文章编号:1004-0609(2012)09-2676-06
含锰废水深度净化的热力学分析
周兆安,甘红祥,李家元,杨天足,楚 广
(中南大学 冶金科学与工程学院,长沙 410083)
摘 要:通过对Mn2+-Ca2+-Mg2+-OH--NH3-NH4+-H2O体系的热力学分析,得到各金属离子浓度与pH 的关系,从而确定镁、锰深度脱除的条件。热力学分析结果表明,溶液中镁、锰的含量随着体系pH的增大而降低,要达到镁、锰的深度脱除必须保证pH值大于11。另外,对Ca2+-CO32--NH3-NH4+-H2O体系的分析得出,当溶液的pH控制在11~11.5之间,碳酸根离子的总浓度[C]T≥0.001 mol/L时就可以使溶液中钙的浓度降低到10-5 mol/L以下。
关键词:含锰废水;热力学分析;深度净化;镁;锰;钙
中图分类号:X703.1 文献标志码:A
Thermodynamic analysis of deep purification in
manganiferous wastewater
ZHOU Zhao-an, GAN Hong-xiang, LI Jia-yuan, YANG Tian-zu, CHU Guang
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: By thermodynamic analysis on Mn2+-Ca2+-Mg2+-OH--NH3-NH4+-H2O system, the relationship between pH value and various metals’ concentrations in the system were obtained, and then the removal condition of manganese and magnesium was established. The thermodynamic analysis results show that both the contents of manganese and magnesium in solution decrease with the system pH value increasing. In order to purify manganese and magnesium deeply, pH value of the system must be greater than 11. In addition, thermodynamic analysis on Ca2+- CO32--NH3-NH4+-H2O system shows that when the pH value of solution is controlled in the range from 11 to 11.5 and the total concentration of carbonate ion [C]T is equal or greater than 0.001 mol/L, the total concentration of calcium in solution can be reduced to below 10–5 mol/L.
Key words: manganiferous wastewater; thermodynamic analysis; deep purification; magnesium; manganese; calcium
基金项目:国家重点基础研究发展计划资助项目(2007CB613604)
收稿日期:2011-05-21;修订日期:2011-10-10
通信作者:楚 广,教授,博士; E-mail: chuguang2006@163.com
四氧化三锰是生产软磁铁氧体的主要原材料,其制成的锰锌铁氧体广泛应用于电子、电器、电力、信息等工业。自20世纪90年代中期开始进入工业生产以来,经过十余年的发展,我国四氧化三锰的生产量和规模均居世界首位,到2007年年底全国产量已达到5.5万余t[1]。目前四氧化三锰的制备方法按原料来源可分为高价锰氧化物法[2]、碳酸锰法[3]、硫酸锰法[4]、锰矿法[5]和金属锰法[6]等。我国现有的四氧化三锰生产工艺,90%以上采用电解金属锰悬浮氧化法,每生产1 t四氧化三锰用水量为5~20 t,废水中含锰离子100~ 600 mg/L[1],远大于国家污水排放标准GB 8978—1996所规定的2 mg/L以下,如果直接排放将会对水体、土壤等生态系统造成极大的污染。
