
Influence of Ga and Hg on microstructure and
electrochemical corrosion behavior of Mg alloy anode materials
FENG Yan(冯 艳), WANG Ri-chu(王日初), YU Kun(余 琨), PENG Chao-Qun(彭超群), LI Wen-xian(黎文献)
School of Materials Science and Engineering, Central South University, Changsha 410083, China
Received 15 July 2007; accepted 10 September 2007
Abstract: The effects of Hg and Ga on the electrochemical corrosion behavior of Mg-5%Hg (molar fraction) alloys were investigated by the measurement of polarization curves and galvanostatic test. The microstructure of the alloys and the corroded surface of the specimens were investigated by scanning electron microscopy, X-ray diffractometry and emission spectrum analysis. It can be concluded that the addition of 1%Ga (molar fraction) reduces corrosion current density from 26.98 mA/cm2 to 2.34 mA/cm2; while the addition of 1%Hg (molar fraction) increases corrosion current density. The addition of Ga and Hg both promotes the electrochemical activity of the alloys and the influence of Ga is more effective than Hg. Mg-5%Hg-1%Ga alloy has the best electrochemical activity, showing mean potential of -1.992 V. The activation mechanism of the magnesium alloy produced by Hg and Ga was put forward. Magnesium atoms are dissolved in liquid Hg and Ga to form amalgam and undergo severe oxidation at the amalgam/electrolyte interface.
Key words: Mg alloy anode; electrochemical measurement; corrosion resistance; activation mechanism
1 Introduction
Magnesium is used extensively as anode material in sea battery systems. Major attributes of the batteries are rapid activation, high cell voltage and wide voltage range, high power density capability, relatively light mass in unactivated state and long unactivated storage life[1-4]. These are reflected in typical applications such as sonobuoys, beacons, emergency equipment, balloon batteries and life jackets[5-8].
In previous studies, Mg-6%Al-5%Pb, Mg-7%Tl- 5%Al alloys[1] and Mg-Hg alloys[9] are mainly used as the high voltage anode materials. Among them the seawater battery using Mg-Hg alloys as anode has specific energy of 150 W?h/kg[10-11]. But there exists some problems such as being hard to process, large self-corrosion velocity, low current efficiency[12-13], the contamination of Hg, Pb and Tl to the environment and the harm to human beings.
So far there is not much report about the alloy component and processing technique for the Mg-Hg anode materials. Experiment demonstrates that the corporation of Ga and Hg leads to uniform dissolution and enhances the electrochemical activity of Al anode [14-17]. The addition of Ga into the dental amalgams promotes the corrosion resistance of the alloy[18]. In this work, Ga is firstly chosen to replace partial Hg in magnesium anode materials and the effects of Ga and Hg on microstructure and electrochemical corrosion behavior of Mg alloys anode materials are studied.
2 Experimental
Mg-Hg-Ga alloys were sealed in iron flask filled with Ar atmosphere, melted in muffle furnace and air cooled. After homogeneous heat treatment, these alloys were taken out. The chemical compositions of the specimens (Table 1) were determined by emission spectrum analysis and atomic absorption spectrometry analysis.
The specimens used for the measurement of electrochemical corrosion behavior were polished with emery paper and buffed to a mirror finish. Each of them was then sealed with epoxy resin except for an exposed surface of 10 mm×10 mm served as an electrode. The auxiliary electrode was Pt sheet. Potential were measured against a SCE reference electrode. Potentiodynamic and galvanostatic experiments were performed with a Potentiostat-Galvanostat(Model 263A). The corrosion solution is 3.5%NaCl and the temperature is 298 K. The current density for the galvanostatic test is 100 mA/cm2.
Table 1 Chemical composites of Mg-Hg-Ga alloys
The microstructures of the alloys with different Hg and Ga contents as well as the corroded surface of each specimen were determined by using scanning electron microscope. The phase of the alloys and the corrosion products were determined by using X-ray diffraction and emission spectrum analysis, respectively.
