DOI:10.19476/j.ysxb.1004.0609.2019.05.21
铝钙粉浸出渣的活化及其在锌冶炼废水处理中的应用
李 安1,郑雅杰1,彭映林2,翟信可1,龙 华1
(1. 中南大学 冶金与环境学院,长沙 410083;2. 湖南城市学院 材料与化学工程学院,益阳 413000)
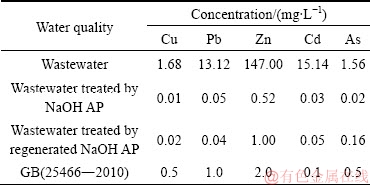
摘 要:以PAC生产过程中铝钙粉浸出渣为原料,采用盐酸和氢氧化钠进行活化,并对其在锌冶炼废水中吸附重金属的性能进行研究。考察铝钙粉浸出渣及其活化产物结构、比表面积、孔结构的变化,分析了pH值、吸附时间和重金属浓度对其吸附性能的影响,并以氢氧化钠活化产物为吸附剂进行了工业实验。结果表明:铝钙粉浸出渣经盐酸和氢氧化钠活化后,其结构均由岛状变为层状。铝钙粉浸出渣、盐酸活化产物和氢氧化钠活化产物的比表面积分别为21.8、63.1、28.1 m2/g,BJH孔径分别为36.06、43.54和236.35 nm,孔容分别为0.03、0.09和0.14 cm3/g。pH=8,吸附温度为25 ℃,吸附时间为150 min时,由Langmuir方程得到铝钙粉浸出渣对Cd2+、Zn2+和As(V)的饱和吸附量分别为2.81、497.57和2.45 mg/g,盐酸活化产物对Cd2+、Zn2+和As(V)的饱和吸附量分别为3.44、516.32和2.04 mg/g,氢氧化钠活化产物对Cd2+、Zn2+和As(V)的饱和吸附量分别为7.64、526.32和4.72 mg/g。工业实验结果表明:吸附过程具有化学吸附特征,废水中Cu2+、Pb2+、Zn2+、Cd2+和As(V)的浓度由1.68、13.12、147.00、15.14和1.56 mg/L降至0.01、0.05、0.52、0.03和0.02 mg/L,达到《铅、锌污染物排放标准》(GB25466— 2010)。
关键词:铝钙粉浸出渣;活化;孔结构;锌冶炼废水;吸附
文章编号:1004-0609(2019)-05-1073-10 中图分类号:TB321 文献标志码:A
PAC是目前在水处理方面应用最广泛的一种无机高分子絮凝剂。20世纪90年代中期,中国成功开发出了铝酸钙粉制备液体PAC生产工艺,使PAC的生产和应用得到了快速发展[1]。2016年,中国液体PAC总产量已经超过300 万t。采用铝酸钙粉法生产PAC时,每生产1 t PAC会压滤出铝钙粉浸出渣45~50 kg。2016年,中国PAC生产过程中产生的铝钙粉浸出渣大约有15 万t,且浸出渣含有大量的Al,具有良好的Al回用价值。铝钙粉浸出渣活化产物为层状硅酸盐 结构,层状硅酸盐因具有特殊的结构、独特的性能,是一类重要的非金属矿物资源。以层状硅酸盐为原 料,采用水热合成法、结构重排法、非水体系合成等方法制备多孔吸附材料作为催化材料、重金属吸附 剂、储藏材料、纳米复合材料的应用研究越来越受到广泛的关注[2-4]。中国每年产生400亿m3左右的工业废水,其中重金属废水约占60%。例如,株洲冶炼厂废水产生总量每年可达到716 万t[5]。由于Pb、Cu、Ni、Cd、Cr、Hg等9种重金属已列入我国水中优先控制的68种污染物名录,含重金属废水的治理一直 是我国及国外环保领域的重大课题[6]。由层状硅酸盐矿物制备的重金属吸附剂具有比表面积高,吸附性能好等优点。分别采用硫酸、氢氧化钠活化高岭石,可得到比表面积为280 m2/g多孔氧化硅和56 m2/g多孔氧化铝,多孔氧化铝对Cu2+和Pb2+饱和吸附量分别可达134 mg/g和450 mg/g[7],矿物吸附剂对Zn2+饱和吸附量最高可达50.43 mg/g[8]。本研究以PAC生产过程中铝钙粉浸出渣为原料,分别采用盐酸和氢氧化钠活化,研究了其在锌冶炼废水中重金属的吸附特性。
1 实验
1.1 实验原料
以洛阳某PAC生产厂铝钙粉浸出渣为原料,本研究中铝钙粉浸出渣CALR(Calcium aluminate leaching residues)。盐酸(AR)、氢氧化钠(AR)、湖南某锌冶炼厂冶炼废水,经ICP检测其重金属含量如表1所示。
1.2 实验过程
盐酸活化[9]:按液固比5:1(mL:g) 将铝钙粉浸出渣加入到8 mol/L的盐酸溶液中,在85 ℃下活化3 h,过滤后烘干得到盐酸活化产物。本研究中盐酸活化产物用HCl AP(HCl activation pruduct)表示。
