氢化球磨Mg-Al-H粉体的显微组织及放氢特征
来源期刊:中国有色金属学报(英文版)2013年第10期
论文作者:王 瑶 曾小勤 邹建新 李德江 吴晓梅 丁文江
文章页码:3112 - 3118
关键词:镁合金;机械球磨;氢化;纳米镁氢化物;双峰
Key words:Mg alloy; reaction milling; hydriding; nanocrystalline magnesium hydride; peak doublets
摘 要:研究Mg-Al粉体在氢气气氛下球磨的显微组织及相变。结果表明:在Mg-3%Al和Mg-9%Al两种粉末中,球磨4 h后都能观察到β-MgH2相生成,并且β-MgH2相的含量在球磨32 h后达到最大(~80%)。在这两种粉末中球磨后都没有Mg17Al12相出现。而将Mg-9%Al球磨后粉末加热到350 °C有Mg17Al12相析出。球磨8~40 h后,DTA分析显示放氢曲线都有双峰现象,这可能是β-MgH2相不同大小颗粒的放氢性能不同产生的。
Abstract: Microstructure and phase evolutions of Mg-Al powders ball milled in hydrogen atmosphere were investigated. Both in Mg-3%Al (mass fraction) and Mg-9%Al systems, β-MgH2 phase was observed upon a short milling time of 4 h and its maximum content of ~80% was reached after 32 h. Neither as-milled powders of the in the two systems contain Mg17Al12. However, heating the milled powders of Mg-9%Al powders to 350 °C resulted in the precipitation of Mg17Al12. DTA/TG analyses of those powders milled for 8-40 h showed that either well-developed peak doublets or shoulders were observed, which plausibly corresponded to the separate hydrogen desorption from different particle fractions of β-MgH2.
Trans. Nonferrous Met. Soc. China 23(2013) 3112-3118
Yao WANG1, Xiao-qin ZENG1,2, Jian-xin ZOU1, De-jiang LI1, Xiao-mei WU1, Wen-jiang DING1,2
1. Shanghai Engineering Research Center of Magnesium Materials and Applications, School of Materials Science and Engineering, Shanghai Jiao Tong University, Shanghai 200240, China;
2. State Key Laboratory of Metal Matrix Composites, School of Materials Science and Engineering, Shanghai Jiao Tong University, Shanghai 200240, China
Received 3 September 2012; accepted 15 March 2013
Abstract: Microstructure and phase evolutions of Mg-Al powders ball milled in hydrogen atmosphere were investigated. Both in Mg-3%Al (mass fraction) and Mg-9%Al systems, β-MgH2 phase was observed upon a short milling time of 4 h and its maximum content of ~80% was reached after 32 h. Neither as-milled powders of the in the two systems contain Mg17Al12. However, heating the milled powders of Mg-9%Al powders to 350 °C resulted in the precipitation of Mg17Al12. DTA/TG analyses of those powders milled for 8-40 h showed that either well-developed peak doublets or shoulders were observed, which plausibly corresponded to the separate hydrogen desorption from different particle fractions of β-MgH2.
Key words: Mg alloy; reaction milling; hydriding; nanocrystalline magnesium hydride; peak doublets
1 Introduction
Nanocrystalline and ultrafine grained materials have received considerable attention during the past decades owing to their improved properties as compared with conventional coarse grained materials [1]. Powder metallurgy is a quite potential way to prepare nanocrystalline or ultrafine grained bulk alloys. For most Mg alloys, however, it is difficult to produce nanocrystalline alloy by means of rapid solidification [2-5]. Another possible technique to refine grain size is hydrogenation-disproportionation-desorption-recombi-nation (HDDR) treatment which was first used to produce Nd-Fe-B permanent magnetic alloy [6]. The key step of this process is to obtain nano-structured hydride alloy powders through hydrogenation. Since Mg can absorb hydrogen to form MgH2 and the reaction is reversible, HDDR technique is believed to be applicable to Mg alloys [7]. However, the application of HDDR in Mg alloys is precluded because of the high thermodynamic stability, high desorption temperature, low plateau pressure, and slow sorption kinetics of MgH2.