3 Results and discussion
3.1 Effect of Hg and Ga on microstructure
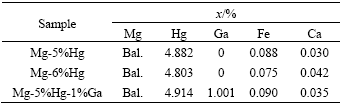
Fig.1 shows the scanning electron micrographs of the specimens. The results from emission spectrum analysis demonstrate that in these specimens the tint second-phase is Mg3Hg and the dark phase is α-Mg, confirmed by X-ray diffraction analysis. The content of Hg increasing from 5% to 6% leads to the size and number of Mg3Hg second-phase increasing. The addition of 1%Ga also increases the size of the second-phase.
3.2 Activation mechanism
Fig.2 shows the scanning electron micrograph of the corrosion surface of Mg-5%Hg-1%Ga after galvanostatic test for 10 s. The alloy is mainly attacked by pitting in the α-Mg solid solution at the early stage. The vesicular corrosion products accumulate on the surface of the pit due to the precipitation of the hydrolysis product H2. When the test time prolongs to 1 000 s, general corrosion occurs and a layer of loose and porous corrosion products covers the surface. The profile observation shows that the general corrosion is even everywhere without pitting in depth. Fig.3 shows that Hg, Ga, HgMg and Ga2Mg exist in the corrosion product except magnesium.
Fig.4 shows the scanning electron micrograph of the pit in Mg-5%Hg-1%Ga alloy after galvanostatic test for 1 000 s. The vesicular corrosion product cracks showing a mud structure at the bottom. Wide circular cavity with incipient feather in surrounding can be seen. At the bottom of the pit there is also cracks that make the activated α-Mg exposed. Spherical Hg and Ga particles identified by X-ray diffraction analysis can be seen at the bottom of the pit. Similar micrographs and products are observed in the Mg-Hg samples except for Ga2Mg and Ga.
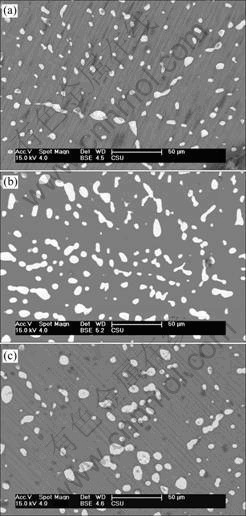
Fig.1 Scanning electron micrographs of Mg-Hg-Ga alloy: (a) Mg-5%Hg; (b) Mg-6%Hg; (c) Mg-5%Hg-1%Ga
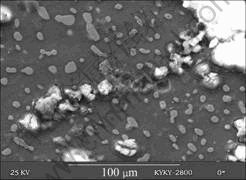
Fig.2 Scanning electron micrograph of surface of Mg-5%Hg- 1%Ga alloys after galvanostatic test in 3.5%NaCl solution at 298 K for 10 s
The activation reaction equations can be written as
Mg(Hg, Ga)→Mg2++Hg++Ga3++6e (1)
Hg++e→Hg (2)
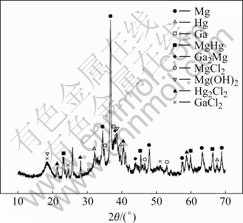
Fig.3 X-ray pattern of corroded products of Mg-5%Hg-1%Ga alloy after galvanostatic test
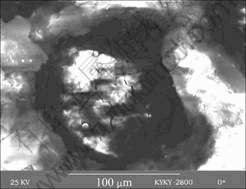
Fig.4 Scanning electron micrograph of bottom zone of pit
Ga3++3e→Ga (3)
Hg, Ga+Mg→Hg, Ga(Mg) (4)
Hg, Ga(Mg)+H2O→MgO·H2O+Hg+Ga+2H+ (5)
Hg, Ga+Mg→Hg, Ga(Mg) (6)
The pure Hg and Ga spherical particles at the bottom of the pit generate from deoxidation accumulation of Hg+ and Ga3+ (Eqns.(2) and (3)), which are produced by the pitting (Eqn.(1)). In the spherical Ga particle liquid appears because of the heat from the exothermic Mg2+ hydrolysis reaction. The mixture liquid of Hg and Ga enter into a metallic contacting with α-Mg, whose atoms diffuse into the liquid to form amalgam, HgMg and Ga2Mg (Eqn.(4)). Magnesium amalgam reacts severely with moisture and forms metal oxide film (feather-like) and pure liquid Hg and Ga (Eqn.(5)). The liquid Hg and Ga strip the corrosion product and react with α-Mg unceasingly (Eqn.(6)), maintaining the activation dissolution process.