氢氧化钠活化:按液固比5:1(mL:g) 将铝钙粉浸出渣加入到3 mol/L的氢氧化钠溶液中,在75 ℃下活化2 h,过滤后烘干得到氢氧化钠活化产物。本研究中氢氧化钠活化产物用NaOH AP(NaOH activation pruduct)表示。
吸附:按一定液固比将铝钙粉浸出渣及其活化产物加入到锌冶炼废水中,在25 ℃、振荡速度为100 r/min下振荡,吸附一定时间后过滤,测定废水中Cu2+、Pb2+、Zn2+、Cd2+、As(V)含量。
表1 锌冶炼废水重金属浓度
Table 1 Concentration of heavy metal in Zn metallurgical wastewater (mg/L)

1.3 分析与检测
采用X射线荧光光谱仪(XRF,S4PIONEER)分析原料中元素成分;采用X射线衍射仪 (XRD,Rigaku D/max-TTR III)分析样品物相 (发光源为Cu Kα靶,管压为40 kV,管流为250 mA,λ=0.154056×10-10 m,2θ为10.0°~80.0°);采用电感耦合等离子体光谱仪(ICP- OES,IRIS Intrepid Ⅱ,Thermo Eleetron Corporation)分析溶液中的元素含量;采用BET比表面积测定仪(BETA201A)分析样品孔结构;在扫描电镜(SEM,FEIQuanta 200)下观察样品表面形貌;采用激光粒度分析仪(LS-POP(6),珠海欧美克仪器有限公司)分析样品粒度组成。
重金属吸附率A(%)和吸附剂的吸附量q(mg/g)分别按式(1)和(2)计算:
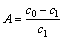 ×100% (1)
×100% (1)
 (2)
(2)
式中:c0为吸附前废水中重金属的质量浓度(mg/L);c1为吸附后废水中重金属的质量浓度(mg/L);V吸附废水体积(L);m为吸附剂质量(g)。
2 结果与讨论
2.1 铝钙粉浸出渣的活化及活化产物结构
根据1.2实验步骤,取500 g铝钙粉浸出渣分别进行盐酸和氢氧化钠活化,铝钙粉浸出渣及其活化产物经XRF检测其主要元素含量如表2所示,其XRD谱如图1所示,盐酸和氢氧化钠浸出液主要元素含量如表3所示。
由图1可知,铝钙粉浸出渣的主要物相组成为Ca3Al2(SiO4)2(OH)4(水钙铝榴石)、Fe3Si2O5(OH)4(铁蛇纹石)、CaTiO3。铝钙粉浸出渣经盐酸活化后Ca3Al2- (SiO4)2(OH)4(水钙铝榴石)物相转化成Al2Si2O5(OH)4 (高岭石),CaAl4O7(二铝酸钙)。由于Ca3Al2(SiO4)2- (OH)4与盐酸反应过程中部分Al3+、Fe3+、Ti4+、Ca2+溶出,岛状结构破坏[8],六配位A13+的 Al—O键键价为3/6=1/2,连六配位Al的O的剩余电价为-2+1/2=-3/2,其键价变化分别如式(3)和(4)所示,部分—Al +0.5与[SiO4]、—OH结构重排,生成了具有层状结构的Al2Si2O5(OH)4(高岭石),高岭石结构单元层为1:1型,由一层硅氧四面体层和一层氢氧化铝层组成的,其结晶有序程度低[11-13]。部分—Al—O-1.5结合Ca2+生成CaAl4O7。
—Al—O-1.5+H+=—Al—OH-0.5 (3)
—Al—O—H-0.5=—Al+0.5+OH- (4)
铝钙粉浸出渣经氢氧化钠活化后Ca3Al2(SiO4)2- (OH)4物相转化成Al2Si2O5(OH)4(高岭石),Al(OH)3,Na2xCa3-xAl2O6。由于Ca3Al2(SiO4)2(OH)4与氢氧化钠反应过程中Si4+溶出,岛状结构破坏,生成具有层状结构的Al2Si2O5(OH)4(高岭石),溶出的Al3+与OH—键合,生成Al(OH)3,溶出的Ca2+与Al(OH)3作用,生成Ca3Al2O6[14],由于Na+取代部分Ca2+,形成分子式为Na2xCa3-xAl2O6的固溶体[15]。
表2 铝钙粉浸出渣及其活化产物主要元素含量
Table 2 Main chemical components of CALR and its activation products


图1 铝钙粉浸出渣及其活化产物XRD谱
Fig. 1 XRD patterns of CALR and its activation products
表3 盐酸和氢氧化钠浸出液主要元素含量
