DOI: 10.11817/j.ysxb.1004.0609.2020-39475
基于硫酸氧铋的硫酸锰溶液深度净化除氯的技术路线
刘伟锋,贾 锐,孙百奇,张杜超,陈 霖,杨天足
(中南大学 冶金与环境学院,长沙 410083)
摘 要:针对硫酸锰电解液中氯离子不断积累并损害电解过程的问题,查阅锰、锌等金属电解液中氟氯的脱除方法并对主要方法进行了归纳总结,结合各方法的优缺点,提出硫酸氧铋作为除氯剂的沉淀除氯新方法,包括“活化-沉淀-再生”三个主要方面,对工艺过程进行了研究。结果表明:在pH=1、温度30 ℃、过量系数1.5、反应时间1 h的条件下,除氯过程的除氯效率达到95.11%,沉淀物主要物相为BiOCl且结晶度良好,处理后液中含氯离子43.96 mg/L,含铋离子110.00 mg/L;再生除氯剂的除氯效率达90.75%,除氯后液含氯降至83.00 mg/L;在除氯后液的深度净化除铋过程中,铋的置换沉淀率可达98.46%,置换后液中含铋1.80 mg/L;最终所得溶液可满足锰电解要求。
关键词:锰电解液;硫酸氧铋;氯氧铋;除氯;活化;再生
文章编号:1004-0609(2020)-03-0648-09 中图分类号:TF792;TF09 文献标志码:A
锰是一种重要的金属[1-3],锰及其化合物的用途非常广泛,几乎涉及到人类生活的各个方面,其应用主要分为以下几个方面:1) 钢铁工业;2) 有色冶金工业;3) 电池工业;4) 电子工业;5) 农业;6) 环保治理;7) 医学;8) 其他行业。
自1920年英国的ALLMAND和CAMPBELL初次采用电解法制得高纯金属锰以来,迄今为止,电解法仍是全世界金属锰的最主要生产方式[1-3]。电解法生产金属锰的工艺过程为:矿物浸出-浸出液净化-电积沉锰-水洗烘干-成品电解锰,常见原料包括菱锰矿和软锰矿等。在锰矿的浸出过程中,铁、铜、锌、氯等杂质元素会进入浸出液中,通常采用水解沉淀法除铁[4-5],采用硫化沉淀法脱除铜锌镍等重金属[6],而锰电解液中氯离子一直未引起重视。
随着锰工业发展,锰矿资源大量消耗,新开采的矿石中杂质含量升高,氯的含量也逐渐增加,在浸出过程中,越来越多的杂质氯将会进入锰电解液[7]。此外,还有少部分来源于生产中使用的工业用水。
如果硫酸锰电解液中含有大量氯离子,会对电解生产造成很大的负面影响。氯离子是一种活性阴离子,在电场作用下会大量富集于阳极,容易从极板表面露出机体金属的缺陷处引起点蚀;当体系pH较低或者温度较高时会增加点蚀的倾向,点蚀严重时会引起穿孔甚至断板,加大阳极板损耗,增加生产成本[8-10]。此外,电解液中大量氯离子的存在会加重电解系统工艺设备的腐蚀,加速设备的损伤老化,从而影响生产效率,增大生产成本[11-12]。
锰电解工业作为我国重要的工业产业之一,对国民经济有着重要的影响。近年来,由于锰冶炼原料中氯含量逐渐增大,导致氯离子在锰电解液中的含量增加,这极大地影响了电解生产过程,增加生产成本,人们逐渐意识到硫酸锰电解液除氯的重要性和必要性。如何开发出一种高效、环保、低成本并且短流程的除氯技术是当务之急。
1 电解液除氯技术研究现状
1.1 电解液除氯技术概括
目前,我国对于电解液除氯技术的研究十分有限,获得工业应用的更是少之又少,仅限于几种方法,有些冶炼企业甚至未建设专门的除氯工序。本节将已有研究的比较重要的电解液除氯技术呈现出来,为冶金工作者提供参考。此外,为了能够提供尽量多的参考和借鉴,文章中还列举了一些其他行业的溶液除氯技术及硫酸盐溶液除氟的技术。根据不同技术的除氯机理,可将它们大致分为三类:物理法、吸附法和沉淀法。这些方法的名称、除氯机理、再生过程及优缺点如表1所列,这些方法的具体应用情况如后文所述。
表1 除氯技术概况
Table 1 Review of Cl removing techniques

1.2 物理除氯法
物理法除氯技术主要用于处理制碱工业产出的淡盐水,可分为吹脱法和真空法[13-14]。这类淡盐水中含有一定量的氯气,而氯气在水溶液中的溶解度与溶液表面气体压力及溶液温度有关。吹脱法就是利用大量流动空气增加气液两相接触面,从而带走大量的氯气。吹脱法是将空气加入脱氯塔内,在填料表面,空气与溶液填料表面接触,利用大量流动的空气带走溶液中的氯气。真空法脱氯的机理与其相同,只是在真空状态下,加热溶液可以进一步降低氯气在水中的溶解度,同时还能使溶液剧烈沸腾,加剧气液两相的接触,加快氯气的脱除。物理法除氯的数据并不是非常完整,具体除氯率文献中并未提及。