目前,含锰废水的处理方法主要以沉淀法为主。姚俊等[7]采用混凝沉淀法处理电解锰生产废水,小试结果表明,在最佳pH=9.5时对锰的去除率为99.76%。樊玉川[8]利用石灰—PAC混凝沉淀法处理含锰废水,小试结果表明,在控制pH=8.5~10的条件下,锰由397 mg/L降到 0.2 mg/L。何强等[9]采用石灰中和/板框压滤机/NaOH反应沉淀/混凝沉淀工艺序批式处理电解锰厂含锰废水,工程调试结果表明可将废水中550~ 700 mg/L的锰离子降低到0.8~1.5 mg/L,出水水质可以达到排放标准,且成本较低。
一般经处理后废水虽然锰离子达到国家污水排放标准,但往往因含有大量的钙和钠,而软磁铁氧体使用四氧化三锰对钙、镁、钠等杂质有严格要求[10]。因此,处理后液难以直接返回系统使用,对含锰废水进行深度处理还需要进一步的研究,本文作者以四氧化三锰工业的含锰废水(Mn:400 mg/L,NH3-NH4+:170 mg/L,Ca:7 mg/L,Mg:20 mg/L,pH:6.8)为例,进行相应的热力学分析。
1 Mn2+-Ca2+-Mg2+-OH--NH3-NH4+-H2O系热力学分析在Mn2+-Ca2+-Mg2+-OH--NH3-NH4+-H2O系中,用氢氧化钙或氧化钙作为沉淀剂,锰、镁等均以沉淀析出。体系中可能存在的化学反应及相应的平衡常数如表1所列。
表1 锰、镁离子与有关配体的配位反应及平衡常数[11–12]
Table 1 Chemical reactions and stability constants of Mn2+ and Mg2+ with relevant complexes[11-12]
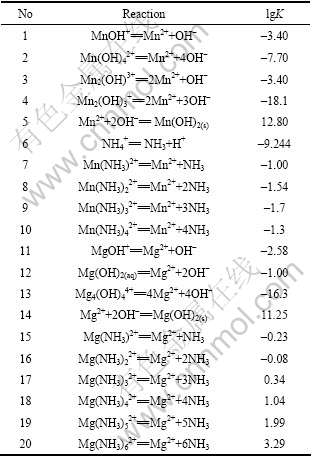
根据化学反应平衡及质量守衡定律可得以下关系式:
[N]T≈[NH3]+[NH4+]=[NH3](1+109.244–pH)=[NH3]αN (令αN=1+109.244–pH) (1)
[Mn]T =[Mn2+]+[MnOH+]+[Mn(OH)42–]+2[Mn2(OH)3+]+2[Mn2(OH)3+]+[Mn(NH3)2+]+[Mn(NH3)22+]+[Mn(NH3)32+]+[Mn(NH3)42+]=[Mn2+]+[Mn2+][OH–]+[Mn2+][OH–]4+2×[Mn2+]2 [OH–]+2×[Mn2+]2[OH–]3+[Mn2+][NH3]+[Mn2+][NH3]2+[Mn2+][NH3]3+[Mn2+][NH3]4 (2)
[Mg]T=[Mg2+]+[MgOH+]+[Mg(OH)2(aq)]+[Mg(NH3)2+]+[Mg(NH3)22+]+[Mg(NH3)23+]+[Mg(NH3)42+]+[Mg(NH3)52+]+[Mg(NH3)62+]=[Mg2+]+[Mg2+][OH–]+[Mg2+][OH–]2+[Mg2+][NH3]+[Mg2+][NH3]2+[Mg2+][NH3]3+[Mg2+][NH3]4+[Mg2+][NH3]5+[Mg2+][NH3]6 (3)
[Mn2+]=10–12.80/[OH–]2 (4)
[Mg2+]=10–11.25/[OH–]2 (5)
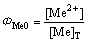 (6)
(6)
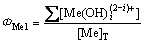 (7)
(7)
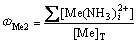 (8)
(8)
式中:[N]T、[Mn]T、[Mg]T分别表示氨、锰离子、镁离子的总浓度;ФMe0、ФMe1、ФMe2分别代表游离的金属离子、金属离子的羟基配合物以及氨基配合物所占金属离子总量的比例;[OH–]=10pH–14。在 [N]T=0.01 mol/L的条件下计算出不同pH值下溶液中各金属离子的各种配离子分布分数及其总浓度,结果如图1~3所示。
由图1和2可知,镁、锰在上述条件下主要以自由离子和与氢氧根的配合物形态存在,几乎不与氨配合,随着pH值的升高以羟合配离子形式存在的比例逐渐增大。由图3可以看出,金属离子的总浓度随着pH值的增大而急剧降低;当pH值等于11时,[Mg]T和[Mn]T均小于10–5 mol/L。因此,要想彻底除去锰、镁,必须要保持较高的pH值。另外,产生的Mn(OH)2易被氧化成为Mn3O4[13],且新生成的Mn3O4有很强的吸附作用[14–15],在此pH值下会吸附阳离子[16]。在实验过程中发现,pH值约为10时,反应1 h之后,锰和镁的含量均小于1 mg/L,实际浓度均比理论计算的小一些,另外还有不少Ca2+被吸附,也验证了它的吸附作用。
