文章编号:1004-0609(2008)07-1358-06
ZrVFe吸气剂激活过程及其机理
熊玉华,顾 为,尉秀英,苑 鹏,秦光荣
(北京有色金属研究总院 先进电子材料研究所,北京 100088)
摘 要:通过X射线光电子能谱仪研究ZrVFe吸气剂激活过程中表面成分的变化,采用四极质谱仪监测激活过程中真空腔体内残余气体成分的变化。结果表明:暴露于过大气的吸气剂表面覆盖着H2O、CO2及碳氢化合物等,Zr和V主要以氧化态存在;在吸气剂加热激活过程中,V的氧化物在200 ℃开始还原;激活过程导致表面近金属态Zr的富集以及部分金属碳化物的生成;随着温度的升高,H2O、CO2及碳氢化合物逐渐从吸气剂表面脱附,在200 ℃以上,主要脱附气体为H2,整个激活过程未发现O2;ZrO2还原为近金属态或金属态,主要是由于ZrO2中的O通过向吸气剂内部扩散而离开吸气剂表面所致。
关键词:ZrVFe吸气剂;激活机理;残气
中图分类号:TB 741 文献标识码:A
Activation process and mechanism of ZrVFe getter
XIONG Yu-hua, GU Wei, WEI Xiu-ying, YUAN Peng, QIN Guang-rong
(Advanced Electronic Materials Institute, General Research Institute for Nonferrous Metals, Beijing 100088, China)
Abstract: During the activation process of ZrVFe getter, the variation for its surface composition and residual gases in the vacuum chamber were studied by XPS and quadrupole mass spectroscopy(QMS), respectively. The results show that the surface layer of the air-exposed ZrVFe getter is covered with H2O, CO2 and hydrocarbons, both Zr and V exist in the oxidized state, and vanadium oxide starts to reduce at 200 ℃. The activation results in the enrichment of Zr on the getter surface and the formation of the metallic carbides. H2O, CO2 and hydrocarbons sequentially desorb from the surface with the increase of temperature. H2 is the main gas which desorbs above 200 ℃ and O2 is not discovered. ZrO2 is reduced to near-metallic or metallic state because O in ZrO2 leaves the getter surface through diffusing into getter bulk.
Key words: ZrVFe getter; activation mechanism; residual gas
非蒸散型吸气剂指激活后形状不改变就直接吸收活性气体的吸气剂。该类吸气剂广泛应用于电光源、热绝缘装置、电真空器件、惰性气体净化、核过程和基本粒子研究等领域,起到维持器件真空度和提纯惰性气体的目的[1?5]。在使用前,非蒸散型吸气剂必须在真空条件下进行加热以去除表面钝化膜—氧化物膜及表面吸附气体,从而获得清洁的活性表面,该过程被称为激活过程[6?7]。对激活过程及激活机理进行研究有利于了解激活本质,为设计新型吸气剂提供了理论依据。目前,有关激活过程中表面成分变化的X射线光电子能谱分析(X-ray photoelectron spectroscopy, XPS)较多[8?10],但尚未见结合XPS研究、四极质谱分析(Quadrupole mass spectroscopy, QMS)及热力学计算说明非蒸散型吸气剂激活本质的相关报道。
在前期的工作中,本文作者已对非蒸散型ZrVFe吸气剂的激活过程开展了一些研究[11]。本文作者利用X射线光电子能谱仪研究ZrVFe吸气剂激活中表面成分及化合价态的变化,采用四极质谱仪监测激活过程中真空腔体内残余气体成分的变化,采用热力学计算方法分析激活过程中表面氧化物的去除机理,以进一步探讨激活现象和机理,为设计低温激活吸气剂提供理论依据,具有重要的理论和实践意义。
1 实验
1.1 样品制备
称取一定质量的38 μm Zr-24.6%V-5.4%Fe(质量分数,%)吸气剂粉,在Ar气保护下将粉末置于Ni基片上压制成d 6 mm?0.5 mm的样品。
1.2 XPS分析
采用Thermo ESCALAB-250型能谱仪原位监测样品激活过程中表面Zr、V、O和C等化学态的变化。X射线光源为双阳极 Al Kα (1 486.60 eV) ,功率为200 W。设备的本底真空度优于10?7 Pa。激活升温过程如下:首先将ZrVFe样品在80 ℃下除气8 h,接着对样品继续加热,在300~340 ℃每隔10 ℃ 保温1 h,同时记录XPS谱。
1.3 质谱分析
采用日本真空技术株式会社生产的RG?202型四极质谱仪监测ZrVFe吸气剂在激活过程中的残气组成和相对含量。设备真空度优于10?5 Pa。激活升温过程分为4段:25~100 ℃、100~200 ℃、200~300 ℃和300~350 ℃。每次升温完毕后,保温10 min,测试真空腔体中残余气体中各组分的相对摩尔浓度,随后将真空抽至起始水平,再次升温测试,以保证释放的气体不出现积累。
2 结果与分析
2.1 XPS谱及分析
2.1.1 XPS谱
图1所示为在25 ℃时测试得到的ZrVFe样品的XPS谱。由图1可知,样品表面主要存在Zr、V、O和Fe和C 5种元素,其中C元素来源于表面吸附和系统污染,O元素主要来源于表面吸附和表面氧化物。