物理法产出的气体不能直接排放,通常引入氯气吸收系统,经过碱液吸收后制成NaClO;然而产生的NaClO价格低廉不易销售且不易保存,存在着造成二次污染的隐患。
1.3 化学吸附法
1.3.1 离子交换法
离子交换法是利用硫酸锰电解液中的氯离子与离子交换树脂中的可交换离子发生交换反应,促使树脂吸附氯离子从而实现除氯的目的。王晓丹等[15]、孙红燕等[16]都曾做过关于离子交换法从锌电解液中除氯实验的研究,但除氯效果较差,除氯率约高于50%,这可能是受到溶液pH值的影响[17]。失效树脂可实现再生,再生过程如下:首先用蒸馏水充分洗涤失效的树脂,直至使用硝酸银溶液无法测出洗涤水中的氯离子为止,随后采用硫酸对失效树脂进行再生处理。
这种方法设备简单,操作便捷,运营成本低;但脱氯率较低,且树脂再生过程耗水量较大、再生后液处理难度较大、树脂再生率不高且再生后树脂的吸附能力会明显减弱。
1.3.2 絮凝沉淀法
絮凝沉淀法是通过加入试剂在溶液中生成具有吸附能力的沉淀物,通过吸附、离子交换和络合沉降三种作用除去溶液中的特定离子[18]。最为典型的例子是利用铝盐水解生成氢氧化铝胶体吸附氟[19-20]。有文献认为是因为氟离子电负性较大,与胶体发生了氢键吸附,同时还有一部分氢氧根会和氟离子交互换位,使氟离子进入胶体;证据是在加入铝盐一段时间后pH值出现上升,可能是氟离子置换出了游离的氢氧根导致的。此外,XPS检测结果显示沉淀物中还有一部分氟形成了氟铝配合物,说明形成配合物也是除氟的另一重要机理,这也说明絮凝沉淀法除氟是多个反应共同进行的复杂过程。
目前可选用的絮凝剂种类繁多,包括铝盐、铁盐、稀土吸附剂及腐殖酸类吸附剂等;然而絮凝剂吸附作用是一次性的,目前还未发现回收再生重新利用吸附剂的研究报道,这势必会增加除杂过程的成本。此外,不同中试剂在不同体系中的吸附效果也存在差异,因此利用絮凝沉淀法脱除锰电解液中氯的过程还需要更具体的研究。
1.3.3 吸附法
参考吸附法除磷的研究,有多种吸附剂可以使用,包括氧化铝[21]、冶炼废渣、人造吸附剂[22]及活性炭等。研究结果表明,具有活性的、大比表面积的、化学稳定性好的吸附剂具有较强的吸附除磷能力,而且吸附剂的再生过程通常比较简单易操作,仅采用加热、光照等方法即可恢复吸附剂的性能。
目前想找到性能优良、成本较低且适用于锰电解液体系的除氯吸附剂[23-24]还是具有一定难度的,在现有的文献及研究成果中,这类吸附剂尚未被发现。一般用于处理重金属废水的吸附剂如沸石、粉煤灰等大多用于吸附金属阳离子,用于吸附阴离子的报道并不多见。虽然吸附剂改性后可用于吸附阴离子,但此方面研究较少且改性过程也可能会给吸附剂带来负面影响。
1.4 化学沉淀法
1.4.1 氯化亚铜沉淀法
氯化亚铜沉淀法是利用一价铜离子与电解液中的氯离子相互作用,生成难溶的氯化亚铜沉淀,从而实现氯的脱除[25]。一价铜离子的来源主要有两种:1) 直接利用铜渣、氧化亚铜、氢氧化亚铜等中的一价铜;2) 利用锌粉、铁粉或是铜粉还原二价铜生成一价铜。黄炳行等[26]就曾利用铁粉还原二价铜生成的一价铜进行过硫酸锰电解液除氯的研究,除氯效率为60.80%。
氯化亚铜脱氯渣经过后期处理可实现再生。其中一种再生方法如下:将所得的氯化亚铜渣加水浆化,随后加入氢氧化钠进行转化,加热反应一段时间后搅拌分离,即可得到氢氧化亚铜,水洗后的氢氧化亚铜可作为脱氯剂再次使用[27]。虽然氯化亚铜脱氯渣的再生在诸多文献中都有提及,但对其循环除氯效果未作说明。氯化亚铜沉淀法工业适应性较好,但工艺参数并不稳定,操作也复杂,流程周期较长,再生过程中氢氧化钠的损耗较大,成本较高。
1.4.2 氯化银沉淀法
氯化银沉淀法是通过向含氯电解液中加入银盐,使氯离子与银离子相互反应,生成难溶于水的氯化银沉淀,从而达到除去氯离子的目的[25]。这种方法操作简单,除氯效率很高,一般能达到95%以上;但因银盐价格昂贵,导致该方法成本较高,且银的再生回收较难,其运用受到限制,很少在工业生产中运用。
1.4.3 氯氧化铋沉淀法
