
Thermodynamic properties of vapor complex Na2ZrCl6
LI Jun-li(李军丽)1, YU Jin(于 锦)1, 2, YANG Dong-mei(杨冬梅)1, WANG Zhi-chang(王之昌)1
1. School of Sciences, Northeastern University, Shenyang 110004, China;
2. School of Sciences, Shenyang University of Technology, Shenyang 110023, China
Received 7 September 2006; accepted 20 December 2006
Abstract: Thermodynamic studies were carried out for the vapor complex of sodium chloride with zirconium tetrachloride at 718-778 K and 0.5-2.5 kPa by using high temperature phase equilibrium-quenching experiments, taking closed Pyrex glass ampoules as the reaction containers. The results show that the sole predominant vapor complex is Na2ZrCl6 for the ZrCl4-NaCl system under the experimental conditions. The thermodynamic equilibrium constants and other thermodynamic functions of the reaction 2NaCl(s)+ZrCl4(g)=Na2ZrCl6(g) have been derived from the measurements. The results for the changes in enthalpy and entropy are ?H0=(-70.1±1.5) kJ/mol and ?S0=(-105.9±2.0) J/(mol?K) in the temperature range.
Key words: ZrCl4; NaCl; Na2ZrCl6; vapor complex; phase equilibrium-quenching experiment
1 Introduction
Transition metal halide vapor complexes have been used as key constituents chemically transported in the high temperature processes such as chemical synthesis, catalysis, extraction and separation, high-intensity discharge lamps, and lasers. To understand the nature of these high temperature processes, it is necessary to know their stoichiometry, structure and thermodynamic properties. There have been relatively more experimental and theoretical studies on the structure, properties and applications of the rare earth (ⅢB group metal) halide vapor complexes (see, for example, Refs.[1-12] and references therein). However, the same information is relatively few for the stoichiometry, structure and thermodynamic properties of the ⅣB group metal halide vapor complexes.
We have determined the thermodynamic properties of the vapor complexes in the AlCl3-LnCl3 systems from Ln=Sc to Ln=Lu at 588-838 K[13-16] and of the NbCl5-NaCl and NbCl5-KCl binary systems at 580-680 K[17] by using the high temperature phase equilibrium- quenching experiments. In this study, we report the formation thermodynamics of the vapor complex Na2ZrCl6 in the ZrCl4-NaCl system determined by the same quenching experiments.
We try to extend the same measurements to the vapor complex K2ZrCl6 in ZrCl4-KCl system. Unfortunately, however, the vapor complex is very unstable and its yield is too low to be quantitatively determined under the same experimental conditions by this method, which makes us unable to report the thermodynamic data for the K2ZrCl6 vapor complex.
2 Experimental
The chemicals used in this study were of 99.5% purity for anhydrous ZrCl4 and of 99.99% purity for NaCl and KCl from Aldrich Chemical Co. The anhydrous ZrCl4 was further purified by repeated sublimations under vacuum. NaCl and KCl were further crystallized using double-distilled water and then dried under vacuum over P2O5. The experiments were carried out in closed ampoules made from Pyrex glass with a special shape as shown in Fig.1. The volumes of the ampoules were determined to be 50-60 mL.
The quenching experiments employed in this study were similar to those described in Refs.[13-16]. An excess of NaCl or KCl, and less ZrCl4 were placed in the deep ditch of the ampoule (see part A in Fig.1) under argon atmosphere and the ampoule was then sealed under vacuum. Four ampoules were placed in a graphite container and then placed in a furnace, where the four isothermal samples with different initial amounts of anhydrous ZrCl4 and NaCl or ZrCl4 and KCl resulted in a set of isothermal complexation data at different pressures. The temperature was kept constant within ±0.5 K measured with a Pt-PtRu10 thermocouple so as to ensure the four samples to be at the same temperature during each run. The maximum temperature difference in the container was always smaller than 1.0 K.