3.3 Effect of Hg and Ga on electrochemical corrosion behavior
Fig.5 shows the polarization curves of the Mg-Hg-Ga alloys in 3.5%NaCl solution. Starting from the rest potential, the anode polarization shows a remarkable increase in the anodic current density. Near the rest potential as the potential rises, anodic dissolution rate increases linearly. The whole reaction is controlled by activation polarization.
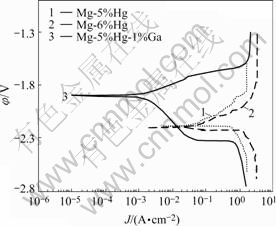
Fig.5 Polarization curves of Mg-Hg-Ga alloys in 3.5%NaCl solution at 298 K
It can be seen that Mg-5%Hg-1%Ga alloy has more positive corrosion potential than Mg-(5, 6)%Hg alloys. The corrosion current densities calculated from the polarization curves in Fig.5 demonstrate that the addition of 1%Ga into Mg-5%Hg alloy greatly reduces corrosion current density from 26.98 mA/cm2 to 2.34 mA/cm2 and Mg-5%Hg-1%Ga alloy has the smallest corrosion current density. Compared with Mg-6%Hg alloy, Mg-5%Hg has the lower corrosion current density, so the increasing content of Hg declines the corrosion resistance.
Fig.6 shows the galvanostatic plots for Mg-Hg-Ga alloys. The φ—t response obtained at current density of 100 mA/cm2 demonstrates that a very active behavior is attained, whereas the potential displays oscillations in
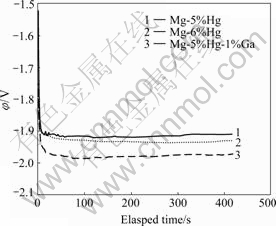
Fig.6 Galvanostatic curves of Mg-Hg-Ga alloys at 100 mA/cm2 current density
0.01 mA/cm2. Mg-5%Hg-1%Ga alloy has the most negative potential (-1.992 V) as well as the best electrochemical activity. The sequential negative potential occurs in Mg-6%Hg alloy and Mg-5%Hg alloy has the most positive potential (-1.915 V). The addition of Ga and Hg promote the electrochemical activity and the influence of 1%Ga is effective than 1%Hg.
In Mg-5%Hg-1%Ga alloy the pitting attack of α-Mg leads to the co-dissolution of Hg and Ga atoms. This accumulates liquid gallium because the exothermic Mg2+ hydrolysis reaction can enlarge the area of the Hg liquid and dissolute more Mg atoms. The Mg-Hg alloys only accumulate Hg liquid and the velocity of dissolution reaction is decreased. So the best electrochemical activity occurs in Mg-5%Hg-1%Ga alloy.
The solution of Ga in α-Mg advances the electrode potential, so the electro-negativity discrepancy between α-Mg and Mg3Hg as well as the driving force of pitting is reduced. Mg-5%Hg-1%Ga alloy has better corrosion resistance than Mg-(5, 6)%Hg alloy. Compare with the Mg-5%Hg alloy, Mg-6%Hg alloy has more cathode phase Mg3Hg, so the corrosion resistance becomes worse and the electrochemical activity becomes better.
4 Conclusions
1) The content of Hg increasing from 5% to 6% leads to the size and number of the second phase Mg3Hg increasing. The addition of 1% Ga increases the size of Mg3Hg.
2) The activation mechanism of the magnesium alloy produced by Hg and Ga is as follows. The dissolution of Hg and Ga atoms leads to the accumulation of liquid Hg and Ga, which makes a true metallic contact with α-Mg. Magnesium atoms diffuse through the liquid mercury and gallium to form magnesium amalgam and undergo severe oxidation at the amalgam/electrolyte interface. The reaction produces pure Hg and Ga again, which continues the activation process.
3) Mg-5%Hg-1%Ga alloy has more positive corrosion potential than Mg-(5, 6)%Hg alloys. The addition of 1%Ga into Mg-5%Hg alloy greatly reduces corrosion current density from 26.98 mA/cm2 to 2.34 mA/cm2 and the increasing content of Hg from 5% to 6% declines the corrosion resistance. The additions of Ga and Hg promote the electrochemical activity and the influence of Ga is effective than Hg. Mg-5%Hg-1%Ga alloy has the best electrochemical activity with the mean potential of -1.992 V.