One efficient approach to produce nano-structure metal hydride powders is mechanically assisted hydrogenation via ball milling at room temperature [8]. The mechanical energy provided by ball milling can be totally converted to the heat energy for driving the reaction of Mg and hydrogen, so the hydrogenation reaction can occur at room temperature rather than much high temperature. Controlled reactive mechanical milling is a one-step method through which the hydride synthesis accompanied by nanostructurization of phases occurs in due course of milling and it facilitates in-situ formation of nanocrystalline hydrides [9,10] at room temperature.
WANG et al [11] have prepared nanocrystalline Mg and Mg alloy powders using pure Mg and ZK60 (Mg-5.5%Zn-0.6%Zr) alloy as the starting material by the process of ball-milling hydrogenation and subsequent thermal dehydrogenation. The hydrogenation kinetics curves of Mg and ZK60 alloy by room temperature mechanical milling in hydrogen indicated that both Mg and ZK60 were featured by a three-stage kinetics, i.e. slow-fast-saturation, which could be explained by the formation of Mg(H) solid solution, the transition of Mg(H) (α phase) to MgH2 (β phase), and gradually the saturation of hydrogen in the crystal lattice, respectively [11]. The milling time to achieve full hydrogenation for Mg and ZK60 suggested that the hydrogenation kinetics of ZK60 was faster than that of pure Mg, which could be attributed to the higher affinity of Zr to hydrogen.
In the work of SUN et al [1] nanocrystalline bulk Mg-3Al-Zn alloy with an average grain size of 48 nm was prepared by powder metallurgy assisted hydriding-dehydriding process. The results showed that by milling in hydrogen for 60 h, using pure Mg, Al and Zn powders mixed in mass ratio of 96:3:1 as the starting material, as-hydrided powders possessed an average grain size of 5.9 nm.
GENNARI et al [12] also found that β and γ phases of MgH2 were synthesized by reactive mechanical alloying (RMA) at room temperature under hydrogen atmosphere with elemental magnesium granules (purity >99.9%) as starting material. The results indicated that the RMA process could result in size reduction of particle and crystalline and the formation of metastable γ-MgH2.
All above results indicated that, nanostructures can be achieved in Mg and Mg alloy by the mechanically driven solid-gas reaction: Mg+H2→MgH2 through mechanically assisted hydrogenation process which is the key step of application of HDDR in Mg alloys. Most researchers have mainly focused on the final state of the powders milled in hydrogen and cared more about alloys after dehydrogenation. However, few researches have studied the process and microstructural evolution during ball-milling induced hydrogenation at room temperature.
Considering the above, a systematic study of microstructure and phase evolutions of mixed Mg-Al powders ball milled in hydrogen atmosphere is carried out in the present work. This work may hopefully help throw light upon the comprehension of the mechanically assisted hydrogenation process via ball milling of magnesium based alloys and hence help promote the application of HDDR in more Mg alloys.
2 Experimental
The reagent grade fine Mg and Al powders (provided by Sinopharm Chemical Reagent Co. Ltd, China) with purity higher than 99.0% were used for reactive mechanical alloying in hydrogen to form magnesium hydride. Their diameters were both approximately 100 μm. Mechanical milling was carried out with a QM-3SP2 planetary ball milling machine operated at 400 r/min for 0.8 h of rolling and 0.2 h of dwelling and the total milling duration varied from 4 to 40 h. The Mg and Al powders were mixed in stainless steel vial in mass ratio of 97:3 and 91:9, respectively. The mass ratio of ball to powder was 40:1. The mass of mixed powder for each milling batch was 3 g and correspondingly 120 g of balls (60 g big ones and 60 g small ones). During the whole milling process, the hydrogen pressure of the vial was charged to 4.5 MPa at the very beginning. To prevent the powders from oxidation, the powders were always handled in a glove box filled with pure argon.
The as-milled and reference powders were characterized by D/Max B X-ray diffractometer equipped with a Cu Kα (λ=0.15406 nm) radiation source. The separation of crystallite size and strain was obtained from broadening of Bragg peaks using approximation and linear regression plots by software Jade 5. The standard reference material Si was used for subtracting the instrumental broadening of Bragg peaks.