Table 3 Main chemical components of hydrochloric acid leachate and sodium hydroxide leachate

铝钙粉浸出渣及其活化产物微观形貌如图2所示。铝钙粉浸出渣中位粒径(D50)为7.72 μm,盐酸活化产物中位粒径(D50)为7.45 μm,氢氧化钠活化产物中位粒径(D50)为7.75 μm。
由图2(a)可知,铝钙粉浸出渣颗粒排布紧密,分散性差,团聚严重,部分颗粒粒径大。由图2(b)可知,盐酸活化产物表面不平,呈锯齿状,孔隙增大,粒径减小,由于盐酸活化过程中部分Al3+、Fe3+、Ti4+、Ca2+被浸出,致使盐酸活化产物表面不平。由图2(c)可知,氢氧化钠活化产物呈絮状,颗粒粒径大,团聚严重,因为铝钙粉浸出渣经氢氧化钠活化后形成Al(OH)3,具有粘结作用,并使空隙变小。

图2 铝钙粉浸出渣及其活化产物的SEM像
Fig. 2 SEM images of CALR and its activation products
铝钙粉浸出渣及其活化产物的BJH孔结构分布如图3所示,孔性能参数如表4所示。
由图3可知,铝钙粉浸出渣及其盐酸活化产物孔径分布主要集中在38 nm且盐酸活化产物孔径分布峰强度降低,宽度增加,表明盐酸活化产物与铝钙粉浸出渣相比在38 nm处的介孔数量减少,在30~43 nm的介孔分布范围增大。氢氧化钠活化产物孔径分布主要集中在38 nm和186 nm,且氢氧化钠活化产物分布峰强度降低,出现了70~415 nm大孔分布范围。这表明与铝钙粉浸出渣相比,氢氧化钠活化产物在38 nm的介孔数量减少,孔结构主要以38 nm的介孔和186 nm的大孔形式存在,由于Si4+的大量溶出,拓展了孔径的范围 。
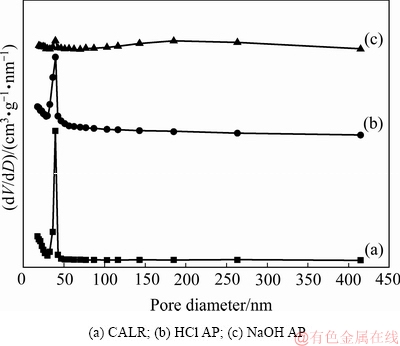
图3 铝钙粉浸出渣及其活化产物孔径分布曲线
Fig. 3 Pore size distribution curves of CALR and its activation products
表4 铝钙粉浸出渣及其活化产物孔性能参数
Table 4 Pore performance parameters of CALR and its activation products

由表4可知,铝钙粉浸出渣、盐酸活化产物和氢氧化钠活化产物的比表面积分别为21.8、63.1、28.1 m2/g,BJH孔径分别为36.06、63.54、236.35 nm,孔容分别为0.03、0.09、0.14 cm3/g。由于盐酸活化过程中Al3+、Fe3+、Ti4+、Ca2+的溶出,盐酸活化产物表面呈锯齿状,比表面积、孔径、孔容随之增加。由于氢氧化钠活化过程中Si4+的大量溶出,氢氧化钠活化产物出现了大孔分布,孔径、孔容随之增加,同时,生成的Al(OH)3和Na2xCa3-xAl2O6填充部分孔道,比表面积增加不明显。
2.2 铝钙粉浸出渣及其活化产物的重金属吸附性能
2.2.1 pH值对重金属吸附性能的影响
取铝钙粉浸出渣及其活化产物各0.5 g分别加入到300 mL锌冶炼废水中,振荡速度为100 r/min,吸附温度为25 ℃,吸附时间为150 min,吸附结束后过滤,测定废水中重金属含量。pH值对铝钙粉浸出渣及其活化产物吸附重金属的影响如图4所示。
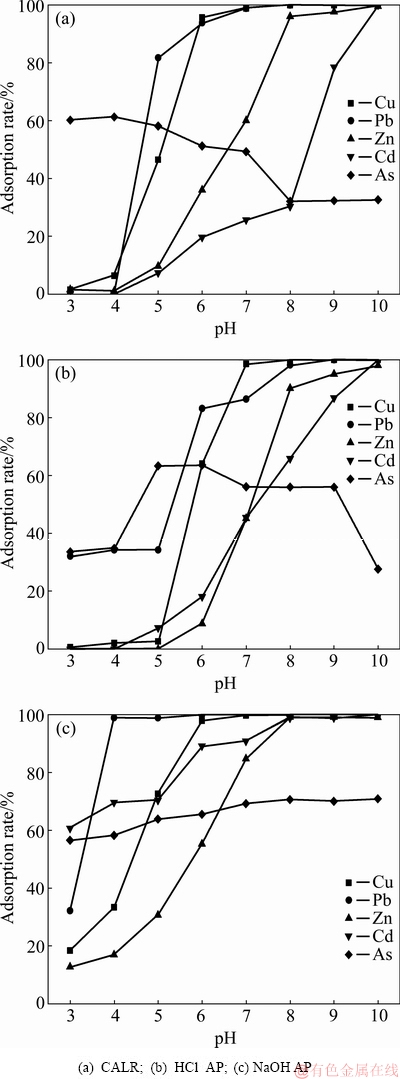
图4 pH值对铝钙粉浸出渣及其活化产物吸附重金属的影响
Fig. 4 Effect of pH value on adsorption of heavy metal by CALR and its activation products
由图4可知,铝钙粉浸出渣及其活化产物对重金属吸附率均随pH增加而增大,铝钙粉浸出渣吸附重金属在pH值为10时,吸附率达到100%;对As(V)吸附率先减小后增加,在pH=4时达到最高,为61.33%。盐酸活化产物吸附重金属在pH值为10时,吸附率达到100%;对As(V)吸附率先减小后增加,在pH=6时达到最高,为63.61%。氢氧化钠活化产物吸附重金属在pH值为8时,吸附率达到100%;对As(V)去除率逐渐增加,在pH=8时趋于稳定,达73.59%。因为盐酸和氢氧化钠活化产物属于层状硅酸盐,表面存在有—AlOH、—SiOH等基团,这些基团通过对H+的解吸和缔和产生可变表面电荷,层状硅酸盐矿物的这些可变电荷表面是产生重金属离子专性吸附的主要位点[16]。使用方程式表示如下:
L—OH+ H+L—
H+L—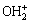 (5)
(5)
L—OH+OH- L—O-+H2O (6)
L—O-+H2O (6)
M2++OH- M(OH)+ (7)
M(OH)+ (7)
M2++L—O- L—OM+ (8)
L—OM+ (8)
L—O-+M(OH)+ L—OM(OH) (9)
L—OM(OH) (9)
式中:L代表A1、Si等表面;L—OH为表面羟基官能团;M为Pb、Cu、Cd、Zn等重金属元素[17-18]。
由式(5)~(9)可知,pH值越高越有利于重金属的吸附,pH值低时发生交换性吸附,pH值高时发生专性吸附;同时随着pH值的增高, Si—O四面体中少量的Si4+被溶出,产生离子交换作用,也将增加吸附剂对重金属离子的吸附能力。
层状硅酸盐矿物含Al配位活性中心,与As(V)可以发生多核配位而使结合更加容易。pH值对As(V)吸附的影响与两方面因素有关,一是As(V)在不同pH溶液中的存在形式;二是不同pH下吸附剂表面的带电情况。砷酸在溶液中的解离如下:
H3AsO4→ +H+ pKa1=2.24 (10)
+H+ pKa1=2.24 (10)
 →
→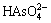 +H+ pKa2=6.76 (11)
+H+ pKa2=6.76 (11)
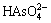 →
→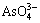 +H+ pKa3=11.60 (12)
+H+ pKa3=11.60 (12)
因此,As(V)主要以下列形式存在:H3AsO4 (pH< 2), (2
(2 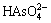 (pH>7)。在弱酸性条件下,表面带正电荷的吸附剂对以阴离子形式存在的
(pH>7)。在弱酸性条件下,表面带正电荷的吸附剂对以阴离子形式存在的 有很强的静电结合力,吸附能力较强。pH升高到一定程度后(大于7),吸附剂表面所带正电荷数减少,静电引力减弱,吸附能力下降。由于氢氧化钠活化产物,Si溶出量大,
有很强的静电结合力,吸附能力较强。pH升高到一定程度后(大于7),吸附剂表面所带正电荷数减少,静电引力减弱,吸附能力下降。由于氢氧化钠活化产物,Si溶出量大,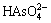 置换出较多的Al,形成了单配位基络合物,因此pH值升高,其对As(V)吸附率趋于稳定[19]。
置换出较多的Al,形成了单配位基络合物,因此pH值升高,其对As(V)吸附率趋于稳定[19]。
2.2.2 吸附时间对重金属吸附性能的影响
在其他条件不变,当废水吸附终点pH值为8时,吸附时间对铝钙粉浸出渣及其活化产物吸附重金属的影响如图5所示。

图5 吸附时间对铝钙粉浸出渣及其活化产物吸附重金属的影响
Fig. 5 Effect of adsorption time on adsorption of heavy metal by CALR and its activation products
由图5可见,铝钙粉浸出渣及其活化产物对重金属吸附率均随吸附时间延长而增大,150 min时其吸附基本达到平衡状态。氢氧化钠活化产物对重金属的吸附效果优于铝钙粉浸出渣及其盐酸活化产物。吸附时间为150 min时,氢氧化钠活化产物对重金属吸附率达到100%,对As(V)吸附率达到73.59%。此现象说明氢氧化钠活化产物表面富含的功能基团能够与重金属快速配合[20-21]。为确保吸附能达到充分平衡,本实验选150 min为吸附平衡时间。
2.2.3 初始浓度对重金属吸附性能的影响
在其他条件不变,当吸附时间为150 min时,Cu2+、Pb2+吸附率均已达到100%。因此,本实验仅考察Cd2+、Zn2+、As(V)初始浓度对铝钙粉浸出渣及其活化产物吸附性能的影响,铝钙粉浸出渣及其活化产物吸附Cd2+、Zn2+、As(V)的情况如图6所示。