图1 25 ℃时ZrVFe样品的XPS谱
Fig.1 XPS spectrum of ZrVFe getter at 25 ℃
2.1.2 Zr 3d5/2的XPS谱
图2所示为不同温度下Zr 3d的能谱。按照Shirley法[12]减去背景常数后,Zr 3d峰可分解为几个基本峰,这些峰对应于Zr不同价态的氧化物,Zr4+对应的结合能为182~182.6 eV,Zr2+对应为180.2~180.6 eV,金属Zr0对应为179.1 eV。
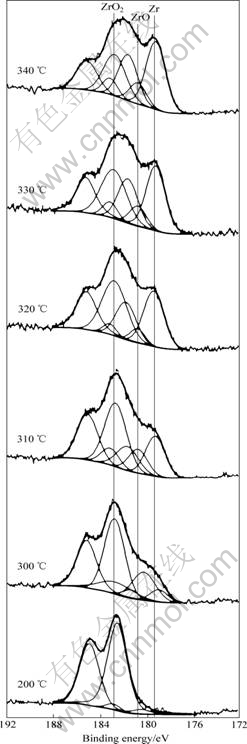
图2 ZrVFe 吸气剂激活过程中Zr 3d5/2的XPS谱
Fig.2 XPS patterns of Zr 3d5/2 during activation process of ZrVFe getter
由图2可知,当温度为200 ℃时,Zr元素在表面基本上以ZrO2的形式存在,当温度升至300 ℃时,表面出现少量的 Zr金属,且亚氧化物ZrO的量也随之增加;当温度从310 ℃升至340 ℃的过程中,样品表面金属 Zr所对应峰的面积不断增大,表明金属Zr的含量在不断增加,即在激活过程中,Zr由氧化态向近金属态转变。
通过灵敏度因子计算法发现,在激活过程结束后,表面虽仍存在部分氧化,但Zr的摩尔分数为66.8%,即表面是富Zr的,这与ICHIMURA等[13]的报道一致。
2.1.3 V、O、C和Fe在激活过程中化合价态的变化
在激活过程中,加热到200 ℃时,金属态V0的含量较多,相比之下,200 ℃时,却没有金属态Zr0的出现,这说明V的氧化物还原所需的温度低于Zr的氧化物。也就是说,激活过程开始于V氧化物的还原,这与MATOL?N等[14]报道的Zr-V吸气剂激活过程中的实验结果一致。在激活过程的初期,V所起的作用较大,该元素的存在是ZrVFe合金具有较低激活温度的主要原因。
O 1s谱线有两个基本峰:一个对应于金属氧化物,另一个一般认为是吸附态的氧。300 ℃以下,较明显减少的是吸附态的氧原子,这主要是由CO2和H2O等
分子在吸气剂表面脱附所造成。在低温200 ℃时,C 1s谱线有两个基本峰:一个是在结合能284.6 eV附近,认为对应于碳氢化合物的C 1s;另一个结合能为288.1 eV处,可以认为是C=O键。这说明暴露过空气后的ZrVFe表面的碳主要以吸附态的形式存在。在激活温度升高至300 ℃的过程中,随着吸附态CO2等气体的不断解吸,出现了新的单个碳峰,根据结合能数据可知,该峰对应于V的碳化物,这说明激活过程中表面碳原子不但参与解吸附过程,还参与了碳化物的生成过程。另外,XPS分析表明,Fe在原位加热后价态上基本没有变化。
2.2 激活过程的残气分析
图3所示为不同温度范围内对ZrVFe吸气剂激活得到的质谱。由图3可看出,激活过程中放出的气体主要为H2O、CH4、H2、CO/N2、C2H6和CO2等,由于CO与N2的分子量相同,这些气体的荷质比(m/e)分别为18×1.04×10?8、16×1.04×10?8、2×1.04×10?8、28×1.04×10?8、30×1.04×10?8和44×1.04×10?8 kg/C。在不同温度下,各气体相对含量有很大差异,这是由于不同气体在不同温度脱附造成的。图中反映了各气体组分相对含量的变化, H2O的摩尔分数达到了94.5%(见图3(a));而在图3(b)中几乎看不到H2O,说明H2O主要在100 ℃以下脱附,图3(b)中的CO2及碳氢化合物的总含量达到71.6%;而图3(c)中几乎没有CO2及碳氢化合物,说明CO2及碳氢化合物主要在100~200 ℃间发生脱附作用。图3(a)和3(b)的结果与XPS分析中O元素在300 ℃以下大量减少相对应。从图3(c)及(d)中可明显看出,H2主要在200 ℃以上脱附,占总气体的摩尔分数分别为98.02%和99.93%,此时,间隙H原子克服深层势阱向表面扩散。