The thermal behavior of the powders was studied by differential thermal analysis (DTA) coupled with thermogravimetry (DTA/TG, NETZSCH STA 449 F3) using a ~20 mg powders sample, a heating rate of 5 K/min and an argon flow rate of 40 mL/min. The mass change and the temperature of the phase change were calculated from the measured DTA/TG profiles. The microstructure characterization of the powders was conducted by transmission electron microscopy (TEM) of JEOL-2100 type.
3 Results and discussion
3.1 Mechanical milling induced hydrogenation in Mg-3%Al system
Figure 1 shows the XRD patterns of the initial mixed powders (3% Al) and the powders milled in hydrogen for various durations. The initial one was well fitted with Mg and Al diffraction peaks. Upon a short milling time of 4 h, tetragonal β-MgH2(JCPDS 12-0697) phase was observed while the relative diffraction intensity of the Al phase didn’t decline, suggesting that the absorption of hydrogen and the hydrogenation of Mg by the mechanically driven solid-gas reaction Mg+H2→MgH2 was prior to the formation of Mg-Al solid solution. With the increase of milling time, the relative diffraction intensity of the β-MgH2 phase increased with a decreasing speed due to the consumption of hydrogen, and that of the Mg (JCPDS 12-0697) phase decreased correspondingly. Indeed, after milling for 32 h, the diffraction peaks of the Mg phase nearly disappeared, meaning that almost all Mg was hydrogenated to form β-MgH2 phase.
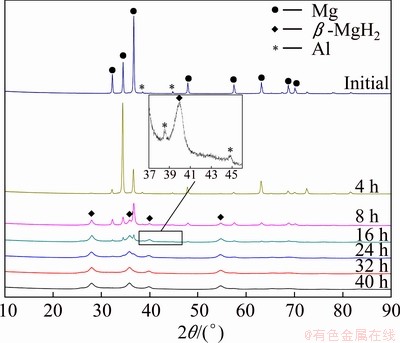
Fig. 1 XRD patterns of Mg-3%Al powder milled in hydrogen with different time
VIGEHOLM et al [13] estimated the enthalpy and corresponding entropy of MgH2 formation as -70.0 kJ/mol and -126 J/(mol·K), respectively. The high energy ball milling is able to create high density of lattice defects and interfaces in the powders and thus increases the free energy of the system. The increased free energy along with the local temperature rising helps to overcome the needed energy barrier for the formation of MgH2 [14].
Figure 2 shows the evolution in percentage of different phases with milling durations, which was calculated using the integrated intensity of diffraction peaks and the value of reference intensity ratio (RIR) of each phase. For powders milled from 8 to 40 h, the percentage of Al phase was always lower than 3% due to the formation of Mg(Al) solid solution, which was reflected in the reduction of the lattice constants a and c of Mg. However, the percentage of Al in the powders milled for 16 h, 1.37%, violated the decreasing tendency with increasing milling time. This may be attributed to the continuous reaction between Mg(Al) solution and hydrogen [7,15,16], which forms β-MgH2 and pushes Al out. After 24 h milling, no diffraction peaks of Al phase were observed, suggesting that the Al element dissolved again either in residual Mg or in MgH2 phases. Indeed, both the lattice parameters of Mg and MgH2 have changed after a long time milling.
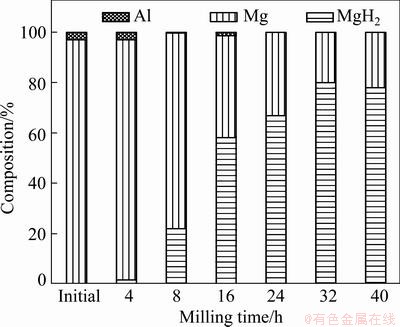
Fig. 2 Phase constitution of powders milled in hydrogen for different time in Mg-3%Al system
By calculation based on the half width of the XRD peaks, the average grain size of β-MgH2 formed by mechanical milling of the Mg-3%Al powders in hydrogen was estimated, which shows a decreasing tendency with the increasing milling duration, as seen in Fig. 3. After milling for 16 h, the average grain size of β-MgH2 was smaller than 10 nm, reaching approximately 8.35 nm. Since the key step of hydrogenation- disproportionation–desorption–recombination (HDDR) treatment is to produce nanostructured hydride powders by hydrogenation, the process to produce nanocrystalline MgH2 powders in the present work is considerable in HDDR, therefore, it is potential to be applied in other Mg alloys.