Fig.1 Schematic of ampoule
Preliminary experiments showed that the glass ampoule broke easily when quenching from an equilibrium pressure higher than 0.25 MPa at high temperature. Ref.[18] showed that the normal sublimation temperature is 605 K and the melting point is 710 K for ZrCl4. Moreover, Ref.[19] showed that the solid and liquid phases might coexist in the ZrCl4-NaCl system at temperatures higher than 812 K, where the activities of ZrCl4 and NaCl are unknown. Therefore, the formal experiments were carried out at pressures lower than 0.25 MPa and at temperatures lower than 800 K but higher than 715 K for the ZrCl4-NaCl system to ensure the complete evaporation of ZrCl4 and the existence of pure solid NaCl in the deep ditch of the ampoule in this study.
Our preliminary experiments showed that the complexation equilibrium might be achieved within 5 h. Therefore, the formal equilibrium period was chosen to be 6 h for each run. After the equilibrium had been achieved, the graphite container was quickly removed from the furnace and the ampoules were covered with asbestos, then quenched with ice water. Thus the equilibrium gas phase was rapidly and uniformly condensed all over the ampoules. The amounts of substance of Zr4+ and Na+ condensed in part B of the ampoules could then be determined by spectrophotometry and atomic absorption spectro- photometry, respectively.
3 Results and discussion
At temperatures below 800 K, the equilibrium pressure of pure solid NaCl is negligible. On the other hand, however, the Na+ ions are condensed in part B of the ampoule after quenching. It is known [20] that there is a dimerization reaction in the ZrCl4 vapor but with the thermodynamic equilibrium constant only in the magnitude of 10-13. Therefore, the Na+ ions condensed in part B of the ampoule may reasonably be assumed to all belong to the vapor complexes NauZrvClu+4v(g) in the ZrCl4-NaCl system formed by the reaction:
uNaCl(s)+vZrCl4(g)= NauZrvClu+4v(g) (1)
with the equilibrium constant

which yields

where p0 =0.1 MPa, pi denotes the partial pressure of i, c(uv) and Kp(uv) denote the vapor complex NauZrvClu+4v and its equilibrium constant, respectively. The relationships between pi and the analysis results of Zr4+ and Na+ may be given by

and the total pressure may be given by
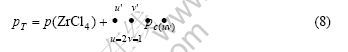
where T is the temperature, V is the volume of part B of the ampoule, ni is the amounts of substance of the component i. After calculating the quantities from the analysis results of n(Zr4+) and n(Na+), the molar Gibbs energy (?G0), molar enthalpy (?H0) and molar entropy (?S0) of reaction (1) may be determined by

The Refs.[19,21-24] suggested the Na2ZrCl6 to be the sole solid and liquid complex in the ZrCl4-NaCl system. Therefore, one may assume the Na2ZrCl6 (or Na2ZrvCl4v+2) to be the sole vapor complex in this study for the same system. In that case, Kp(uv), nc(uv) and pc(uv) may simply be rewritten as Kp, nc and pc, and Eqns.(6) and (7) may be simplified to
n(Zr4+)=n(ZrCl4)+vnc (10)
n(Na+)=2nc (11)
The calculation results of Eqns.(3)-(5) and (9)-(11) should lead to the straight lines of ln(pc/p0) vs ln(p(ZrCl4)/p0) with the slope of v and of RlnKp vs 1/T for the ZrCl4-NaCl system.
On the other hand, SCH?FER[25] and PAPATHEODOROU et al[1,26] suggested the u=v=1 type vapor complex to be the only vapor complex in many alkali metal chloride-containing binary systems, such as KMgCl3, NaSnCl3, KLuCl4 and NaFeCl4. According to this, one may also assume the Na2ZrCl6 vapor complex to coexist with the NaZrCl5 vapor complex in the ZrCl4-NaCl system. In this case, however, the calculation results of Eqns.(3)-(5) and (9)-(11) may result in the nonlinear ln(pc/p0)—ln(p(ZrCl4)/p0) and/or RlnKp—1/T relationships in the ZrCl4-NaCl system, similar to the situations of LaAl3Cl12-LaAl4Cl15 or CeAl3Cl12-CeAl4Cl15 coexistence[16] and of DyAl3Cl12- DyAl2Cl9[14] or HoAl3Cl12-HoAl2Cl9 coexistence[16].
The calculation results of Eqns.(3)-(5) and (8)-(10) are listed in Table 1 for the ZrCl4-NaCl system. It can be seen that the values of the apparent stoichiometric factor v are within 1.03-1.08 at 718-778 K for the ZrCl4-NaCl system, being very close to the theoretical value of v=1.0.
Fig.2 also shows that the plots of ln(pc/p0) vs ln(p(ZrCl4)/p0) are all straight lines with the slope of v=1. It may be therefore concluded that Na2ZrCl6 is the predominant vapor complex for the ZrCl4-NaCl system at 718-778 K. The values of Kp of reaction (1) at v=1.0 can then be calculated in terms of Eqn.(3) for Na2ZrCl6.
The results are given in the last column of Table 1. The plots of RlnKp vs 1/T are shown in Fig.3 for Na2ZrCl6, which are also straight lines.

