.99%) as carrier gas. A 5A molecular sieve column (3 mm×1.5 m) was used to separate H2, O2, N2 and CH4, and a TDX-01 column (3 mm×1.5 m) was used to separate CO2 and CH4. The hydrocarbons contained in COG were also analyzed on the Agilent-7890A GC with a KB-Al2O3/Na2SO4 column (0.53 mm×30 m×20 μm) and a flame ionization detector using nitrogen (99.99%) as carrier gas.
The concentration of the all components except for sulfur-containing compounds was determined in terms of calibrated area normalization method which uses CH4 as the common component to scale the GC areas obtained from three columns, i.e. 5A molecular sieve, TDX-01 and KB-Al2O3/Na2SO4. The calibration coefficients of the components were determined by analyzing standard calibration gases.
2.6 Internal and external diffusion effect test
In this work, determination of the presence of internal and external diffusion effect was a complicated task since the conversion of COG on the T-202 catalyst involves many compounds that may participate in different types of reactions such as hydrogenation of unsaturated hydrocarbons, formation and subsequent HDS of the sulfur-containing compounds. In this process, the hydrogenation of ethylene is one of the most concerned reactions, and its relatively high concentration allows its conversion to be precisely determined in a wide range of space time. Therefore, hydrogenation of ethylene was selected for the test of the presence of internal and external diffusion effect.
The effect of external diffusion was examined by varying the mass of the catalyst. Two series of experiments were carried out at 2.0 MPa and 300 °C with 3.85 and 7.70 g catalyst. The influence of the catalyst mass on the ethylene conversion is shown in Fig. 1. It can be seen that the effect of external diffusion on hydrogenation of ethylene is not obvious when the space time is in the range of 0.5-7.2 s.
The effect of internal diffusion was examined using 3.85 g catalysts with particle sizes of 0.18-0.25 mm and 0.25-0.42 mm. The experiments were carried out at 2.0 MPa and 300 °C. The results shown in Fig. 2 indicate that the effect of internal diffusion for the hydrogenation of ethylene can be eliminated when the particle size of the catalyst is smaller than 0.42 mm. To ensure the internal diffusion effect on ethylene hydrogenation being eliminated, the subsequent experiments were carried out with catalyst of 0.18-0.25 mm. The conditions determined above, therefore, warrant that the kinetics of ethylene hydrogenation on the T-202 catalyst is diffusion-free and intrinsic. However, the kinetics for transformation of the sulfur-containing compounds can only be regarded as apparent.

Fig. 1 External diffusion effect test for hydrogenation of ethylene on T-202 catalyst (Reaction conditions: p=2.0 MPa, T= 300 °C)
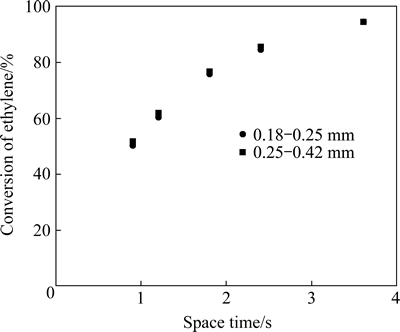
Fig. 2 Internal diffusion effect test for hydrogenation of ethylene on T-202 catalyst (Reaction conditions: p=2.0 MPa, T= 300 °C)
3 Results and discussion
3.1 Conversion of COG
In this work, the conversion and reaction kinetics of the COG over the T-202 catalyst were investigated at pressures of 1.6-2.8 MPa, space time of 1.2-3.55 s, and temperatures of 240-360 °C. Under these conditions, the compounds contained in the COG can be involved in different reactions, depending on their molecular structures.
3.1.1 Conversion of O2, CO, CO2 and H2
As listed in Table 1, the concentration of O2 is about 3.3 mmol/mol in the COG feed and 1.1 mmol/mol in the reaction product. Theoretically, O2 may react with all the components in the COG except N2 under the conditions used. The reduced O2 may be consumed in the oxidation of H2, hydrocarbons and CO. Based on the composition of the reaction product it is difficult to distinguish which kind of reaction is predominate.