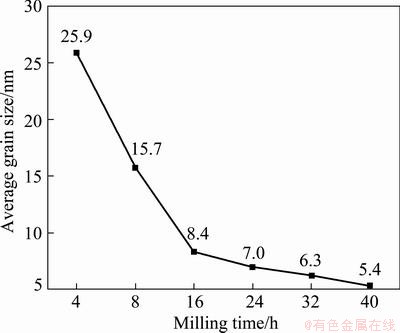
Fig. 3 Evolution of average grain size of β-MgH2 during mechanical milling in Mg-3%Al system
3.2 Mechanical milling induced hydrogenation in Mg-9%Al system
Figure 4 shows the XRD patterns of the initial mixed powders (9% Al) and the powders milled in hydrogen for various durations. With increasing the milling time, the tendency of structural evolution in the Mg-9%Al system was just like that in the Mg-3%Al system as described above. However, Mg17Al12 intermetallic phase was observed in milled Mg-9%Al powders after heat treatment at 350 °C, as shown in Fig. 5. Both the facts that the integrated intensity of diffraction peak 35.9° (the second strong peak of β-MgH2) was bigger than that of 27.9° (the strongest peak of β-MgH2) and the abnormal broadening of peak 35.9° suggested that there was a peak ~36.1° matching the strongest diffraction peak of Mg17Al12 phase. Besides, the three peaks, located at 33.86°, 41.72° and 43.46° in XRD patterns, confirmed the existence of Mg17Al12. Based on the percentage of Mg17Al12 phase obtained by the same way as mentioned above, the mass ratio of Al to Mg was determined to be 0.01084, which was much smaller than the nominal value, 9%/91%=0.09890. That is to say, although there was precipitation of Mg17Al12 phase during the heat treatment, most Al still existed in the solid solution. In addition, mechanical alloying was known to extend the solubility between metals. In the present case, the maximum solubility of Al in Mg may be higher than 3%, so that no precipitation of Mg17Al12 phase occurred in the heat treated Mg-3%Al system.
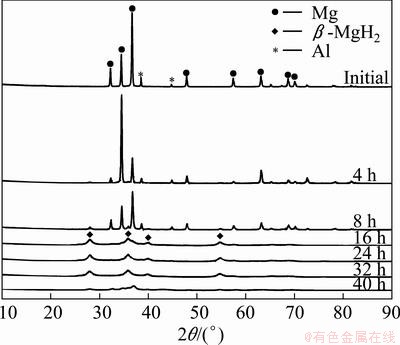
Fig. 4 XRD patterns of Mg-9%Al powder milled in hydrogen with different time
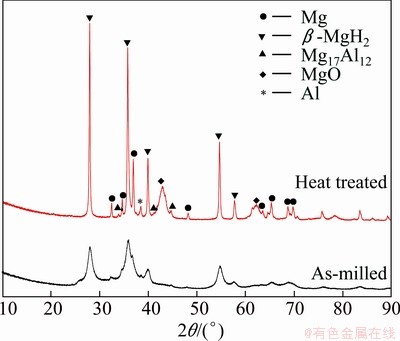
Fig. 5 XRD patterns of Mg-9%Al powders milled for 16 h before and after heat treatment at 350 °C
Figures 6(a) and (b) show a typical bright field TEM image and the corresponding dark field image for the Mg-9%Al powders milled for 16 h in hydrogen atmosphere followed by heat treatment at 350 °C for 20 min. The selected area electron diffraction (SAED) pattern obtained from a region with a diameter of 500 nm is shown in the inset of Fig. 6(a). Based on the indexation of each diffraction ring, Mg, β-MgH2 and Mg17Al12 phases were detected, as shown in Fig. 6(a). To reveal the morphology and distribution of Mg17Al12 phase, the Mg17Al12 (332) plane was chosen to do a dark field image, as shown in Fig. 6(b). It shows that the precipitated Mg17Al12 particles distributed quite homogenously with grain size ranging from several to 20 nm.