氯氧化铋沉淀法是利用氧化铋在酸性条件下解离出的铋离子,与硫酸锰电解液中的氯离子反应生成溶度积较小的氯氧化铋,从而实现硫酸锰电解液中氯离子的脱除。封志敏等[28]进行氧化铋法从硫酸锌溶液中除氯的研究时,曾探究过部分影响因素,但未明确提及其除氯效率。文剑[29]在金狮冶金化工厂电锌系统除氯方案中选择了氯氧化铋沉淀法,但所得除氯效率不高,约为70%左右。
氯氧化铋脱氯渣可以与氢氧化钠反应实现除氯剂再生,二者在高温高碱条件下可生成氧化铋,而在低温低碱性条件下可生成氢氧化铋。封志敏等[28]、文剑[29]、吴文花等[30]、郑国渠等[31]、苏莎等[32]的研究中均提到过通过碱液转化实现脱氯剂的再生,文剑[29]的研究表明再生脱氯剂的除氯效率为70%以上,其他研究均未明确提及其循环除氯效果。该法除氯工艺效果一般,尚待完善提高,且其使用氢氧化钠实现再生时所得的高碱高氯后液较难处理,氯氧化铋脱氯渣的再生工艺尚待优化。
除了以上几种较为常见的除氯方法以外,萃取法[33]、蒸馏法、氧化法等同样可以实现氯离子的脱除;但因为其存在除氯效率低下、原料要求高、会引入杂质、成本高、无法实现除氯剂再生、工业适应性差等问题,在实际中运用较少。
1.5 除氯新工艺的提出
虽然现在可借鉴的除氯技术种类较多,但是目前还没有一种方法能够兼顾除氯效率、再生效率及再生除氯剂性能。综合对比了各种除氯方法后可知,氯氧化铋沉淀法除氯具有一定的优势:1) 该法所用设备简单,操作便捷,且工艺尚有较大的完善空间,潜力巨大;2) 铋在我国的资源储量丰富,占世界总储量的70%左右,除氯剂原料充足,成本不会过高。此外,铋号称绿色金属,较其他重金属如铜、铅、锌等产生的污染和危害较小,环保性较高,使用铋作为脱氯剂具有极大的经济性和环保性[34-36]。
目前的氯氧化铋沉淀法除氯工艺还存在一些问题。首先,该法通常采用稀硫酸对氧化铋进行转化,转化效果较差。其次,目前使用该法除氯的效果一般,除氯最佳条件尚待探索。再者,现有的氯氧铋脱氯渣再生一般采用碱法转化,成本较高,且所得的高碱高氯再生后液难以处理。此外,除氯后液中可能存在部分铋离子,其浓度是否符合电解生产的需求尚待研究。
基于此,本文提出一种基于氯氧化铋沉淀的锰电解液除氯新工艺,采用浓硫酸活化浸煮、中和除氯、浓硫酸再生及除氯后液深度净化4个步骤。该方法实现了锰电解液中氯离子的有效脱除,对硫酸电解液中氯离子的高效脱除有积极的借鉴作用。
2 硫酸锰电解液除氯新工艺
2.1 实验原料
实验原料为某企业提供的硫酸锰电解液,pH≈2.3,其主要成分如表2所列。
表2 硫酸锰电解液的主要成分
Table 2 Main composition of MnSO4 electrolyte (g/L)
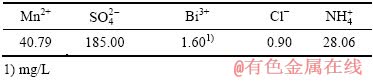
所用三氧化二铋(99.9%)、三氧化二锑(99.9%)、碳酸锰(≥99.6%)均为分析纯,硫酸(98%)为工业纯,锰粉纯度为99.9%。
2.2 工艺过程与原理
本文提出的硫酸氧铋沉淀除氯新工艺如下:首先采用浓硫酸浸煮的方法将氧化铋转化为硫酸氧铋,同时实现物料活化,如式(1)所示;然后将硫酸氧铋加入到锰电解液中,并调整pH值进行中和除氯,氯将以氯氧化铋的形式沉淀除去,过程如式(2);除氯剂再生过程则是通过浓硫酸浸煮氯氧化铋的方式再生成硫酸氧铋并返回使用,反应过程如式(3);最后为了保证除氯后电解液的品质,还加入锰粉进行置换净化的工序以脱除溶液中的铋,如式(4)所示。整个工艺流程如图1所示。
Bi2O3+H2SO4=(BiO)2SO4+H2O (1)
(BiO)2SO4+2HCl=2BiOCl↓+H2SO4 (2)
2BiOCl+H2SO4=(BiO)2SO4+2HCl↑ (3)
(BiO)2SO4+3Mn+2H2SO4=2Bi+3Mn2SO4+2H2O (4)
2.3 检测手段及数据分析
本实验主要的分析检测产物为活化产物、氯氧铋渣、再生除氯剂、脱氯后液及置换后液。固体产物取一定量样品进行溶解并稀释后制成溶液进行检测,液体样品则是取样后直接稀释制成待测溶液。铋浓度的测定主要采用ICP-AES分析仪测定(美国热电公司IRIS Interprid Ⅲ XRS型电感耦合等离子体发射光谱仪),氯离子则采用硫氰酸钾间接容量法进行滴定,固体样品全元素分析采用XRF检测,沉淀物物相则采用XRD衍射仪识别(日本理学公司TTRAX-3型,所用靶材为铜靶,自带单色器,Kα波长为1.54184 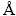 。测试用电压为40 kV,测试电流为250 mA,扫描条件为10 (°)/min)。