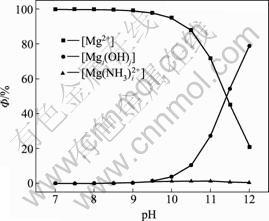
图1 镁的各种配离子分布分数与pH值的关系
Fig. 1 Relationship between distribution of complex anions of magnesium and pH value
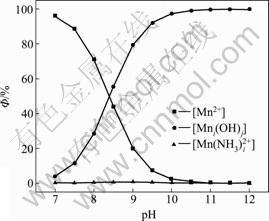
图2 锰的各种配离子分布分数与pH值的关系
Fig. 2 Relationship between distribution of complex anions of manganese and pH value
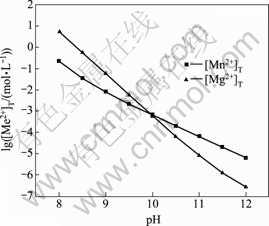
图3 金属离子总浓度与pH值的关系
Fig. 3 Relationship between [Me]T and pH value
2 Ca2+-CO32--NH3-NH4+-H2O系热力学分析在Ca2+-CO32--NH3-NH4+-H2O系中,用碳酸铵作为沉淀剂,氨水调节pH值,钙将以碳酸钙的形式沉淀析出。当反应平衡后,体系中可能存在的配位反应及相应的平衡常数如表2所列。
表2 钙离子与有关配体的配位反应及平衡常数[11–12]
Table 2 Chemical reactions and stability constants of Ca2+ with relevant complexes[11–12]

在水溶液中碳酸根为弱酸根离子,它在溶液中可以与H+发生加质子反应,有关的反应及平衡常数见表2的反应式(1)和(2)。溶液中碳酸根离子的总浓度[C]T可表示为
[C]T=[CO32–]+[HCO3–]+[H2CO3] (9)
[CO32–]、[HCO3–]、[H2CO3]所占[C]T的浓度分数ФH0、ФH1、ФH2分别为
 (10)
(10)
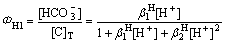 (11)
(11)
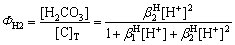 (12)
(12)
将表2中CO32–与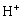 的积累加质子常数带入式(10)、(11)和(12),可得ФH0、ФH1、ФH2与pH值的关系,结果如图4所示。
的积累加质子常数带入式(10)、(11)和(12),可得ФH0、ФH1、ФH2与pH值的关系,结果如图4所示。
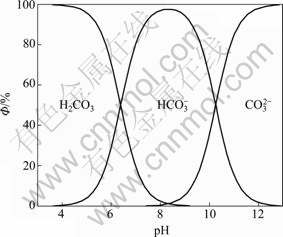
图4 ФH0、ФH1、ФH2与pH值的关系
Fig. 4 Relationships between ФH0, ФH1, ФH2 and pH value
由图4可知,[CO32–]的比例随着pH的增加而增加,当pH=10.5的时候,约占了50%。钙离子除去的彻底,必须要保证溶液中有足够的游离CO32–,这不仅要有足量的[C]T,还必须保持较高的pH值。
根据化学反应平衡及质量守衡定律可得以下关系式:
[Ca]T =[Ca2+]+[CaHCO3+]+[CaCO3(aq)]+[CaOH+]+[Ca(OH)2(aq)]+[Ca(NH3)2+]+[Ca(NH3)22 +]= ×{1+1011.329-pH([C])TΦH0}+103.15([C]TΦH0)+10pH-12.6+102pH-24.17+
×{1+1011.329-pH([C])TΦH0}+103.15([C]TΦH0)+10pH-12.6+102pH-24.17+
10-0.2([N]T/αN)+10-0.8([N]T/αN)2} (13)
式中:[Ca]T 、[C]T 、[N]T分别表示溶液中钙、碳酸根、氨的总浓度;αN=1+109.244–pH (见式(1))。
根据式(13)计算出不同条件下钙的各种配离子分布分数和浓度以及[Ca]T随pH、[C]T 和[N]T等影响因素的变化趋势。结果见图5~8。
由图5和图6可以看出,[N]T对[Ca]T的影响不大,主要是由于钙与氨形成的可溶性配合物比较少,所以 [Ca]T不会随着[N]T的增大而有明显的变化;而[C]T和pH值对[Ca]T的浓度影响较大。随着pH值增大,游离的碳酸根浓度增大,游离的钙相应会减少,导致[Ca]T降低,当pH=11时,[Ca]T达到最低值。如果pH继续增高,由图8可知钙和氢氧根形成配合物急剧增加,[Ca]T也随之增加。