TAKAMURA et al [17] investigated the hydrogenation of AZ31 and showed that the AZ31 alloy exhibited a disproportionation reaction, resulting in the formation of MgH2, Mg0.42Al0.58 and Al phases. The results also indicated that most of solute atoms in the AZ31 alloy were expelled from the matrix phase. According to the Mg-Al binary phase diagram, the meta-stable phase Mg0.42Al0.58 can be formed by a peritectic reaction of Mg17Al12 and Mg2Al3. While in the present work, after milling for 24 h and longer, neither Al nor Mg-Al phase were detected by XRD analysis. That is to say, considering the solubility of Al in Mg at 100 °C (1.9%), all Al existed in Mg-Al solution and were preserved in the supersaturated lattice after milling in both Mg-3%Al and Mg-9%Al systems due to the very high mechanical energy provided by ball milling.
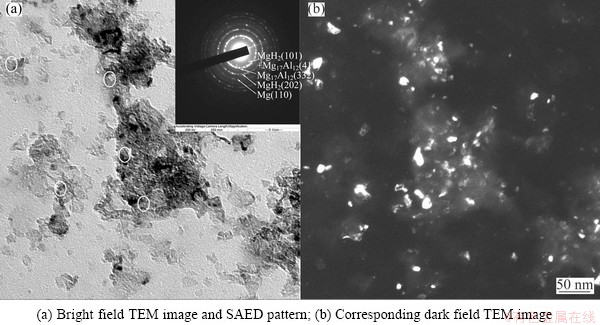
Fig. 6 TEM images of Mg-9%Al powders milled for 16 h in hydrogen and heat treated at 350 °C for 20 min
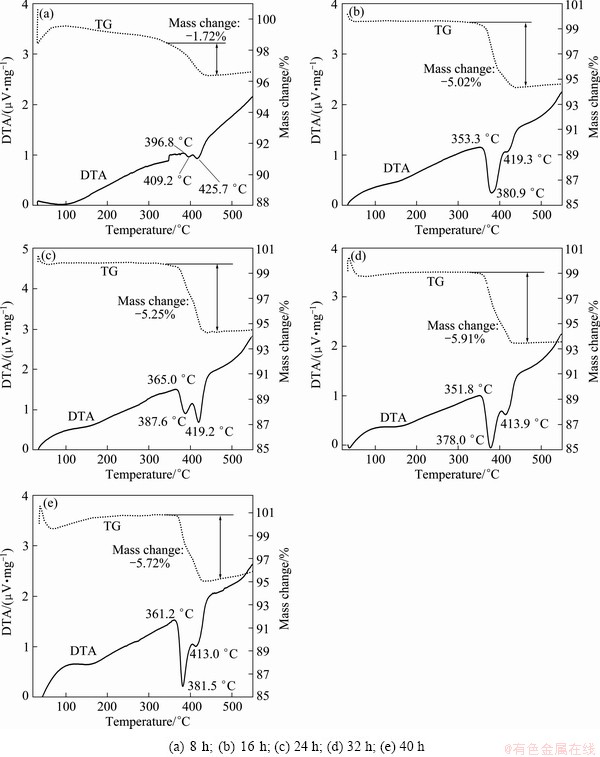
Fig. 7 TG/DTA curves for Mg-3%Al powders ball milled for
3.3 Hydrogen desorption behaviors of as-milled powders
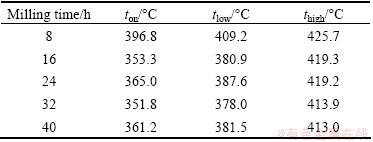
Figure 7 shows the DTA/TG curves of the ball milled Mg-3%Al powders. Since in as-milled powders, H element only exists in MgH2, the percentage of H can be calculated according to the percentage of MgH2, which actually agrees quite well with the mass change in DTA/TG as shown in Table 1 and Fig. 7, indicating that the calculated percentage of each phase in Fig. 2 was reliable. In all the DTA/TG curves of powders milled from 8 h to 40 h, either well-developed peak doublets or shoulders were observed. The onset dehydrogenation temperature (ton), the peak temperature for low temperature dehydrogenation (tlow) and the peak temperature for high temperature dehydrogenation (thigh) in the doublet, as marked in Fig. 7, are listed in Table 2. The peak splitting phenomenon suggested an overlap of two types of hydrogen desorption reactions. In contrast, a single endothermic peak with the maximum at ~415 °C characterized hydrogen desorption from a coarse particle of as-received commercial MgH2 [18]. All milled powders in the present work had a lower ton than the as-received commercial MgH2. The shorter milling time (8 h) gave a relatively higher ton, tlow and thigh than other longer milling times. While after milling for 16 h and longer, powders had similar ton, tlow and thigh, indicating that the desorption behavior of MgH2 in that powders was fairly similar, though the grain size of MgH2 decreased with the increasing milling time, as shown in Fig. 3. In the work of VARIN et al [18,19], quantitative evidence showed that two factors, namely the refined powder particle size and the γ-MgH2 phase residing within the powder particles, acting additively, were responsible for a substantial reduction of the hydrogen desorption temperature of MgH2 hydride. However, in the present work, no γ-MgH2 was observed beside the size reduction of MgH2 after milling for 16 h.