。测试用电压为40 kV,测试电流为250 mA,扫描条件为10 (°)/min)。

图1 硫酸锰电解液除氯过程的工艺流程图
Fig. 1 Flow sheet of MnSO4 electrolyte Cl removing process
除氯效率计算如下:
 (5)
(5)
式中:R为除氯效率;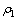 和
和 分别为原液和除氯后液中氯离子浓度,mg/L;V1和V2为原液和除氯后液体积,L。
分别为原液和除氯后液中氯离子浓度,mg/L;V1和V2为原液和除氯后液体积,L。
铋的置换沉淀率计算如下:
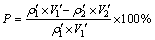 (6)
(6)
式中:P为置换沉淀率; 和
和 分别表示原液和除氯后液中氯离子浓度,mg/L;
分别表示原液和除氯后液中氯离子浓度,mg/L;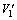 和
和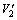 为原液和除氯后液体积,L。
为原液和除氯后液体积,L。
3 结果与讨论
3.1 硫酸浸煮活化过程
在温度150 ℃条件下,使用硫酸浸煮Bi2O3约0.5 h,以进行除氯剂的转换及活化过程;浸煮过程不断用玻璃棒进行搅拌确保反应充分进行,转化结束后沉淀物全部转化为淡黄色或白色。取转化产物进行XRD检测,检测结果如图2所示。由图2可知,经过该过程处理,沉淀物物相大部分转化为硫酸氧铋,只有极少部分残余的Bi2O3物相存在。
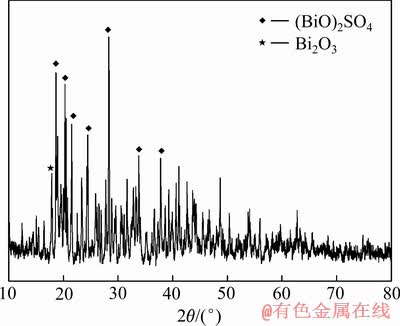
图2 活化除氯剂的XRD谱
Fig. 2 XRD pattern of activated removal agent
3.2 中和除氯过程
在完成了除氯剂的制备和活化后,趁热将活化产物全部倒入500 mL硫酸锰电解液中,在除氯剂过量系数1.5、pH=1、温度30 ℃及反应时间1 h的条件下进行中和除氯实验,测试除氯渣及除氯后液中的氯含量,其结果见表3。由表3可知,在最优条件下,除氯效率达到95.11%。除氯后液残余氯离子浓度仅为43.96 mg/L,满足电解要求。沉淀物中氯含量为6.04%。对沉淀物进行X射线衍射以检测其物相组成,检测结果如图3所示。
表3 除氯实验结果
Table 3 Results of Cl- removing experiment
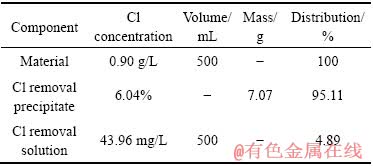
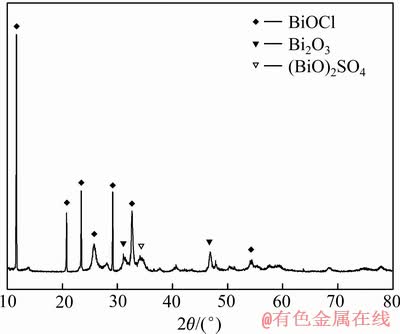
图3 除氯沉淀物的XRD谱
Fig. 3 XRD pattern of Cl removal precipitate
由图3可知,沉淀物物相组成为BiOCl、Bi2O3及(BiO)2SO4。其中主要物相是BiOCl,Bi2O3相可能是活化过程未被完全转化的Bi2O3,(BiO)2SO4则可能是除氯过程未反应的残余组分。
3.3 除氯剂再生过程
除氯剂再生过程实验条件为反应温度150 ℃、浸煮时间30 min。浸煮过程中用玻璃棒进行搅拌以保证再生过程充分进行,反应完成后取样品检测其物相组成,再生产物的XRD谱如图4所示。
由图4可知,再生产物中主要物相包括Bi2O3和(BiO)2SO4,且不存在BiOCl相,这说明再生过程将除氯产物完全转化为可用于除氯过程的Bi2O3和(BiO)2SO4相,即能够有效实现除氯剂的再生。对活化产物进行化学成分分析,结果如表4所列。
由成分表4可看出,再生产物主要成分是Bi、O和S,未检测到Cl含量,说明再生过程彻底将氯脱除。

图4 再生产物的XRD谱
Fig. 4 XRD pattern of regeneration product
表4 最优条件下再生硫酸氧铋的成分