图3 ZrVFe吸气剂激活过程中的残气质谱分析
Fig.3 QMS analysis of residual gases during activation process of ZrVFe getter: (a) 25?100 ℃; (b) 100?200 ℃; (c) 200?300 ℃; (d) 300?350 ℃
2.3 激活机理的热力学分析
由QMS谱可知,整个激活过程中都没有发现O2,说明O2在残气中的比例较小,基本可以忽略不计。因此,认为激活过程中ZrO2没有分解为Zr和O2,这一点可根据本研究的激活条件,对ZrO2分解反应的热力学可行性进行分析而得以证明。
对分解反应ZrO2(s)=Zr(s)+O2(g)的热力学可行性分析如下:
以温度为变量的热容公式比较复杂,在一定的温度范围内,任何相的热容可用下式表示[15]:

根据兰氏化学手册[9],不同温度下ZrO2、Zr和O2的cp值如表1所列。根据表1中的数据分别求出ZrO2、Zr和O2的cp 中的经验参数a、b和c,结果如表2所列。根据如下热力学方程[15?16]可计算不同温度下ZrO2分解反应的吉布斯自由能变:
表1 不同温度下ZrO2、Zr和O2的定压比热容
Table 1 Heat capacity cp of ZrO2, Zr and O2 at different temperatures

表2 ZrO2、Zr和O2定压比热容cp的经验参数
Table 2 Empirical constants of heat capacity of ZrO2, Zr and O2


在本研究条件下: =1.1 MJ/mol,
=1.1 MJ/mol, =193.8 J/(K?mol),p= 1μPa,
=193.8 J/(K?mol),p= 1μPa, =101 kPa,则最终计算得到该分解反应的吉布斯自由能变? rG (J/mol)与温度T (K)的关系如下:
=101 kPa,则最终计算得到该分解反应的吉布斯自由能变? rG (J/mol)与温度T (K)的关系如下:

则可计算不同温度下吉布斯自由能变? rG,结果列于表3。
表3 不同温度下反应的吉布斯自由能变化
Table 3 Gibbs free energy variation at different temperatures