Due to the presence of the higher amount of H2 in COG, the most possible reactions that CO and CO2 may be involved are their hydrogenation. However, the concentration of CO and CO2 in the reaction product does not show significant change as compared to that of the COG feed. This indicates that the T-202 catalyst does not have good catalytic activity for the hydrogenation of CO and CO2. Besides hydrogenation, another important reaction of CO is its reaction with H2S to form COS. As will be discussed in the later section, the presence of CO in the COG may prevent the complete HDS of COS. As compared to that in the COG feed, the concentration of H2 in the product shows a slight decrease. The consumption of H2 can be attributed to the reaction with O2, the hydrogenation of unsaturated hydrocarbons and the HDS of sulfur-containing compounds.
3.1.2 Conversion of hydrocarbons
The hydrocarbons contained in COG range from C1 to C6 as listed in Table 1. CH4 is the main component in the hydrocarbon group and its concentration amounts to approximate 0.22 mol/mol. After reaction, the concentration of CH4 in the product remains practically the same as that in the COG feed, supporting the observation that the hydrogenation of the CO and CO2 on the T-202 catalyst does not take place to a significant degree.
The C2 hydrocarbons contained in COG gas are ethane, ethylene and acetylene. The concentrations of C2 hydrocarbons in the product as a function of the reaction temperature are shown in Fig. 3. As can be seen from this figure, acetylene has been converted completely. With increasing reaction temperature, the concentration of ethane in the product increases while that of ethylene decreases, indicating that ethylene can be progressively hydrogenated to ethane.

Fig. 3 Concentration of C2 hydrocarbons in product as a function of reaction temperature (Reaction conditions: p=1.6 MPa, space time=1.82 s)
The C3 hydrocarbon compounds in the COG feed are propane, propylene, propadiene and propyne. The possible reactions that involve the unsaturated C3 are hydrogenation and reaction with H2S. Since no propyl mercaptan was detected in the product, it can be assumed that the reaction between unsaturated C3 and H2S is not significant. The concentration of C3 in the product observed at pressure of 1.6 MPa and space time of 1.82 s as a function of the reaction temperature is presented in Fig. 4. Under the reaction conditions, conversion of propadiene and propyne reaches complete. Increasing the reaction temperature results in an increase of the propane concentration and a decrease of the propylene concentration in the product, indicating that propylene can also be progressively hydrogenated.

Fig. 4 Concentration of C3 hydrocarbons in product as a function of reaction temperature (Reaction conditions: p=1.6 MPa, space time=1.82 s)
The conversion of C4 hydrocarbons on the T-202 catalyst may involve isomerization, hydrogenation, and reaction with H2S to form sulfur-containing compounds. Since no butanethiols was detected in the product, the sulfur-containing compounds formed from C4 can be assumed to be only C4H4S.
To exam the isomerization degree of C4 hydrocarbons, the concentration of isobutane and isobutene in the product was checked as shown in Fig. 5. As can be seen from Fig. 5, increasing the reaction temperature results in a decrease of the concentration of isobutene and an increase of the concentration of isobutane in the product. This means that isobutene can be also hydrogenated on the T-202 catalyst. The total concentration of iso-C4 in the product shows only a slight increase after the reaction, implying that the skeletal isomerization of the C4 hydrocarbons may not occur to a significant degree. This is in line with the weak acidic property of the T-202 catalyst since skeletal isomerization of hydrocarbons needs strong acidic sites.