Table 4 Components of optimal regenerated (BiO)2SO4 (mass fraction, %)

氯离子主要进入了气相,可以认为氯离子与氢离子结合后以氯化氢的形式挥发了。这些氯化氢可以被收集并制备成盐酸溶液。
为了验证再生除氯剂的除氯效果是否良好,将该产物作为除氯剂再次进行除氯实验,实验条件与3.2节中的相同。实验结果显示:除氯后液中氯离子含量83.00 mg/L,二次除氯效率达到90.78%,说明再生后仍具有较好的除氯能力。
3.4 深度净化除铋过程
深度净化过程所用原料为除氯后液,经检测其铋浓度为0.11 g/L。在锰粉过量系数2.0,反应温度30 ℃及反应时间1 h的条件下,置换后液中残余铋离子浓度仅为1.80 mg/L,置换沉淀率高达98.46%。深度净化后液中铋离子浓度与原料基本相同(1.60 mg/L),没有引入杂质且不会影响后续步骤。置换产物主要成分是铋,可返回活化步骤做除氯剂使用。
硫酸氧铋除氯工艺能够高效除氯,同时能够实现除氯剂再生循环的目标。该工艺过程除氯效率高,再生过程简单易操作且再生除氯产物具有良好的除氯能力。再生过程产生的氯化氢气体可收集并制成盐酸并加以利用,置换产物可返回活化步骤使用。整个过程没有废物外排,不仅解决了除氯的问题,而且避免环境的污染。该工艺满足一切工业化生产需要满足的条件,具有实际应用价值。此外,该工艺还为“硫酸溶液脱除氯离子”及类似课题提供了一种新的思路。
4 结论
1) 提出了基于硫酸氧铋的除氯新工艺,实现硫酸锰电解液高效除氯的同时也实现除氯剂的循环再生使用。采用硫酸浸煮活化工艺将三氧化二铋转化为硫酸氧铋完成活化,XRD检测结果显示转化后产物的主要物相与预期相同。除氯过程控制硫酸氧铋过量系数1.5、pH=1、反应温度30 ℃和反应时间1 h,除氯后液中含氯离子43.96 mg/L,除氯效率达到95.11%,沉淀物主要物相为BiOCl且结晶度良好。
2) 采用浓硫酸浸煮的方法使BiOCl转化为(BiO)2SO4,并使其中的氯离子以氯化氢形式挥发,再生产物中氯含量低于0.01%。用再生后的除氯剂进行中和除氯,除氯后液含氯降至83.00 mg/L,除氯效率达90.75%,完成了除氯剂的循环再生使用。
3) 对除氯后液(含铋离子110.00 mg/L)使用锰粉置换法净化除去残余铋离子。在置换剂过量系数2.0,温度30 ℃,反应时间1 h的条件下实验,置换后液含铋降低至1.80 mg/L,深度净化除铋过程中铋的置换沉淀率可达98.46%。
4) 该工艺以硫酸氧铋为除氯剂,通过中和沉淀的方法实现了高效除氯;同时采用硫酸浸煮的方法实现了除氯剂的循环再生;除氯后液的深度净化亦能保证净化后的硫酸锰溶液能够达到电解的需求。该方法不仅集高效除氯和除氯剂循环再生于一体,更是实现了生产废物的零排放并杜绝了环境污染,具有实际应用的价值。该工艺将为硫酸水溶液中氯离子的脱除提供一种新的思路。
REFERENCES
[1] 谭柱中, 梅光贵, 李维健. 锰冶金学[M]. 长沙: 中南大学出版社, 2004.
TAN Zhu-zhong, MEI Guang-gui, LI Wei-jian. Metallurgy of manganese[M]. Changsha: Central South University Press, 2004.
[2] 王运正, 王吉坤, 谢红艳. 现代锰冶金[M]. 北京: 冶金工业出版社, 2015.
WANG Yun-zheng, WANG Ji-kun, XIE Hong-yan. Modern metallurgy of manganese[M]. Beijing: Metallurgical Industry Press, 2015.
[3] 梅光贵, 张文山, 曾湘波. 中国锰业技术[M]. 长沙: 中南大学出版社, 2011.
MEI Guang-gui, ZHANG Wen-shan, ZENG Xiang-bo. Technology of China manganese industry[M]. Changsha: Central South University Press, 2011.
[4] 陈家墉, 于淑秋, 伍志春. 湿法冶金中铁的分离与运 用[M]. 北京: 冶金工业出版社, 1991.