Fig.2 Plots of ln(pc/p0) vs ln(p(ZrCl4)/p0) for NaCl-ZrCl4 binary system

Fig.3 Plots of RlnKp vs 1/T for NaCl-ZrCl4 binary system
Table 1 Quenching experimental results for vapor complexes Na2ZrCl6

The thermodynamic quantities of reaction (1) can be determined from the experiments by a least squares computation within the reaction temperature ranges. The results are ΔH0=-70.1 kJ/mol and ?S0= -105.9 J/(mol?K) at about 718-778 K for Na2ZrCl6.
The probable overall errors of the ?G0, ?H0, ?S0 values should be computed from the statistical errors and the estimated probable uncertainties[13-16]. The statistical errors may be not more than ±0.2 kJ/mol for ?G0 at every temperature, ±0.7 kJ/mol for ?H0 and ±1.0 J/(mol?K) for ?S0 for the complex Na2ZrCl6 in the reaction temperature ranges shown in this study. The estimated probable uncertainties may result from assuming absolute errors of the chemical analysis for Zr4+ and Na+ as ±5 %, of the volume measurement of the ampoules as ±0.5%, and of the temperature measurement as ±2.0 K. These uncertainties together with the error from the scatter of the experimental points in Figs.2 and 3 may give rise to the probable overall errors of ±0.5 kJ/mol for ?G0 at every temperature, ±1.5 kJ/mol for ?H0 and ±2.0 J/(mol?K) for ?S0 for the vapor complex Na2ZrCl6 in the reaction temperature range shown in this study.
4 Conclusions
The vapor complex Na2ZrCl6 was formed by the reaction of solid NaCl with gaseous ZrCl4. The formation thermodynamics is ?H0=(-70.1±1.5) kJ/mol and ?S0=(-105.9±2.0) J/(mol?K) for Na2ZrCl6(g) at 718-778 K. The vapor complex formation ability in the ZrCl4-KCl system is much lower than that in the ZrCl4-NaCl system.
References
[1] BOGHOSIAN S, PAPATHEODOROU G N. Halide vapors and vapor complexes[A]. GSCHNEIDNER K A Jr, EYRING L. Handbook on the Physics and Chemistry of Rare Earths[M]. Amsterdam: Elsevier, 1996: 435-496.
[2] JIANG J, OZAKI T, MACHIDA K, ADACHI G. Separation and recovery of rarte earth via a dry chemical vapour transport based on halide gaseous complexes [J]. J Alloys Comp, 1997, 260: 222-235.
[3] OPPERMANN H, SCHMIDT P. The thermochemical behaviour of halides, oxidehalides, aluminiumhalides and ammoniumhalides of rare-earth-elements [J]. Z Anorg Allg Chem, 2005, 631: 1309-1340.
[4] HILPERT K, NIEMANN U. High temperature chemistry in metal halide lamps [J]. Thermochim Acta, 1997, 299: 49-57.
[5] KAPALA J, LISEK I, ROSZAK S, MILLER M. Mass spectrometric and theoretical study of the mixed complex NaNdCl4(g) [J]. Polyhedron, 1999, 18: 2845-2851.
[6] AKDENIZ Z, ?NEM Z C, TOSI M P. Structure of rare-earth/alkali halide complexes [J]. Z Naturforch A: Phys Sci, 2001, 56: 721-724.
[7] ?NEM Z C, AKDENIZ Z, TOSI M P. Structure of rare-earth/group-IIIA chloride complexes [J]. Z Naturforch A: Phys Sci, 2002, 57: 937-942.
[8] GROEN C P, OSKAM A, KOVACS A. Theoretical study of mixed LiLnX4 (Ln=La, Dy; X=F, Cl, Br, I) rare earth/alkali halide complexes [J]. Inorg Chem, 2000, 39: 6001-6008.
[9] GROEN C P, OSKAM A, KOVACS A. Theoretical study of mixed MLaX4 (M=Na, K, Cs; X=F, Cl, Br, I) rare earth/alkali metal halide complexes [J]. Inorg Chem, 2003, 42: 851-858.