Table 1 Comparison of hydrogen content in as-milled Mg-3%Al powders from calculation and DTA/TG test

Table 2 Results of DTA/TG test: ton, tlow and thigh of as-milled powders in Mg-3%Al system
Peak doublets and shoulders on DSC curves of hydrogen desorption have been observed by some other researchers. GENNARI et al [12] demonstrated that the low-temperature DSC peak was due to the total decomposition of γ-MgH2 and the partial decomposition of β-MgH2, which was destabilized by the metastable phase γ-MgH2, whereas the high-temperature DSC peak corresponds to β-MgH2. Another explanation was based on the findings of SCHIMMEL et al [20], they reported that in a ball milled nanostructured MgH2 there may exist a non-stoichiometric nanostructured MgHx hydride phase (where x≤2), which likely exhibited a much faster diffusion of hydrogen due to the large concentration of vacancies and could release hydrogen at lower temperature than the stoichiometric β-MgH2. However, in the present work, there was no γ-MgH2, which was always formed during milling through the transformation of β-MgH2 at a high pressure [21]. Besides, the possibility of the existence of such a non-stoichiometric nanostructured MgHx hydride phase in powders milled for only 40 h or shorter duration was fairly low.
However, an alternative explanation was proposed by VARIN et al [19]. In their postulation, the coexistence of two fractions of powder particles: small ones and large ones, is believed to be responsible for the presence of two desorption peaks. In order to demonstrate such a two-step hydrogen desorption occurring owing to the co-existence of two fractions of small and large particles rather than the separate desorption of γ-MgH2 and β-MgH2, the 40 h-milled Mg-3%Al powders were heated to the temperature between the lower and higher peaks, 395 °C. As shown in Fig. 8, apart from Mg, there were only some β-MgH2 left and MgO generated during the heat treatment. In addition, the milled particles tend to form agglomerates due to cold welding. As a result, it is quite plausible that low and high temperatures DTA peaks in doublet correspond to the hydrogen desorption from smaller and larger particle fractions. This intriguing phenomenon of this two-step dehydrogenation behavior of single-phase β-MgH2 is worth further studies.