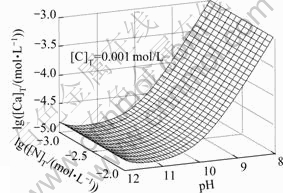
图5 lg[Ca]T随pH、lg[N]T变化的平衡曲面
Fig. 5 Equilibrium curved face of lg[Ca]T with pH and lg[N]T
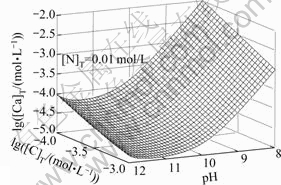
图6 lg[Ca]T随pH、lg[C]T变化的平衡曲面
Fig. 6 Equilibrium curved face of lg[Ca]T with pH and lg[C]T
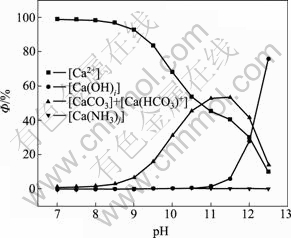
图7 钙的各种配离子分布分数与pH值的关系
Fig. 7 Relationship between distribution of complex anions of calcium and pH value ([C]T=0.001 mol/L, [N]T=0.01 mol/L)
综上所述,用氨水适当调节pH值,以碳酸铵作沉淀剂,理论上可以达到深度除钙的目的。
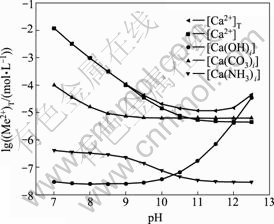
图8 钙的各种配离子浓度与pH值的关系
Fig. 8 Relationship between concentration of complex anions of calcium and pH value [C]T=0.001 mol/L, [N]T=0.01 mol/L
3 结论
1) 对Mn2+-Ca2+-Mg2+-OH--NH3-NH4+-H2O系的热力学分析后发现,四氧化三锰工业产生的含锰废水,可以先用氢氧化钙或氧化钙等作为沉淀剂,调节pH值为10~11之间进行深度净化除锰、镁以及其他一些重金属离子,且各离子浓度均可以小于10–5 mol/L。
2) 通过Ca2+-CO32--NH3-NH4+-H2O系的热力学分析得出,用氨水调节pH值在11~11.5之间,以碳酸铵为沉淀剂进行除钙,反应完毕后保持[C]T≥0.001 mol/L,就可以使[Ca]T的浓度小于10–5 mol/L。
3) 热力学分析得出经过深度净化的废水满足返回四氧化三锰生产使用的要求。
REFERENCES
[1] 曾克新. 近几年我国四氧化三锰工业发展状况与展望[J]. 中国锰业, 2009, 27(2): 1-3.
ZENG Ke-xin. The development and prospect of Mn3O4 in current years of China [J]. China’s Manganese Industry, 2009, 27(2):1-3.
[2] 连锦明, 童庆松, 吴全发, 张晓勤, 郑思宁. 微晶电解二氧化锰制备Mn3O4的研究[J]. 林化工学院学报, 2002, 19(3): 20-23.
LIAN Jin-ming, TONG Qing-song, WU Quan-fa, ZHANG Xiao-qin, ZHENG Si-ning. Study on the preparation of Mn3O4 from microcrystal electrolysis dioxide manganese [J]. Journal of Jilin Institute of Chemical Technology, 2002, 19(3): 20-23.
[3] 吴义添, 张其昕. 四氧化三锰的研制[J]. 中国锰业, 1990(4): 38-41.
WU Yi-Tian, ZHANG Qi-xing. Preparation of manganese oxide [J]. China’s Manganese Industry, 1990(4): 38-41.
[4] 陈飞宇, 梅光贵, 谭柱中. 硫酸锰溶液制备电子级四氧化三锰的研究[J]. 中国锰业, 2003, 21(3): 14-16.
CHEN Fei-yu, MEI Guang-gui, TANG Zhu-zhong. Study on producing mangano-manganic oxide from manganous sulphate solution [J]. China’s Manganese Industry, 2003, 21(3): 14-16.