[10] YU Jin, YANG Dong-mei, JIANG Jun-hui, WANG Zhi-chang. Thermodynamic properties of the rare earth vapour complex NdAl3Br12 [J]. The Chinese Journal of Nonferrous Metals, 2004, 14(4): 702-705. (in Chinese)
[11] YU Jin, WANG Lin-shan, YANG Dong-mei, JIANG Jun-hui, WANG Zhi-chang. Thermodynamic study on the rare earth vapour complex GdAl3Br12 [J]. Journal of The Chinese Rare Earth Society, 2004, 22(3): 326-330. (in Chinese)
[12] YU Jin, YANG Dong-mei, WANG Zhi-chang. Thermodynamic properties of the rare earth vapour complex PrAl3Br12 [J]. Journal of Northeastern University (Natural Science), 2005, 26(2): 201-204. (in Chinese)
[13] WANG Z C, WANG L S, GAO R J, SU Y. Thermodynamic study of the rare earth vapour complexes: ScAl2Cl9 and YAl2Cl9 [J]. J Chem Soc Faraday Trans, 1996, 92(11): 1887-1890.
[14] WANG L S, GAO R J, SU Y, WANG Z C. Formation thermodynamics of the rare earth vapor complexes: DyAl3Cl12 and DyAl2Cl9 [J]. J Chem Thermody, 1996, 28: 1093-1102.
[15] WANG Z C, WANG L S. Thermodynamic properties of the rare earth element vapor complexes LnAl3Cl12 from Ln=La to Ln=Lu [J]. Inorg Chem, 1997, 36: 1536-1540.
[16] WANG Z C, WANG L S. Thermodynamic properties of the rare earth element vapor complexes LaAl4Cl15, CeAl4Cl15 and HoAl2Cl9 [J]. J Alloys Comp, 1998, 265: 153-159.
[17] LI Jun-li, YANG Dong-mei, ZENG Fan-wu, WANG Zhi-chang. Thermodynamic properties of NbCl5-NaCl and NbCl5-KCl binary systems by quenching experiments [J]. The Chinese Journal of Nonferrous Metals, 2006, 16(6): 1-5. (in Chinese)
[18] BARIN I, KNACKE O, Thermochemical Properties of Inorganic Substances[M]. Berlin: Springer-Verlag, 1973: 902.
[19] LEVIN E M, ROBBINS C R, MCMURDIE H F. Phase Diagrams for Ceramists [M]. Columbus: Amer Ceram Soc, 1969: 347.
[20] PHOTIADIS G M, PAPATHEODOROU G N. Vibrational modes and structure of liquid and gaseous zirconium tetrachloride and of molten ZrCl4-CsCl mixtures [J]. J Chem Soc, Dalton Trans, 1998, 6: 981- 989.
[21] LISTER R L, FLENGAS S N. The synthesis and properties of the anhydrous hexachlorozirconates of sodium and potassium [J]. Can J Chem, 1964, 42: 1102-1105.
[22] LISTER R L, FLENGAS S N. On the relationship between equilibrium pressures and the phase diagram of a reactive system [J]. Can J Chem, 1965, 43: 2947-2969.
[23] DUTRIZAC J E, FLENGAS S N. Thermal properties of the compounds Li2ZrCl6, Li2HfCl6, Na2ZrCl6, and K2ZrCl6 [J]. Can J Chem, 1967, 45: 2313-2316.
[24] KIPOUROS G J, FLENGAS S N. Equilibrium decomposition pressures of the compounds Na2ZrCl6 and Na2HfCl6 [J]. Can J Chem, 1981, 59: 990-995.
[25] SCHAFER H. Gaseous chloride complexes with halogen bridges—homo-complexes and hetero-complexes [J]. Angew Chem Int Ed Engl, 1976, 15(12): 713-727.
[26] PAPATHEODOROU G N. Spectroscopy, structure and bonding of high-temperature metal halide vapor complexes [A]. KALDIS E. Current Topics in Materials Science [C]. Amsterdam: North-Holland, 1982: 249-352.
Foundation item: Project(50274027) supported by the National Natural Science Foundation of China
Corresponding author: WANG Zhi-chang; Tel: +86-24-83675010; E-mail: wangzc@mail.neu.edu.cn
(Edited by YUAN Sai-qian)