The concentrations of n-butane, n-butenes and butadiene in the product observed at 2 MPa and 1.2- 3.55 s are presented in Fig. 6. The conversion of butadiene over the T-202 catalyst is completed. Butadiene can be consumed by either hydrogenation or reaction with H2S. However, based on the results of this work, it is difficult to distinguish which reaction is predominant in the conversion of butadiene. With increasing reaction temperature the concentration of total straight chain butene isomers in the product decreases while that of n-butane increases. This indicates that the straight chain butene isomers can be progressively hydrogenated just like ethylene and propylene. Since the cis-, trans- and double-bond isomerization of straight chain butene isomers does not require higher activation energy, these reactions may take place easily.
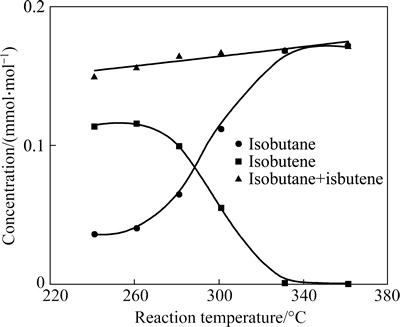
Fig. 5 concentration of iso-C4 in product as a function of reaction temperature (Reaction conditions: p=1.6 MPa, space time=1.82 s)

Fig. 6 Concentration of butane, straight chain butenes and butadiene in product as a function of reaction temperature (Reaction conditions: p=2 MPa, space time=1.2-3.55 s)
The conversion of olefins and diolefins on sulfide catalysts has been investigated previously [4-7]. On sulfide catalyst, hydrogenation of butadiene was found to be a fast reaction [4]. Double bond shift also occurred easily [4-5]. Skeletal isomerization depended on the composition of the catalysts, as well as on the structure of the olefins [4-5]. The hydrogenation rate of olefins was also found to be associated with the structure and composition of the sulfide catalysts and with the molecular structure of olefins [4-7]. The hydrogenation rate of stereo-hindered olefins was found to be lower than that of the unhindered ones [4-7]. The conversion of the hydrocarbons in the COG on the T-202 catalyst is in general consistent with that reported in the above mentioned previous studies and can be summarized as follows: the reaction rate of diolefins and alkynes is fast and complete conversion of them can be reached even at relatively mild conditions; with increasing temperature and pressure, olefins can be progressively hydrogenated; skeletal isomerization of C4 hydrocarbons is not significant.
3.1.3 Conversion of sulfur-containing compounds
The sulfur-containing compounds in the COG feed are COS, CS2 and thiophene (C4H4S). After reaction, the detectable sulfur-containing compounds were COS, H2S, ethyl mercaptan (C2H5SH) and C4H4S. No CS2 was detected in all of the experimental runs. The absence of CS2 in the product means that reaction of CS2 on the T-202 catalyst is very fast and its conversion can reach 100% in the whole range of conditions used in this work. The most likely reaction for the conversion of CS2 is its HDS as shown in Eq. (1), which produces CH4 and H2S.
 (1)
(1)
The COG feed contains 8.9×10-7 mol/mol C4H4S but no C2H5SH. Figure 7 presents the concentration of the sulfur containing compounds in the product as a function of reaction temperature at pressure of 2.0 MPa and space time of 1.82 s. As can be seen from this figure, the concentration of COS decreases from 2.06×10-5 to 5×10-6 mol/mol at 240 °C, indicating that HDS of COS can take place easily on the T-202 catalyst. On the other hand, the concentration of C2H5SH and C4H4S in the product increases to 4.6×10-5 and 5×10-6 mol/mol respectively at temperature of 240 °C. This means that C2H5SH and C4H4S can be produced on the T-202 catalyst. Moreover, the significantly higher concentration of C2H5SH at the lower temperature means that the formation rate of C2H5SH is fast.
The formation of sulfur-containing compounds in fluid catalytic cracking (FCC) gasoline has been investigated [8-10]. Mercaptans, thiophenes, and benzothiophenes were found to be the main sulfur- containing compounds [8]. Reactions of H2S with olefins or diolefins were one of the important ways that could be responsible for the entire FCC gasoline sulfur- compounds formation [8-9]. Addition of H2S to olefins or diolefins resulting from the cracking of hydrocarbons could produce thiols. The thiols then cyclized into tetrahydrothiophenic compounds and their subsequent dehydrogenation formed thiophenic compounds [8-10].