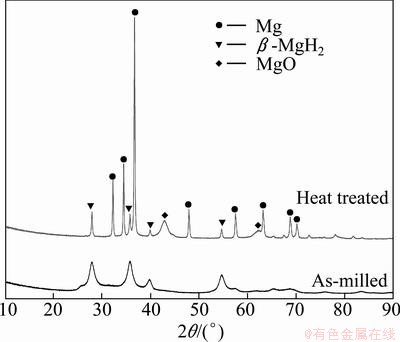
Fig. 8 XRD patterns of Mg-3%Al powders milled for 40 h before and after heat treatment at 395 °C
4 Conclusions
The microstructure and phase evolutions in the Mg-Al powders milled under high pressure hydrogen were investigated. The reaction of Mg with hydrogen during milling formed the stable β-MgH2, which reached its maximum percentage of about 80% after 32 h milling and declined when milling time increased. Al atoms were found to dissolve completely into either Mg or MgH2 after a long time milling. In the Mg-9%Al system, when the milled powders were heated to 350 °C, precipitation of Mg17Al12 occurred, while it was not observed in the Mg-3%Al system. The average grain size of β-MgH2 formed by mechanical milling in hydrogen decreased with increasing milling duration. DTA/TG curves revealed that the onset dehydrogenation temperature of the milled Mg-Al-H powders was much lower than that of the commercial MgH2. In addition, powders milled for 8 h and longer durations showed a two-step hydrogen desorption behavior, which might be attributed to the hydrogen desorption from smaller and larger particle fractions in the milled Mg-Al-H powders.
References
[1] SUN Hong-fei, FANG Wa, FANG Wen-bin. Producing nanocrystalline bulk Mg-3Al-Zn alloy by powder metallurgy assisted hydriding-dehydriding [J]. J Alloys Compd, 2011, 509: 8171-8175.
[2] GOVIND K, SUSEELAN N, M C MITTAL, KISHORI L, R K MAHANTI, C S SIVARAMAKRISHNAN. Development of rapidly solidified (RS) magnesium-aluminium-zinc alloy [J]. Mater Sci Eng A, 2001, 304-306: 520-523.
[3] SHENG Shao-ding, CHEN Ding, CHEN Zhen-hua. Effects of Si addition on microstructure and mechanical properties of RS/PM (rapid solidification and powder metallurgy) AZ91 alloy [J]. J Alloys Compd, 2009, 470(1-2): L17-L20.
[4] CAI Jing, MA Guo-hong, LIU Zheng, WANG Ai-min, HU Zhuang-qi. Influence of rapid solidification on the mechanical properties of Mg-Zn-Ce-Ag magnesium alloy [J]. Mater Sci Eng A, 2007, 456(1-2): 364-367.
[5] CZERWINSKI F. Magnesium alloy particulates for thixomolding applications manufactured by rapid solidification [J]. Mater Sci Eng A, 2004, 367(1-2): 261-271.
[6] KAMEGAWA A, FUNAYAMA T, TAKAHASHI J, TAKAMURA H, OKADA M. Grain refinement of Al-Mg alloy by hydrogen heat-treatment [J]. Mater Trans, 2005, 46(11): 2449-2453.
[7] YUAN Yuan, WANG Heng, HU Lian-xi, SUN Hong-fei, FANG Wen-bin. Hydriding and microstructure nanocrystallinzation of ZK60 Mg alloy by reaction milling in hydrogen [J]. Transactions of Nonferrous Metals Society of China, 2009, 19: s363-s367.
[8] WANG Heng, HU Lian-xi, YUAN Yuan, WANG Er-de. Desorption behavior and microstructure change of nanostructured hydrided AZ31 Mg alloy powders [J]. Transaction of Nonferrous Metals Society of China, 2010, 20: 597-601.
[9] BOBET J L, CHEVALIAR B, SONG M Y, DARRIET B, ETOURNEAU J. Hydrogen sorption of Mg-based mixtures elaborated by reactive mechanical grinding [J]. J Alloys Compd, 2002, 336: 292-296.
[10] VARIN R A, LI S, CHIU C, GUO L, MOROZOVA O, KHOMENKO T, WRONSKI Z. Nanocrystalline and non-crystalline hydrides synthesized by controlled reactive mechanical alloying/ milling of Mg and Mg-X (X=Fe, Co, Mn, B) systems [J]. J Alloys Compd, 2005, 404-406: 494-498.
[11] WANG Xin, WANG Heng, HU Lian-xi, WANG Er-de. Nanocrystalline Mg and Mg alloy powders by hydriding-dehydriding processing [J]. Transaction of Nonferrous Metals Society of China, 2010, 20: 1326-1330.