References
[1] KING J F, UNSWORTH W. Magnesium in seawater batteries [J]. Light Metal Age, 1978, 36(7/8): 22-24.
[2] RENUKA R. Influence of allotropic modifications of surphur on the cell voltage in Mg-CuI(S) seawater activated batter [J]. Materials Chemistry and Physics, 1999, 59(1): 42-48.
[3] HIROI M. Pressure effects on the performance and the e.m.f. of the Mg-AgCl seawater battery [J]. Journal of Applied Electrochemistry, 1980, 10(2): 203-211.
[4] RENUKA R. AgCl and Ag2S as additives to CuI in Mg-CuI seawater activated batteries [J]. Journal of Applied Electrochemistry, 1997, 27(12): 1394-1397.
[5] ZHANG Yao, ZHANG Shu-kai, CHEN Li-xin, LEI Yong-quan, WILCOCK W S D, KAUFFMAN P C. Development of a seawater battery for deep-water applications [J]. Journal of Power Sources, 1997, 66(1/2): 71-75.
[6] BAGSHAW N E, THOMPSON J, WARRELL S. Anodic dissolution of magnesium alloys related to lead chloride-magnesium seawater batteries [C]// THOMPSON J. Power Sources: Research and Development in Non-Mechanical Electrical Power Sources, London: Academic Press, 1981: 117-139.
[7] FIRA S S, KIBL L, LIW L W. Water-activated disposable and long shelf-life microbatteries [J]. Sensors and Actuators A, 2004, 111: 79-86.
[8] VENKATESARA R K. Performance evaluation of Mg-AgCl batteries for under water propulsion [J]. Defense Science Journal, 2001, 5(2): 161-170.
[9] WANG Zong-shu. Summary of development of torpedo propulsion technique [J]. Journal of Naval Academy of Engineering, 1994, 1(36): 95-102.
[10] XI Bei-hua, XIA Tian. Survey of power battery for torpedo propulsion [J]. Torpedo Technology, 2005, 13(2): 7-12.
[11] FONT S, DESCROIX J P, SARIE. Advanced reserve batteries for torpedoes proplsion [C]// Proceedings of the Power Sources Symposium, Atlantic: 1984: 362-368.
[12] KIM J G, JOO J H, KOO S J. Development of high-driving potential and high-dfficiency Mg-based sacrificial anodes for cathodic protection [J]. Journal of Materials Science Letters, 2000, 19: 477-479.
[13] MA Zheng-qing, LI Wen-xian, YU Kun. Electrochemical characteristics of magnesium alloys in synthetic seawater [J]. Materials Protection, 2002, 35(12): 16-18.
[14] FLAMINI D O, SAIDMAN S B, BESSONE J B. Aluminium activation produced by gallium [J]. Corrosion Science, 2006, 48: 1413-1425.
[15] DENG Shu-hao, YI Dan-qing, ZHAO Li-hong, ZHOU Ling-ling, WANG Bin, JI Cheng-nian, LAN Bo. Study on Mg alloy anode material for seawater battery [J]. Battery Technology, 2007, 131(5): 402-405.
[16] TUCK C D, HUNTER J A, SCAMANS G M. Electrochemical behavior of Al-Ga alloys in alkaline and neutral electrolytes [J]. Journal of the Electrochemical Society, 1987, 134(12): 2970-2981.
[17] BRESLIN C B, CARROLL W M. Electrochemical behaviour of aluminium activated by gallium in aqueous electrolytes [J]. Corrosion Science, 1992, 33(11): 1735-1746.
[18] PINSSCO M R, ANGELINI E, CORDANO E, PRSALBINO F. Structural characterisation and corrosion resistance of Ga-precious metal alloys formed by liquid-solid reaction at room temperature [J]. Journal of Alloys and Compounds, 2001, 317/318: 411-418.
(Edited by YANG Bing)
Foundation item: Project(MKPT-02-181) supported by the National Defense Science and Technology Industry Committee of China
Corresponding author: FENG Yan; Tel: +86-731-8836638; E-mail: fengyanmse@yahoo.com.cn