[5] 张三田. 用原生锰矿石制取高纯四氧化三锰[J]. 中国锰业, 2000, 18(3): 22-24.
ZHANG San-tian. Prepare high-purity Mn3O4 from primary manganese ores [J]. China’s Manganese Industry, 2000, 18(3): 22-24.
[6] 银 瑰, 谭柱中, 曾克新. 高比表面积Mn3O4制备研究[J]. 中国锰业, 2004, 22(2): 25-27.
YIN Gui, TAN Zhu-zhong, ZENG Ke-xin. A study on preparation of high specific surface area manganous manganic oxide(Mn3O4) [J]. China’s Manganese Industry, 2004, 22(2): 25- 27.
[7] 姚 俊,田宗平, 姚祖风, 陈 上, 方继辉. 电解金属锰废水处理的研究[J]. 中国锰业,2000, 18(3):25-27.
YAO Jun, TIAN Zong-ping, YAO Zhu-feng, CHEN Shang, FANG Ji-hui. Study of treatment of wastewater from electrolying manganese [J]. China’s Manganese Industry, 2000, 18(3):25-27.
[8] 樊玉川. 含锰废水处理研究[J]. 湖南有色金属, 1998, 14(3): 36-38.
FAN Yu-chuan. Study on the treatment of wastewater with manganese [J]. Hunan Nonferrous Metals, 1998, 14(3): 36-38.
[9] 何 强, 王韧超, 柴宏祥, 韩 乔. 化学沉淀/混凝沉淀工艺序批式处理电解锰废水[J]. 中国给水排水, 2007, 23(10): 62- 64.
HE Qiang, WANG Ren-chao, CHAI Hong-xiang, HAN Qiao. Study on chemical precipitation/coagulation sedimentation process for sequencing batch treatment of electrolytic manganese wastewater [J]. China Water & Wastewater, 2007, 23(10): 62-64.
[10] GB/T 21836-20081. 软磁铁氧体使用四氧化三锰[S].
GB/T 21836-20081. Manganous manganic oxide for soft magnetic ferrites [S].
[11] SMITH R M. Critical Stability Constants [M]. New York, 1979: 4.
[12] 张保平, 唐谟堂, 杨声海. 共沉淀法制备锰锌软磁铁氧体前躯体共沉过程中钙, 镁深度脱除的热力学分析[J]. 湿法冶金, 2003, 22(4): 200-203.
ZHANG Bao-ping, TANG Mo-tang, YANG Sheng-hai. Thermodynamic analysis on removal of calcium and magnesium during preparation of Mn-Zn ferrite precursor by coprecipitation [J]. Hydrometallurgy of China, 2003, 22(4): 200-203.
[13] 谭柱中, 梅光贵, 李维健, 曾克新, 梁汝腾, 曾湘波. 锰冶金学[M]. 长沙: 中南大学出版社, 2004: 546-547.
TANG Zhu-zhong, MEI Guang-gui, LI Wei-jian, ZENG Ke-xin, LIANG Ru-teng, ZENG Xiang-bo. Metallurgy of manganese [M]. Changsha: Central South University Press, 2004: 546-547.
[14] ZHAO Zhong-wei, LIU Jing, XIA Wen-tang, CAO Cai-fang, CHEN Xing-yu, HUO Guang-sheng, CHEN Ai-liang, LI Hong-gui. Removal of molybdenum from MnSO4solution with freshly precipitated “nascent” Mn3O4 [J]. Hydrometallurgy,2009, 99(1/2): 67-71.
[15] 赵中伟, 李洪桂, 霍广生, 孙培梅, 李运娇, 孙召明. 硫酸锰溶液的深度净化方法: 中国专利ZL02150085.1 [P]. 2004- 06-09.
ZHAO Zhong-wei, LI Hong-gui, HUO Guang-sheng, SUN Pei-mei, LI Yun-jiao, SUN Zhao-ming. The methods of deep removal in manganese sulfate solution: CN ZL02150085.1 [P]. 2004-06-09
[16] 夏文堂. 硫酸锰溶液深度除钼新工艺及其基础理论研究[D]. 长沙: 中南大学, 2007: 59-61.
XIA Wen-tang. Study on novel technology and basic theory of removing molybdenum from manganese sulfate solution [D]. Changsha: Central South University, 2007: 59-61.
(编辑 何学锋)