CHEN Jia-yong, YU Shu-qiu, WU Zhi-chun. The separation and usage of iron in hydrometallurgy[M]. Beijing: Metallurgical Industry Press, 1991.
[5] 阳卫军, 屈晓娟, 朱利军. 低品位软锰矿浸出液中铁的去除方法研究[J]. 湖南大学学报(自然科学版), 2014, 41(1): 107-111.
YANG Wei-jun, QU Xiao-juan, ZHU Li-jun. Study on the removal of iron of low-grade pyrolusite leaching solution[J]. Journal of Hunan University (Natural sciences), 2014, 41(1): 107-111.
[6] 彭爱国, 贺周初, 郑贤福, 余长艳, 刘昱霖, 皮银安. 硫酸锰深度除杂研究[J]. 精细化工中间体, 2001, 32(2): 52-53.
PENG Ai-guo, HE Zhou-chu, ZHENG Xian-fu, YU Chang-yan, LIU Yu-lin, PI Yin-an. The study of MnSO4 solution purification[J]. Fine Chemical Intermediats, 2001, 32(2): 52-53.
[7] 程亚亚. 高氯菱锰矿预处理及浸出液脱氯工艺研究[D]. 重庆: 重庆大学, 2015.
CHENG Ya-ya. Study on high chlorine rhodochrosite pretreatment and dechlorination from leaching solution[D]. Chongqing: Chongqing University, 2015.
[8] 吴安东. 菱锰矿粉和锰电解液中氯离子的脱除及脱氯铜盐的回收研究[D]. 兰州: 兰州大学, 2015.
WU An-dong. Study on the dichlorination of rhodochrosite powder and manganese electrolyte and copper salt recovery[D]. Lanzhou: Lanzhou University, 2015.
[9] 胡一航, 王海北, 王玉芳. 锌冶炼中氟氯的脱除方法[J]. 矿冶, 2016, 25(1): 36-40.
HU Yi-hang, WANG Hai-bei, WANG Yu-fang. Removal of fluorine and chlorine in zinc extraction process[J]. Mining & Metallurgy, 2016, 25(1): 36-40.
[10] 谭 青, 李启厚, 刘志宏, 李玉虎, 刘智勇, 刘付朋. 湿法炼锌过程中氟氯脱除技术研究现状[J]. 湿法冶金, 2015, 34(4): 264-269.
TAN Qing, LI Qi-hou, LIU Zhi-hong, LI Yu-hu, LIU Zhi-yong, LIU Fu-peng. Current situation on removal of fluorine and chlorine in zinc hydrometallurgy[J]. Hydrometallurgy of China, 2015, 34(4): 264-269.
[11] 王文录. 湿法炼锌中氯的危害及控制[J]. 湖南有色金属, 2007, 23(1): 22-24.
WANG Wen-lu. The behaviors and control of chlorine in zinc hydrometallurgical extraction[J]. Hunan Nonferrous Metals, 2007, 23(1): 22-24.
[12] 唐道文, 毛小浩, 黄碧芳, 赵平原. 硫酸锌溶液中氟氯净化的实验研究[J]. 贵州工业大学学报(自然科学版), 2004, 33(3): 15-17.
TANG Dao-wen, MAO Xiao-hao, HUANG Bi-fang, ZHAO Ping-yuan. Study on F-, Cl- purification process from zinc sulfuric solution[J]. Journal of Guizhou University of Technology (Natural Science Edition), 2004, 33(3): 15-17.
[13] 李文云, 王 鑫. 淡盐水脱氯工艺[J]. 氯碱工业, 2009, 45(9): 12-15.
LI Wen-yun, WANG Xin. A dechlorination process for diluted brine[J]. Chlor Alkali Industry, 2009, 45(9): 12-15.
[14] 刘亚杰. 淡盐水脱氯工艺[J]. 中国氯碱, 2005(9): 10-11.
LIU Ya-jie. Dechlorination process of depleted brine[J]. China Chlor-Alkali, 2005(9): 10-11.
[15] 王晓丹, 饶金元, 牛旭斐, 朱 云. 离子交换法从锌电解液中除氯的实验研究[J]. 云南冶金, 2010, 39(4): 33-36.
WANG Xiao-dan, RAO Jin-yuan, NIU Xu-fei, ZHU Yun. Experimental study on chlorine removal in zinc electrolyte by ion exchange[J]. Yunnan Metallurgy, 2010, 39(4): 33-36.
[16] 孙红燕, 森 维, 孔 馨, 刘贵阳, 赵龙生. 离子交换法从锌电解液中脱除氯试验研究[J]. 湿法冶金, 2017, 36(2): 133-136.
SUN Hong-yan, SEN Wei, KONG Xin, LIU Gui-yang, ZHAO Long-sheng. Removal of chlorine from zinc electrolyte by ion exchange[J]. Hydrometallurgy of China, 2017, 36(2): 133-136.
[17] 杨 涛. 高浓度氯离子去除技术研究[D]. 兰州: 兰州交通大学, 2017.