In this work, C2H5SH can be assumed to be formed from the reaction of ethylene with H2S according to Eq.(2).
 (2)
(2)
In additions, reaction between acetylene and H2S can form CH2=CH—SH and subsequent hydrogenation of CH2=CH—SH also gives C2H5SH as shown in Eq. (3).
 (3)
(3)

Fig. 7 Concentration of sulfur-containing compounds in product as a function of reaction temperature (Reaction conditions: p=2.0 MPa, space time=1.82 s)
C4H4S can be assumed to be formed from the reactions between H2S and butylene or butadiene according to Eq. (4) and Eq. (5).
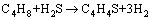 (4)
(4)
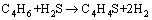 (5)
(5)
In the above reactions, the H2S required for the formation of C2H5SH and C4H4S comes from the HDS of CS2 and COS. However, formation of C2H5SH and C4H4S consumes only part of H2S. Therefore, there is a certain amount of H2S left in the product.
With increasing temperature, the concentrations of COS, C2H5SH and C4H4S in the product decrease, accordingly the concentration of H2S increases. This means that increasing the reaction temperature favors the HDS reactions of the sulfur-containing compounds. When the reaction temperature is higher than 330 °C, the concentration of C2H5SH in the product approaches zero. In contrast, the concentration of COS and C4H4S in the product cannot reachs zero even at higher temperatures and pressures. The observation for the conversion of C4H4S is consistent with the previous report [11]. C4H4S is a refractory sulfur-containing compound and complete HDS of C4H4S cannot be easily realized.
The most likely reactions that involve COS are the HDS of COS and the reverse reaction as shown in Eq. (6).
 (6)
(6)
The equilibrium constant of Eq. (6) was calculated using the thermodynamic data provided in ref. [12]. The equilibrium constant increases with increasing temperature, which favors the HDS of COS. However, the equilibrium constant does not change significantly with temperature. For instance, increasing temperature from 240 °C to 360 °C results in an increase of the equilibrium constant of Eq. (6) by only 1.7 times. On the other hand, the relatively high concentration of CO in the COG gas favors the reverse reaction of Eq. (6). Both of the two factors lead the complete HDS of COS to be impossible.
The influence of reaction pressure on the concentration of the sulfur-containing compounds in the product observed at 300 °C is presented in Fig. 8. Increasing the reaction pressure favors the HDS of C5H5SH and C4H4S. In contrast, the pressure does not have any significant influence on the conversion of COS. The increased concentration of H2S in the product can be attributed to the formation of H2S from the HDS of C5H5SH and C4H4S.
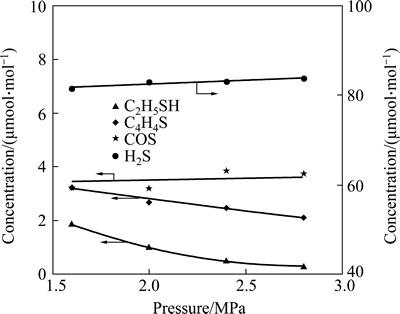
Fig. 8 Concentration of sulfur-containing compounds in product as a function of reaction pressure (Reaction conditions: T=300 °C, space time=3.55 s)
The influence of space time on the concentration of sulfur-containing compounds in the product is shown in Fig. 9. Increasing space time does not have significant influence on the concentration of C4H4S, but results in a sharp decrease of the concentration of C5H5SH in the product. The concentration of COS in the product shows a slight increase with increasing the space time. These mean that higher space time only slightly favors the formation of COS but significantly favors the HDS reaction of C5H5SH, which leads to an increase of the concentration of H2S in the product.
Under the reaction conditions, HDS of C2H5SH produces H2S and ethane according to Eq. (7).