由图6可知,铝钙粉浸出渣及其活化产物对Cd2+、Zn2+、As(V)的吸附率随初始浓度的增大而减小。对于Cd2+、As(V)的吸附效果,氢氧化钠活化产物最好,铝钙粉浸出渣次之,盐酸活化产物再次之。对于Zn2+吸附效果,氢氧化钠活化产物最好,盐酸活化产物次之,铝酸钙浸出渣再次之。这是因为氢氧化钠活化产物Si含量低,其n(Al)/n(Si)达到0.76,铝钙粉浸出渣n(Al)/ n(Si)为0.46,盐酸活化产物n(Al)/n(Si)仅为0.24,Al含量越高,吸附剂表面—AlOH可产生更多重金属离子专性吸附位点,有利于重金属吸附。但盐酸活化产物比表面积大,也能增强重金属的吸附性能。
2.2.4 重金属吸附的Langmuir等温线方程
将2.2.3吸附实验结果采用Langmuir(式(13))等温线方程进行拟合,结果如图7所示,拟合特征参数值和线性相关系数如表7所示。吸附剂的吸附性能通过分离因子RL来判断,分离因子定义见式(14)。0L<1,表明有利于吸附,RL>1,表示不利于吸附,RL=1时属于线性分配,RL趋于0表示不可逆吸附。
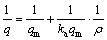 (13)
(13)
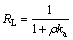 (14)
(14)
式中:ka为Langmuir常数(L/mg);qm为吸附剂的饱和吸附量(mg/g);q为吸附量(mg/g);ρ为吸附质浓度(mg/L)。
由图7可知,铝钙粉浸出渣及其活化产物对Cd2+、Zn2+、As(V)吸附过程均符合Langmuir模型,且0L< 1均,Langmuir方程描述均相表面的单分子层吸附,表明铝钙粉浸出渣及其活化产物吸附重金属为单分子层吸附[16]。
由表5可知,铝钙粉浸出渣、盐酸活化产物、氢氧化钠活化产物对Zn2+的饱和吸附量分别为497.51、516.32、526.32 mg/g,远高于文献报道的土壤矿物吸附剂对Zn2+的吸附能力(如表6所列)[8]。
2.3 氢氧化钠活化产物处理锌冶炼废水工业应用

图6 初始浓度对铝钙粉浸出渣及其活化产物吸附Cd2+、Zn2+、As(V)的影响
Fig. 6 Effect of initial concentration on adsorption of Cd2+、Zn2+、As(V) by CALR and its activation products
铝钙粉浸出渣及其活化产物处理锌冶炼废水实验研究表明,氢氧化钠活化产物吸附重金属在pH值为8时,总饱和吸附量达到538.68 mg/g,表明其在锌冶炼废水处理中是一种优越的重金属吸附剂。根据单因素实验结果,将170 kg氢氧化钠活化产物加入到100 m3 锌冶炼废水中,控制吸附pH值为8,在25℃下,吸附150 min后过滤得到吸附渣,其主要元素含量如表7所示,原料耗量及成本如表8所示,吸附渣XRD谱如图8所示。
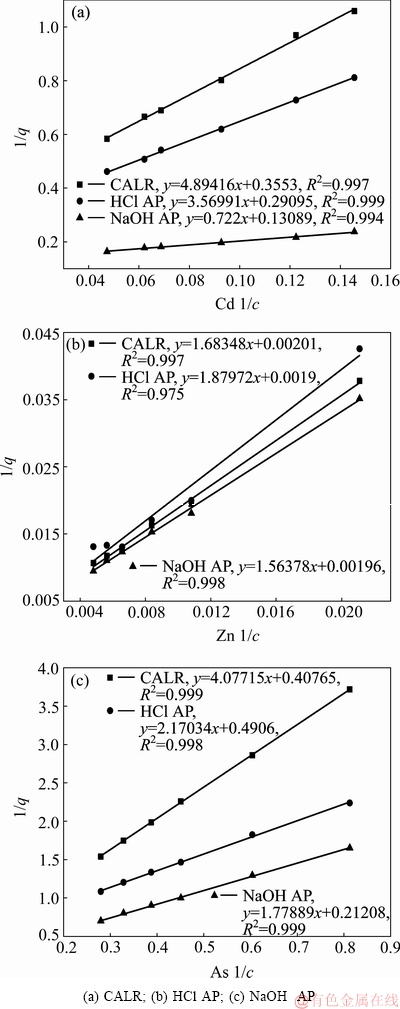
图7 铝钙粉浸出渣及其活化产物对Cd2+、Zn2+、As(V)的Langmuir吸附等温线
Fig. 7 Langmuir isotherm of Cd2+, Zn2+ and As(V) by CALR and its activation products
表5 Langmuir吸附等温线线性拟合参数
Table 5 Langmuir isotherm parameters for adsorption of heavy metals

将吸附渣按盐酸与吸附渣液固比(L:kg) 5:1加入到浓度为1.5 mol/L的盐酸溶液中,在25 ℃下,解附120 min后过滤得解附渣,盐酸解附液经ICP测定其主要成分如表9所示。
将解附渣按1.2实验步骤活化得到再生氢氧化钠活化产物,按上述条件处理锌冶炼废水,氢氧化钠活化产物及再生氢氧化钠活化产物废水处理结果如表10所示。
由表7和8可知,氢氧化钠活化产物对锌冶炼废水中Zn2+、Cd2+、Cu2+、Pb2+、As(V)具有良好的吸附效果,吸附渣锌含量达10.29%,可用于回收锌,且工艺简单,处理成本为1.39 元/m3,废渣量为1.84 kg/m3。对比石灰中和沉淀法[5]处理成本为1.83元/m3,废渣量为3.0 kg/m3,本工艺成本低、废渣量少,工艺简单,具有良好的经济效益和环境效益。由图8可知,氢氧化钠活化产物吸附重金属后,重金属主要物相为CuAl2O4、ZnAl2O4、CdAl2O4、Pb5(AsO4)3OH、Cu2Al2- (AsO4)2(OH)4·H2O,这与2.2研究结果一致。