[12] GENNARI F C, CASTRO F J, URRETAVIZCAYA G. Hydrogen desorption behavior from magnesium hydrides synthesized by reaction mechanical alloying [J]. J Alloys Compd, 2001, 321: 46-53.
[13] VIGEHOLM B,  J, LARSEN B. Magnesium for hydrogen storage [J]. J Less-Comm Metals, 1980, 74: 341-350.
J, LARSEN B. Magnesium for hydrogen storage [J]. J Less-Comm Metals, 1980, 74: 341-350.
[14] NORITAKE T, TOWATA S, AOKI M, SENO Y, HIROSE Y, NISHIBORI E, TAKATA M, SAKATA M. Charge density measurement in MgH2 by synchrotron X-ray diffraction [J]. J Alloys Compd, 2003, 356-357: 84-86.
[15] MIYAZAWA T, KOBAYASHI Y, KAMEGAWA A, TAKAMURA H, OKADA M. Grain size refinement of Mg alloys (AZ61, AZ91, ZK60) by HDDR treatment [J]. Mater Trans, 2004, 45(2): 384-387.
[16] WRONSKI Z, VARIN R A, CHIU C, CZUJKO T, CALKA A. Mechanical synthesis of nanostructured chemical hydrides in hydrogen alloying mills [J]. J Alloys Compd, 2007, 434-435: 743-746.
[17] TAKAMURA H, MIYASHITA T, KAMEGAWA A, OKADA M. Grain size refinement in Mg-Al based alloy by hydrogen treatment [J]. J Alloys Compd, 2003, 356-357: 804-808.
[18] VARIN R A, CZUJKO T, WRONSKI Z. Particle size, grain size and γ-MgH2 effects on the desorption properties of nanocrystalline commercial magnesium hydride processed by controlled mechanical milling [J]. Nanotech, 2006, 17: 3856-3865.
[19] VARIN R A, CZUJKO T, CHIU C, WRONSKI Z. Particle size effects on the desorption properties of nanostructured magnesium dihydride (MgH2) synthesized by controlled reactive mechanical milling (CRMM) [J]. J Alloys Compd, 2006, 424: 356-364.
[20] SCHIMMEL H G, HOUT J, CHAPON L C, TICHELAAR F D, MULDER F M. Hydrogen cycling of niobium and vanadium catalyzed nanostructured magnesium [J]. J Am Chem Soc, 2005, 127: 14348-14354.
[21] VARIN R A, CZUJKO T, WRONSKI Z. Nanomaterials for solid state hydrogen storage [M]. New York: Springer Science and Business Media, 2009: 120-124.
王 瑶1,曾小勤1,2,邹建新1,李德江1,吴晓梅1,丁文江1,2
1. 上海交通大学 材料科学与工程学院,上海镁材料及应用工程技术研究中心,上海 200240;
2. 上海交通大学 材料科学与工程学院,金属基复合材料国家重点实验室,上海 200240
摘 要:研究Mg-Al粉体在氢气气氛下球磨的显微组织及相变。结果表明:在Mg-3%Al和Mg-9%Al两种粉末中,球磨4 h后都能观察到β-MgH2相生成,并且β-MgH2相的含量在球磨32 h后达到最大(~80%)。在这两种粉末中球磨后都没有Mg17Al12相出现。而将Mg-9%Al球磨后粉末加热到350 °C有Mg17Al12相析出。球磨8~40 h后,DTA分析显示放氢曲线都有双峰现象,这可能是β-MgH2相不同大小颗粒的放氢性能不同产生的。
关键词:镁合金;机械球磨;氢化;纳米镁氢化物;双峰
(Edited by Chao WANG)
Foundation item: Projects (10JC407700, 11ZR1417600) supported by the Science and Technology Committee of Shanghai, China; Project (12zz017) supported by the Shanghai Education Committee, China
Corresponding author: Xiao-qin ZENG; Tel: +86-21-54742301; Fax: +86-21-34203730; E-mail: xqzeng@sjtu.edu.cn
DOI: 10.1016/S1003-6326(13)62841-1