YANG Tao. Study on removal of high concentration chlorine ion[D]. Lanzhou: Lanzhou Jiaotong University, 2017.
[18] 彭映林, 余 旺, 郑雅杰, 李长虹. 复合磁絮凝剂的制备及其对黄药废水的处理[J]. 中国有色金属学报, 2018, 28(8): 1676-1687.
PENG Ying-lin, YU Wang, ZHENG Ya-jie, LI Chang-hong. Preparation of composite magnetic flocculant and its application in treatment of butyl xanthate wastewater[J]. The Chinese Journal of Nonferrous Metals, 2018, 28(8): 1676-1687.
[19] 李连香, 刘文朝, 孙瑞刚, 李铁光. 无定形氢氧化铝吸附剂的制备及其除氟性能研究[J]. 水利水电技术, 2017, 48(3): 93-98.
LI Lian-xiang, LIU Wen-xiang, SUN Rui-gang, LI Tie-guang. Study on preparation of amorphous aluminum hydroxide adsorbent and its fluoride removal performance[J]. Water Resources and Hydropower Engineering, 2017, 48(3): 93-98.
[20] 苏荣梅. 高氟地下水除氟研究[D]. 吉林: 吉林大学, 2007.
SU Rong-mei. Study on disposing of high-flouride containing groundwater[D]. Jilin: Jilin University, 2007.
[21] 刘桂华, 陈斌斌, 齐天贵, 周秋生, 彭志宏, 李小斌, 董文波. 氧化铝粉尘吸附脱除铝酸钠溶液中的腐殖酸钠[J]. 中国有色金属学报, 2017, 27(11): 2356-2362.
LIU Gui-hua, CHEN Bin-bin, QI Tian-gui, ZHOU Qiu-sheng, PENG Zhi-hong, LI Xiao-bin, DONG Wen-bo. Removal of sodium humate from sodium aluminate solution by adsorption with alumina dust[J]. The Chinese Journal of Nonferrous Metals, 2017, 27(11): 2356-2362.
[22] 杨欣欣, 徐源来, 马 晨, 郭 格, 周 芳, 池汝安. 纤维状材料对模拟高效废液中钯的吸附行为[J]. 中国有色金属学报, 2018, 28(7): 1453-1461.
YANG Xin-xin, XU Yuan-lai, MA Chen, GUO Ge, ZHOU Fang, CHI Ru-an. Adsorption behavior of Pd onto fibrous adsorbent from simulated high level liquid waste[J]. The Chinese Journal of Nonferrous Metals, 2018, 28(7): 1453-1461.
[23] AL-RAWAJFEH A. Enhancement of hardness and chloride removal and reduction of Cl2 release and corrosion in electrodeionization units[J]. Journal of Water Process Engineering, 2015, 5: 160-165.
[24] AL-RAWAJFEH A, AL-SHAMAILEH E, AL-WHOOSH K, AL-MA’ABRAH A, AL-ZORQAN R, ZANOON R, RAWAJFEH K, AL-JUFOUT S. Adsorption desalination of chloride ions on composite natural-synthetic material: An approach for the reduction of chlorine corrosion in electrodeionization units[J]. Journal of Industrial and Engineering Chemistry, 2013, 19: 1895-1902.
[25] 王明辉, 未立青, 郭天立, 程永强. 高含氯硫酸锌溶液中氯的脱除工艺研究[J]. 有色矿冶, 2013, 29(3): 32-34.
WANG Ming-hui, WEI Li-qing, GUO Tian-li, CHENG Yong-qiang. Process study on removing chloride from zinc sulfuric solution containing high concentration chloride[J]. Non-ferrous Mining and Metallurgy, 2013, 29(3): 32-34.
[26] 黄炳行, 邓永光, 王雨红, 粟海锋. 锰电解液中氯离子的脱除研究[J]. 有色金属(冶炼部分), 2013(6): 11-14.
HUANG Bing-xing, DENG Yong-guang, WANG Yu-hong, SU Hai-feng. Study on removal of chloride ions from manganese electrolyte[J]. Nonferrous Metals (Extractive Metallurgy), 2013(6): 11-14.
[27] 李 春, 李自强. 氯化亚铜沉淀脱氯反应平衡的研究[J]. 湿法冶金, 2001, 20(3): 152-155.
LI Chun, LI Zi-qiang. Study on reaction equilibriums of removing chloride by cuprous chloride precipitation[J]. Hydrometallurgy of China, 2001, 20(3): 152-155.
[28] 封志敏, 宁顺明, 王文娟, 佘宗华, 万洪强, 吴江华. 氧化铋法从硫酸锌溶液中除氯的研究[J]. 矿冶工程, 2015, 35(4): 63-66.
FENG Zhi-min, NING Shun-ming, WANG Wen-juan, SHE Zong-hua, WAN Hong-qiang, WU Jiang-hua. Dechlorination of zinc sulfuric solution by bismuth oxide[J]. Mining and Metallurgical Engineering, 2015, 35(4): 63-66.