由表9和10可知,采用氢氧化钠活化产物及再生氢氧化钠活化产物处理锌冶炼废水,处理后水质达到《铅、锌污染物排放标准》(GB25466—2010)中各项指标。吸附渣经稀盐酸解附,氢氧化钠再活化可再生,其吸附性能与氢氧化钠活化产物吸附性能一致,解附溶液锌浓度达8.89g/L,与锌冶炼废水锌浓度比较富集了60倍,可直接回收锌,实现了变废为宝。
表6 矿物吸附剂对锌离子的吸附能力[8]
Table 6 Zn2+ adsorption capacity of mineral adsorbent (mg/g)[8]

表7 重金属吸附渣主要元素含量
Table 7 Main chemical components of adsorption residues on heavy metals (mass fraction, %)

表8 氢氧化钠活化产物处理锌冶炼废水原料耗量及成本
Table 8 Material consumption and cost of treatment in zinc metallurgical wastewater by NaOH AP
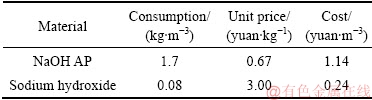

图8 重金属吸附渣XRD谱
Fig. 8 XRD patterns of adsorption residues on heavy metals
表9 盐酸解附液主要元素含量
Table 9 Main chemical components of hydrochloric acid desorption solution (g/L)
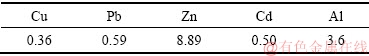
表10 氢氧化钠活化产物及再生氢氧化钠活化产物处理锌冶炼废水工业实验结果
Table 10 Industrial experiment results of treatment in zinc metallurgical wastewater by NaOH AP and regenerated NaOH AP (mg/L)
3 结论
1) 铝钙粉浸出渣经盐酸和氢氧化钠活化后结构均由岛状变为层状。铝钙粉浸出渣、盐酸活化产物、氢氧化钠活化产物比表面积分别为21.8、63.1、28.1 m2/g,BJH孔径分别为36.06、43.54、236.35 nm,孔容分别为0.03、0.09、0.14 cm3/g ,粒径分别为7.72、7.45和7.75 μm。氢氧化钠活化产物表面生成絮状Al(OH)3,颗粒发生团聚现象。
2) 在pH=8、吸附温度为25 ℃、吸附时间为150 min时,根据Langmuir吸附等温线方程计算得到铝钙粉浸出渣对Cd2+、Zn2+、As(V)理论饱和吸附量分别为2.81、497.57、2.45 mg/g,盐酸活化产物对Cd2+、Zn2+、As(V)理论饱和吸附量分别为3.44、516.32、2.04 mg/g,氢氧化钠活化产物对Cd2+、Zn2+、As(V)的理论饱和吸附量分别为7.64、526.32、4.72 mg/g。氢氧化钠活化产物对重金属具有良好的吸附效果。
3) 氢氧化钠活化产物处理锌冶炼废水工业实验表明:采用氢氧化钠活化产物处理锌冶炼废水,重金属主要物相为CuAl2O4、ZnAl2O4、CdAl2O4、Pb5(AsO4)3OH、Cu2Al2(AsO4)2(OH)4·H2O,吸附过程具有化学吸附特征。废水中Cu2+、Pb2+、Zn2+、Cd2+、As(V)浓度由1.68、13.12、147.00、15.14、1.56 mg/L降至0.01、0.05、0.52、0.03、0.02mg/L,达到《铅、锌污染物排放标准》(GB25466—2010),处理成本为1.39 元/m3,废渣量为1.84 kg/m3。
REFERENCES
[1] YANG Zhong-lian, GAO Bao-yu, YUE Qin-yan. Coagulation performance and residual aluminum speciation of Al2(SO4)3 and polyaluminum chloride (PAC) in Yellow River water treatment[J]. Chemical Engineering Journal, 2010, 165(1): 122-132.