 (7)
(7)
HDS of C4H4S can be assumed to produce H2S, butenes or butane according to Eq. (8) or Eq. (9).
 (8)
(8)
 (9)
(9)
The kinetics of HDS of C4H4S on a commercial CoMo/γ-Al2O3 catalyst was studied by Parijd and Froment [13] in a bench-scale tubular reactor at 0.2- 3.0 MPa and 260-350 °C. The measurable products from the hydrogenolysis of thiophene were H2S, butenes, and butane, and the butenes were proposed to be reaction intermediates which gave butane upon further hydrogenation.

Fig. 9 Concentration of sulfur containing compounds in product as a function of space time (Reaction conditions: T=300 °C, p=2.4 MPa)
3.2 Reaction kinetics of COG
In this process, the most concerned reactions are the hydrogenation of ethylene and the transformation of the sulfur-containing compounds. Therefore, kinetics models involving only these most concerned reactions are developed in this work. The results presented above indicate that the reactions of COG on the T-202 catalyst are complicated. To simplify the kinetics models, the following assumptions are made based on the experimental observations: 1) Upon the contact with the catalyst, CS2 in COG feed is immediately converted to H2S and CH4. 2) Transformation of COS involves its HDS and the reverse reaction. 3) C2H5SH is generated from the reaction between ethylene and H2S, and subsequent HDS of C2H5SH forms H2S and ethane. 4) C4H6 is formed by reactions of H2S with straight chain butene isomers and HDS of C4H4S forms H2S and butane.
Based on the above assumptions, the following reactions (10a) to (10g) are used to describe the reaction kinetics of the conversion of ethylene and sulfur containing compounds on the T-202 catalyst.
 (10a)
(10a)
 (10b)
(10b)
 (10c)
(10c)
 (10d)
(10d)
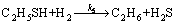 (10e)
(10e)
 (10f)
(10f)
 (10g)
(10g)
To derive the rate equations in the HDS process in terms of Hougen-Watson mechanism, two types of active sites, one (s) for the hydrogenolysis and the other (τ) for the hydrogenation, have been proposed [11, 13]. However, the obtained rate equations have generally complicated forms and discrimination of the rival models is difficult [13]. In this work, the reaction rates of reactions (10a) to (10g) are expressed using power law type equations as shown in Eq. (11) to Eq. (17).
 (11)
(11)
 (12)
(12)
 (13)
(13)
 (14)
(14)
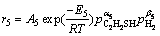 (15)
(15)
 (16)
(16)
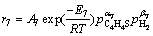 (17)
(17)
where Ai is the pre-exponential factor; Ei is the activation energy; and
and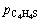 are the pressure of H2, ethylene, H2S, CO, COS C2H5SH, straight chain butene isomers and C4H4S, respectively; αi, and βi are the reaction orders.
are the pressure of H2, ethylene, H2S, CO, COS C2H5SH, straight chain butene isomers and C4H4S, respectively; αi, and βi are the reaction orders.
The data analysis was performed based on the integral method of kinetic analysis of Froment and BISCHOFF [14]. The mole flow rates of the concerned components as shown in Eq. (18) were obtained through integration of the continuity equations in a tubular reactor with plug flow using the Merson method [15-16].
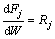 (18)
(18)
where Fj is the molar flow rate of concerned component j, representing respectively ethylene, H2S, COS, C2H5SH and C4H4S; m is the mass of catalyst and Rj is the net formation rate of component j, which can be derived from reactions (10a) to (10g).
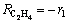 (19)
(19)
 (20)
(20)
 (21)
(21)
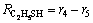 (22)
(22)
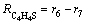 (23)
(23)
The cyclic coordinate method [16] was used to minimize the objective function:
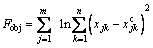 (24)
(24)
where xjk and 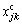 are respectively the experimental and the calculated mole concentration of j in the product; M is the number of the concerned components in Eq.(18) and n is the number of experiments. The obtained values of the parameters for the reaction kinetics models are given in Table 2.
are respectively the experimental and the calculated mole concentration of j in the product; M is the number of the concerned components in Eq.(18) and n is the number of experiments. The obtained values of the parameters for the reaction kinetics models are given in Table 2.