[29] 文 剑. 金狮冶金化工厂电锌系统除氯方案选择研究[J]. 湖南有色金属, 2008, 24(6): 34-36.
WEN Jian. Study on the choice of dechlorination in the production of electric zinc of Jinshi metallurgy chemical plant[J]. Hunan Nonferrous Metals, 2008, 24(6): 34-36.
[30] 吴文花, 刘吉波, 田思远, 王志坚, 苏正夫. 锌电解液除氯渣氯氧化铋再生循环使用研究[J]. 中国有色冶金, 2015, 44(1): 71-73.
WU Wen-hua, LIU Ji-bo, TIAN Si-yuan, WANG Zhi-jian, SU Zheng-fu. Research of regeneration and recycle use of BiOCl in chlorine removal slag of zinc electrolyte[J]. China Nonferrous Metallurgy, 2015, 44(1): 71-73.
[31] 郑国渠, 曹华珍, 唐谟堂. 氯氧铋制备高纯氧化铋过程中除氯的研究[J]. 有色技术, 2001, 53(2): 52-54.
ZHENG Guo-qu, CAO Hua-zhen, TANG Mo-tang. Study on dechlorination in process of preparing high purity Bi2O3 from BiOCl[J]. Nonferrous Metals, 2001, 53(2): 52-54.
[32] 苏 莎. 硫酸废液中氟氯的去除[D]. 长沙: 中南大学, 2012.
SU Sha. The removal of fluoride and chloride from sulfuric acid waste[D]. Changsha: Central South University, 2012.
[33] 窦传龙. 溶剂萃取法从硫酸锌溶液中萃取脱氯的试验研究[J]. 湖南有色金属, 2009, 25(4): 21-24.
DOU Chuan-long. Experiment study on extracting dechlorination by solvent extraction from zinc sulfate solution[J]. Hunan Nonferrous Metals, 2009, 25(4): 21-24.
[34] 龙 涛, 陈其慎, 于汶加, 余 倩, 张艳松. 中国铋供需形势分析及对策建议[J]. 中国矿业, 2016, 25(5): 11-15.
LONG Tao, CHEN Qi-shen, YU Wen-jia, YU Qian, ZHANG Yan-song. The analysis and suggestions of the bismuth’s supply and demand in China[J]. China Mining Magazine, 2016, 25(5): 11-15.
[35] 汪立果. 铋冶金[M]. 北京: 冶金工业出版社, 1986.
WANG Li-guo. Bismuth metallurgy[M]. Beijing: Metallurgical Industry Press, 1986.
[36] U.S.Geological Survey. Mineral Yearbook[R]. 2015.
Technical idea on dechlorination in MnSO4 electrolyte by using (BiO)2SO4
LIU Wei-feng, JIA Rui, SUN Bai-qi, ZHANG Du-chao, CHEN Lin, YANG Tian-zu
(School of Metallurgy and Environment, Central South University, Changsha 410083, China)
Abstract: A new method of removing Cl- as BiOCl by precipitation was proposed to weaken the negative effect during Mn electrolytic deposition which was taken by Cl-, after reviewing and summarizing relevant references. The new method can be divided into three steps: transforming Bi2O3 to (BiO)2SO4, removing Cl- by precipitation and regeneration. The results show that (BiO)2SO4 is the most ideal Cl- removal agent and the process of boiling in sulfuric acid is necessary. In precipitation, the factors of pH, temperature, excess coefficient and duration time affect the results significantly. Moreover, decreasing pH can dramatically drive the reaction of Cl- removing. The Cl- concentration decreases from 900.00 mg/L to 43.96 mg/L and the Cl- removal rate reaches 95.11% under the optimal condition experiment, and the Cl removal precipitate consists of BiOCl mainly. In regeneration process, BiOCl can be transferred to (BiO)2SO4 by boiling in hot sulfuric acid, and the Cl- removal rate can reach 90.78% when the regeneration removal agent is used. The concentration of remined Bi3+ in Cl- removal solution is declined to 1.80 mg/L by cemented using Mn powders and the cementation rate reaches 98.46%.
Key words: manganese electrolyte; (BiO)2SO4; BiOCl; Cl removal; activation; regeneration
Foundation item: Projects(2018YFC1901604, 2018YFC1901605)supported by the National Key Research and Development Program of China; Project(201806375047) supported by the Visiting Scholar of China Scholarship Council; Project(51404296) supported by the Young Scientists Fund of National Natural Science Foundation of China
Received date: 2019-05-05; Accepted date: 2019-05-17
Corresponding author: LIU Wei-feng; Tel: +86-13548654403; E-mail: liuweifeng@csu.edu.cn
(编辑 龙怀中)
基金项目:国家重点研发计划资助项目(2018YFC1901604, 2018YFC1901605);国家留学基金委访问学者项目(201806375047);国家自然科学青年基金资助项目(51404296)
收稿日期:2019-05-05;修订日期:2019-05-17
通信作者:刘伟锋,副教授,博士;电话:13548654403;E-mail:liuweifeng@csu.edu.cn