[2] 杨华明, 杨武国, 张向超, 金胜明, 张 科. 层状硅酸盐制备多孔材料的研究进展[J]. 材料导报, 2004, 18(3): 13- 16.
YANG Hua-ming, YANG Wu-guo, ZHANG Xiang-chao, JIN Sheng-ming, ZHANG Ke. Progress in preparation of porous materials from layer silicates[J]. Materials Review, 2004, 18(3): 13-16.
[3] 李光辉, 艾玲凤, 姜 涛, 邱冠周. 热活化过程中高岭石中铝的结构变化及酸溶特性[J]. 硅酸盐学报, 2008, 36(9): 1200-1204.
LI Guang-hui, AI Ling-feng, JIANG Tao, QIU Guan-zhou. Structure transformation and acid dissociation behaviors of aluminum from kaolinite under thermal activation[J]. Journal of the Chinese Ceramic Society, 2008, 36(9): 1200-1204.
[4] TEMUUJIN J, OKADA K, MACKENZIE K J D, JADAMBAA T S. Characterization of porous silica prepared from mechanically amorphized kaolinite by selective leaching[J]. Powder Technology, 2001, 121(2/3): 259-262.
[5] 王绍文, 王海东, 孙玉亮. 冶金工业废水处理技术及回 用[M]. 北京: 化学工业出版社, 2015: 296-300.
WANG Shao-wen, WANG Hai-dong, SUN Yu-liang. Technical treatment and reutilization of wastewater in metallurgical industry[M]. Beijing: Chemical Industry Press, 2015: 296-300.
[6] 王庆伟, 柴立元, 王云燕, 李青竹. 锌冶炼含汞污酸生物制剂处理新技术[J]. 中国有色金属学报, 2008, 18(S1): s416-s421.
WANG Qing-wei, CHAI Li-yuan, WANG Yun-yan, LI Qing-zhu. Novel technology for treatment of acidic wastewater containing Hg by biologics in zinc smelter[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(S1): s416-s421.
[7] 程 伟. 应用层状硅酸盐矿物制备多孔材料及其吸附性能研究[D]. 长沙: 中南大学, 2011: 23-33.
CHENG Wei. Studies on preparation and adsorption characters of mesoporous materials by using layered structure silicates[D]. Changsha: Central South University, 2011: 23-33.
[8] 王浩杰. 五种土壤矿物胶体的合成及其对镉、铅、锌吸附特征的研究[D]. 沈阳: 东北大学, 2012: 66-71.
WANG Hao-jie. Study on the synthesis of five clay mineral colloids and their adsorption characteristics of Cd2+, Pb2+, Zn2+[D]. Shengyang: Northeastern University, 2012: 66-71.
[9] 郑雅杰, 李 安, 孙召明, 彭映林, 龙 华. 一种利用铝钙粉反应渣制备制备P型沸石分子筛的方法: 中国, 201810472833[P]. 2018-05-17.
ZHENG Ya-jie, LI An, SUN Zhao-ming, PENG Ying-lin, LONG Hua. A method for preparing zeolite P by utilizing calcium aluminate leaching residues: China, 201810472833[P]. 2018-05-17.
[10] 赵清杰, 杨巧芳, 陈启元, 尹周澜, 吴争平, 殷振国. 硅矿物在拜耳法溶出中的行为[J]. 中国有色金属学报, 2008, 18(S1): s172-s182.
ZHAO Qing-jie, YANG Qiao-fang, CHEN Qi-yuan, YIN Zhou-lan, WU Zheng-ping, YIN Zhen-guo. Behavior of silicon minerals during Bayer digestion[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(S1): s172-s182.
[11] MILLER J D, NALASKOWSKI J, ABDUL B, DU Hao. Surface characteristics of kaolinite and other selected two layer silicate minerals[J]. Canadian Journal of Chemical Engineering, 2010, 85(5): 617-624.
[12] RAI B, SATHISH P, TANWAR J, PRADIP, MOON K S, FUERSTENAU D W. A molecular dynamics study of the interaction of oleate and dodecylammonium chloride surfactants with complex aluminosilicate minerals[J]. Journal of Colloid and Interface Science, 2011, 362(2): 510-516.
[13] YIN Xi-hui, GUPTA V, DU Hao, WANG Xu-ming, MILLER J D. Surface charge and wetting characteristics of layered silicate minerals[J]. Advances in Colloid and Interface Science, 2012, 179(S1): 43-50.
[14] BROWN I W M, MACKENZIE K J D, MEINHOLD R H, BOWDEN M E. Outstanding problems in the kaolinite- mullite reaction sequence investigated by 29Si and 27Al solid-state nuclear magnetic resonance: I, Metakaolinite[J]. Journal of the American Ceramic Society, 2010, 68(6): 293- 297.
[15] 杨守磊, 肖国庆, 丁冬海, 刘科燕. CaO-Al-Al2O3-CaCO3- O2体系燃烧合成铝酸钙热力学研究[J]. 硅酸盐学报, 2016, 44(6): 908-913.