Table 2 values of parameters in reaction kinetics models for conversion of COG over T-202 catalyst

The significance of the parameters of the kinetic models was evaluated in terms of the correlation coefficient (ρ) and the Fisher’s value (F) as shown in Table 3. The values of the correlation coefficient for Eq. (19) to Eq. (23) are higher than 0.99, indicating that the kinetic models are highly relative to the experimental data. The F values are all higher than 10 times of the F0.05, indicating that the correlation results are significant at confidential level of 0.95.
Figure 10 gives the comparison between the experimentally measured and the calculated conversions of ethylene. Figures 11 and 12 present the comparison between the experimentally measured and the calculated concentrations of the sulfur-containing compounds in the product. As can be seen from these figures, the calculated conversions of ethylene and the concentration of the sulfur-containing compounds in the product agree well with the experimentally measured values.
Table 3 Statistical tests of reaction kinetic models for conversion of COG over T-202 catalyst

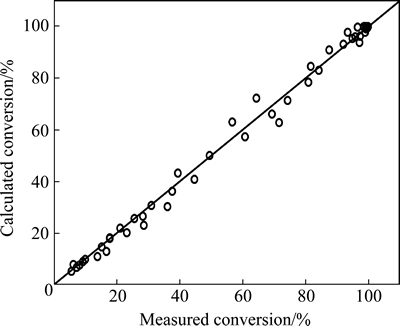
Fig. 10 calculated versus experimentally measured conversions of ethylene in mole fraction (Reaction conditions: p=1.6- 2.8 MPa, t=240-360 °C, space time=1.82-3.55 s)
For the hydrogenation of ethylene, the reaction order with respect to H2 and ethylene is 0.92 and 1.01 respectively. The activation energy is 140.7 kJ/mol. Parijs and Froment [13] studied the hydrodesulfurization kinetics of C4H4S on a CoMo/γ-Al2O3 catalyst and reported that the activation energy for the hydrogenation of butenes could be 119.2 or 159.5 kJ/mol. The value of the activation energy depended on the kinetic model derived from the Hougen- Watson reaction mechanism. As pointed previously [5-7], the hydrogenation activity of olefins on sulfide catalysts was associated with the composition of the catalyst and the structure of the olefins. As compared with the values reported by PARIJS and Froment [13], the activation energy for the hydrogenation of ethylene on the T-202 catalyst is reasonable.
The activation energy for the HDS of COS is 28.59 kJ/mol, which is much higher than that of the reverse reaction, 6.55 kJ/mol. The activation energy for the HDS of C2H5SH is 37.43 kJ/mol. For the formation of C2H5SH from ethylene and H2S, it is only 6.71 kJ/mol. The activation energy for the HDS of C4H4S is 76.67 kJ/mol. For formation reaction from straight chain butene isomers and H2S, it is only 8.35 kJ/mol.