由表3可知,当温度逐渐达到2 735 K(2 462 ℃)时,? rG逐渐减小至零,即在此温度以上,ZrO2的分解反应才能进行。而在本研究的激活温度613 K (340 ℃)时,? rG为853.014 52 kJ/mol,远大于零,不会发生ZrO2分解生成O2的反应。
从XPS谱可知,当温度低于200 ℃时,Zr在吸气剂表面以氧化态形式存在;从QMS分析可知,H2O在100 ℃以下脱附,这说明ZrVFe表面的ZrO2不是以ZrO2+H2→Zr+H2O的方式还原。
此外,ZrO2的饱和蒸汽压极低,在本研究的激活条件下不会蒸发出来。在Zr 3d的XPS谱中可清楚看出,随着温度升高,吸气剂表面ZrO和Zr的出现及O的减少。因此,根据元素守恒定律,可以认为激活过程中ZrO2中的O通过向吸气剂内部扩散而离开吸气剂表面,从而使吸气剂表面成为近金属态或金属态。
3 结论
1) 暴露于过大气的ZrVFe吸气剂表面吸附着H2O、CO2及碳氢化合物等气体,吸气剂表面的Zr和V主要以氧化态存在。
2) ZrVFe非蒸散型吸气剂的激活过程是由Zr、V氧化物向Zr、V的亚氧化物和金属Zr、V的混合物转变的过程。激活过程还导致了在表面近金属态锆的富集以及部分碳化物的生成。
3) 在激活过程中,随着温度的升高,H2O、CO2及碳氢化合物逐渐脱附出吸气剂表面,在200 ℃以上,主要脱附气体为H2,整个过程没有O2的释放。
4) 结合XPS、质谱分析和热力学计算可知,在激活过程中,金属氧化物中的氧元素主要以向内部扩散为主要方式而离开吸气剂表面,从而出现氧化态向近金属态或金属态的转变。
REFERENCES
[1] GIORGI T A, FERRARIO B, STOROY B. An updated review of getters and gettering[J]. Journal of Vacuum Science & Technology A: Vacuum, Surfaces and Films, 1985, 3(2): 417?423.
[2] 庄寿全. 现代吸气材料及其应用技术[J]. 真空电子技术, 1994, 5: 42?49.
ZHUANG Shou-quan. Modern getters and their applications[J]. Vacuum Electronics, 1994, 5: 42?49.
[3] GRANGEON F, MONNIN C, MANGEARD M, PAULIN D. Development of an ultra-high vacuum system for space application[J]. Vacuum,2004, 73(2): 243?248.
[4] MAHNER E. The vacuum system of the low energy ion ring at CERN: Requirements, design, and challenges[J]. Vacuum,2007, 81(6): 727?730.
[5] HAHN M. Operational experience and relation to deposition process for NEG-coated chambers installed on the ESRF electron storage ring[J]. Vacuum,2007, 81(6): 759?761.
[6] 万玉金. 吸气剂[M]. 南京: 江苏科学技术出版社, 1982: 63?66.
WAN Yu-jin. Getter[M]. Nanjing: Jiangsu Science and Technology Press, 1982: 63?66.
[7] LI C C, HUANG J L, LIN R J, CHEN C H, LII D F. Characterization of activated non-evaporable porous Ti and Ti-Zr-V getter films by synchrotron radiation photoemission spectroscopy[J]. Thin Solid Films,2006, 515(3): 1121?1125.
[8] MELI F, SHENG Z, VEDEL I, SCHLAPBACH L. XPS analysis of the getter mechanism and getter activation process[J]. Vacuum,1990, 41(7/9): 1938?1940.
[9] ZEMEK J, JIRICEK P. XPS and He II photoelectron yield study of the activation process in Ti-Zr NEG films[J]. Vacuum,2003, 71(1/2): 329?333.
[10] 陈 旭, 张德胜, 齐 京, 陈丕瑾, 朱秀珍. 新型非蒸散型消气剂德激活温度和吸气性能的实验研究[J]. 真空电子技术, 2003, 46(5): 46?48.
CHEN Xu, ZHANG De-sheng, QI Jing, CHEN Pi-jing, ZHU Xiu-zhen. Experimental study on the activation temperature and sorption characteristics of the new nonevaporable getter material[J]. Vacuum Electronics, 2003, 46(5): 46?48.
[11] 顾 为, 熊玉华, 杜 军, 尉秀英, 秦光荣. ZrVFe吸气剂激活过程研究[J]. 稀有金属, 2007, 31(4): 501?505.
GU Wei, XIONG Yu-hua, DU Jun, WEI Xiu-ying, QIN Guang-rong. Investigation of activation process on ZrVFe getter[J]. Chinese Journal of Rare Metals, 2007, 31(4): 501?505.
[12] MATOL?N V, DRBOHLAV J, MASEK K. Mechanism of non-evaporable getter activation XPS and static SIMS study of Zr44V56 alloy[J]. Vacuum, 2003, 71(1/2): 317?322.
[13] ICHIMURA K, ASHIDA K, WATANABE K. Surface characterization of a Zr-V-Fe getter by XPS-SIMS-activation process and D2O exposure[J]. Journal of Vacuum Science & Technology A: Vacuum, Surfaces and Films, 1985, 35(2): 346?350.
[14] MATOL?N V, MASEK K, MATOL?NOV? I, SK?LA T, VELTRUSK? K. XPS and SIMS study of the ageing mechanism of Zr-V non-evaporable getter films[J]. Applied Surface Science, 2004, 235(1/2): 202?206.
[15] DEAN J A. 兰氏化学手册[M]. 魏俊发, 张安运, 杨祖培, 高子伟, 杨义方, 胡道道, 译. 北京: 科学出版社, 2003: 1?6, 166.
DEAN J A. Lange’s handbook of chemistry[M]. WEI Jun-fa, ZHANG An-yun, YANG Zu-pei, GAO Zi-wei, YANG Yi-fang, HU Dao-dao, transl. Beijing: Science Press, 2003: 1?6, 166.
[16] 朱传征, 许海涵. 物理化学[M]. 北京: 科学出版社, 2004: 230?236.
ZHU Chuan-zheng, XU Hai-han. Physical chemistry[M]. Beijing: Science Press, 2004: 230?236.
收稿日期:2007-10-23;修订日期:2008-02-24
通讯作者:熊玉华, 高工, 博士; 电话:010-82241345; E-mail: xiongyh@griaem.com
(编辑 李艳红)