YANG Shou-lei, XIAO Guo-qing, DING Dong-hai, LIU Ke-yan. Thermodynamic analysis of combustion synthesis of calcium aluminate in CaO-Al-Al2O3-CaCO3-O2 system[J]. Journal of the Chinese Ceramic Society, 2016, 44(6): 908- 913.
[16] MALAMIS S, KATSOU E. A review on zinc and nickel adsorption on natural and modified zeolite, bentonite and vermiculite: examination of process parameters, kinetics and isotherms[J]. Journal of Hazardous Materials, 2013, 252/253(10): 428-461.
[17] 姜腾达. 粘土矿物对水中Pb2+、Cu2+、Cd2+的吸附及机理研究[D]. 长沙: 中南大学, 2014: 16-18.
JIANG Teng-da. Study of Pb2+, Cu2+, Cd2+ adsorbed on clay minerals under aqueous solution and adsorption mechanism[D]. Changsha: Central South University, 2014: 16-18.
[18] 何宏平. 粘土矿物与金属离子作用研究[M]. 北京: 石油工业出版社, 2001: 46-47.
HE Hong-ping. Study on the interaction of clay minerals with metal ions[M]. Beijing: Petroleum Industry Press, 2001: 46-47.
[19] 刘海玲, 梁美娜, 朱义年. 复合铁铝氢氧化物对As(V)的吸附作用[J]. 环境化学, 2006, 25(6): 743-747.
LIU Hai-ling, LIANG Mei-na, ZHU Yi-nian. Adsorption of As(V) on complex Fe-Al hydroxides[J]. Environmental Chemistry, 2006, 25(6): 743-747.
[20] NASERNEJAD B, ZADEH T E, POUR B B, BYGI M E, ZAMANI A. Comparison of biosorption modeling of heavy metals (Cr(VI), Cu(II) and Zn(II)) adsorption from wastewater by carrot residues[J]. Process Biochemistry, 2005, 40(3): 1319-1322.
[21] WU X L, ZHAO Dong-lin, YANG S T. Impact of solution chemistry conditions on the sorption behavior of Cu (Ⅱ) on Lin an montmorillonite[J]. Desalination, 2011, 269(3): 84-91.
Activation of calcium aluminate leaching residues and their application in treatment of zinc metallurgical wastewater
LI An1, ZHENG Ya-jie1, PENG Ying-lin2, ZHAI Xin-ke1, LONG Hua1
(1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. School of Chemistry and Environmental Engineering, Hunan City University, Yiyang 413000, China)
Abstract: The calcium aluminate leaching residues(CALR), which were from the process of polyaluminum chloride production, were activated by hydrochloric acid and sodium hydroxide, respectively, and the adsorption properties CALR and activation products on heavy metals in zinc metallurgical wastewater were researched. The change of structure, specific surface area, pore structure of CALR and activation products were investigated. The effects of pH value, adsorption time and heavy metal concentration on its adsorption performance were analyzed. The industrial experiments were also conducted with NaOH activation products(NaOH AP). The results show that the structure changes from island to layer after CALR are activated by HCl and NaOH. The specific surface areas of CALR, HCl activation products(HCl AP) and NaOH AP are 21.8, 63.1, 28.1 m2/g, respectively, the BJH pore diameters of CALR, HCl activation products (HCl AP) and NaOH AP are 36.06, 43.54, 236.35 nm, respectively, and the pore volumes of CALR, HCl activation products (HCl AP) and NaOH AP are 0.03, 0.09, 0.14 cm3/g, respectively. According to Langmuir isotherm, the saturation Cd2+, Zn2+, As(V) adsorption capacities are 21.8, 497.57, 2.45 mg/g, respectively, the adsorption capacities of HCl AP are 3.44, 516.32, 2.04 mg/g, respectively, and the adsorption capacities of NaOH AP are 7.64, 526.32, 4.72 mg/g, respectively, when pH value is 8, the adsorption temperature is 25 ℃, adsorption time is 150 min. The industrial experiment results show that the chemical adsorption is in possession of adsorption process and that the concentrations of Cu2+, Pb2+, Zn2+, Cd2+, and As(V) in zinc metallurgical wastewater treated with NaOH AP decrease from 1.68, 13.12, 147.00, 15.14, and 1.56 mg/L to 0.01, 0.05, 0.52, 0.03, and 0.02 mg/L, respectively. The water qualities reach up to the “Emission Standard of Pollutants for Lead and Zinc Industry” (GB25466—2010).
Key words: calcium aluminate leaching residues; activation; hole structure; zinc metallurgical wastewater; adsorption
Foundation item: Project(2017SK2254) supported by Key Research and Development Project of Hunan Province, China
Received date: 2018-06-22; Accepted date: 2018-12-30
Corresponding author: LI An,Tel: +86-731-88836285; E-mail: zyj@csu.edu.cn
(编辑 李艳红)
基金项目:湖南省重点研发计划项目(2017SK2254)
收稿日期:2018-06-22;修订日期:2018-12-30
通信作者:郑雅杰,教授,博士;电话:0731-88836285; E-mail: zyj@csu.edu.cn