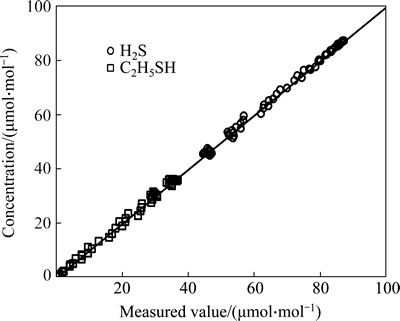
Fig. 11 calculated versus experimentally measured concentration of H2S and C2H5SH in the product (Reaction conditions: p=1.6-2.8 MPa, t=240-360 °C, space time=1.82-3.55 s)

Fig. 12 calculated versus experimentally measured concentration of COS and C4H4S in product (Reaction conditions: p=1.6-2.8 MPa, t=240-360 °C, space time=1.82-3.55 s)
Though the activation energies for the conversion of the sulfur-containing compounds obtained in this study are apparent values, some implications can still be outlined. The low activation energies for the formation of the sulfur-containing compounds imply that the sulfur- containing compounds are easily formed under the reaction conditions. The experimentally observed higher concentration of C2H5SH at lower temperatures and pressures obviously supports this idea. With increasing temperature and pressure, HDS of the sulfur-containing compounds becomes important. Whether a sulfur-containing compound is easy or not to be desulfurized depends on its molecular structure and is reflected by the values of the activation energies. The HDS of C4H4S is difficult [11]. In this work, an activation energy of 76.67 kJ/mol is obtained. this value is close to 72.5 kJ/mol, an intrinsic activation energy observed on a commercial CoMoNi/Al2O3 catalyst [17] and to 83.5 kJ/mol, an intrinsic activation energy observed on a sulfide NiMo/SiO2 planar model catalyst [18]. The activation energies for the HDS of COS and C2H5SH are 28.59 kJ/mol and 37.43 kJ/mol respectively, which are significantly lower than that of C4H4S and are consistent with the fact that HDS of COS and C2H5SH is easier than C4H4S. For COS, complete conversion is difficult due to the relatively high concentration of CO in the COG and the restriction of thermodynamics.
4 Conclusions
the conversion and reaction kinetics of an industrial COG on a commercial Fe-Mo/Al2O3 catalyst (T-202) have been studied in a continuous flow fixed bed reactor. Under the reaction conditions used, conversion of CO and CO2 in the COG is not significant; reaction of diolefins and alkynes is fast and complete conversion of these compounds is reached even under mild conditions (T=240 °C, p=1.6 MPa). With increasing temperature and pressure, olefins can be progressively hydrogenated. The HDS of CS2 on the T-202 catalyst is easy, and complete conversion is reached under the conditions used in this study. The HDS of COS is also easy. However, complete HDS of COS is difficult due to the relatively high concentration of CO in the COG and the restriction of thermodynamics. H2S produced from the HDS of CS2 and COS reacts with unsaturated hydrocarbons, forming significant amount of C2H5SH and C4H4S at low temperature and pressure. With increasing temperature and pressure, HDS of C2H5SH and C4H4S is favored and progressive HDS of these compounds takes place. Based on the experimental observations, kinetic models for the hydrogenation of ethylene and transformation of the sulfur-containing compounds are proposed. The values of the parameters in the kinetic models are estimated by regression of the experimental data. The calculated conversion of ethylene and the concentration of the sulfur-containing compounds in the product agree well with the experimentally observed values. The results obtained in this work can be used to optimize the operation of the HDS reactors in the process of COG purification.
References
[1] BERM DEZ J M, Arenillas A, Luque R, MEN
DEZ J M, Arenillas A, Luque R, MEN NDEZ J A. An overview of novel technologies to valorise coke oven gas surplus [J]. Fuel Process Technol, 2013, 110: 150-159.
NDEZ J A. An overview of novel technologies to valorise coke oven gas surplus [J]. Fuel Process Technol, 2013, 110: 150-159.
[2] YI Qun, WU Yan-li, FAN Yang, HU Chang-chun, CHU Qi, FENG Jie, LI Wen-ying. Economic evaluation of industrial chain extension solutions for coke oven gas to methanol and chemicals [J]. J Chem Ind Eng, 2014, 65(3): 1003-1011. (in Chinese)
[3] QI Jing-li, KONG Fan-rong. Status and prospect for chemical utilization of coke oven gas in China [J]. Nature Gas Chem Ind (C1 Chem Chem Ind), 2013, 38(1): 60-64.
[4] Meerbott W K, Hinds G P Jr. Selective hydrotreating over tungsten nickel sulfide catalyst: Reaction studies with mixtures of pure compounds [J]. Ind Eng Chem, 1955, 47(4): 749-752.
[5] Badawi M, Vivier L, P ROT G, Duprez D. Promoting effect of cobalt and nickel on the activity of hydrotreating catalysts in hydrogenation and isomerization of olefins [J]. J Mol Catal A: Chem, 2008, 293: 53-58.
ROT G, Duprez D. Promoting effect of cobalt and nickel on the activity of hydrotreating catalysts in hydrogenation and isomerization of olefins [J]. J Mol Catal A: Chem, 2008, 293: 53-58.
[6] Badawi M, Vivier L, Pérot G, Duprez D. Kinetic study of olefin hydrogenation on hydrotreating catalysts [J]. J Mol Catal A: Chemical, 2010, 320: 34-39.
[7] Toba M, Miki Y, Matsui T, Harada M, Yoshimura Y. Reactivity of olefins in the hydrodesulfurization of FCC gasoline over CoMo sulfide catalyst [J]. Appl Catal B: Environmental, 2007, 70: 542-547.
[8] Stratiev D S, Shishkova I, Tzingov T, Zeuthen P. Industrial investigation on the origin of sulfur in fluid catalytic cracking gasoline [J]. Ind Eng Chem Res, 2009, 48(23): 10253-10261.
[9] Leflaive P, Lemberton J L, Pérot G, Mirgain C, Carriat J Y, Colin J M. On the origin of sulfur impurities in fluid catalytic cracking gasoline—Reactivity of thiophene derivatives and of their possible precursors under FCC conditions [J]. Appl Catal A-Gen, 2002, 227: 201-215.
[10] Bruneta S, Meya D,  G, Bouchyb C, Diehl F. On the hydrodesulfurization of FCC gasoline: a review [J]. Appl Catal A-Gen, 2005, 278: 143-172.
G, Bouchyb C, Diehl F. On the hydrodesulfurization of FCC gasoline: a review [J]. Appl Catal A-Gen, 2005, 278: 143-172.
[11] Startsev A N. The mechanism of HDS catalysis [J]. Catal Rev-Sci Eng, 1995, 37(3): 353-423.
[12] NIST Chemistry WebBook, NIST Standard Reference Database Number 69 [EB/OL]. [2015-04-18]. http://webbook.nist.gov/ chemistry/.
[13] van Parijs I A, Froment G F. Kinetics of hydrodesulfurization on a CoMo/γ-Al2O3 catalyst. 1. Kinetics of the hydrogenolysis of thiophene [J]. Ind Eng Chem Prod Res Dev, 1988, 25(3): 431-436.
[14] Froment G F, Bischoff K B. Chemical reactor analysis and design [M]. 2nd ed. New York: JohnWiley & Sons Inc, 1990.
[15] ZHOU Pin, HE Zheng-feng. Matlab numerical analysis [M]. Beijing: China Mechine Press, 2009. (in Chinese)
[16] GONG Chun, WANG Zheng-lin. Matlab optimization calculations [M]. Beijing: Publishing House of Electronics Industry, 2009. (in Chinese)
[17] YANG Dong, CHENG Zhen-min, ZHOU Zhi-ming, YUAN Wei-kang. Pyrolysis gasoline hydrogenation in the second-stage reactor: Raction kinetics and reactor simulation [J]. Ind Eng Chem Res, 2008, 47(4): 1051-1057.
[18] Borgna A, Hensen E J M, van Veen J A R, Niemantsverdriet J W. Intrinsic kinetics of thiophene hydrodesulfurization on a sulfided NiMo/SiO2 planar model catalyst [J]. J Catal, 2004, 221: 541-548.
(Edited by YANG Hua)
Received date: 2014-12-22; Accepted date: 2015-04-20
Corresponding author: Qu Yi-xin, Professor; Tel: +86-10-64436998; Fax: +86-10-64436781; E-mail: quyx@mail.buct.edu.cn

