J. Cent. South Univ. (2012) 19: 911-917
DOI: 10.1007/s11771-012-1092-4
Synthesis and properties of UV curable polyurethane acrylates based on
two different hydroxyethyl acrylates
LIAO Feng(廖峰)1, ZENG Xing-rong(曾幸荣)1, LI Hong-qiang(李红强)1,
LAI Xue-jun(赖学军)1, ZHAO Fu-chun(赵富春)1, 2
1. College of Materials Science and Engineering, South China University of Technology, Guangzhou 510640, China;
2. School of Materials and Chemical Engineering, Hainan University, Haikou 570228, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract: Two kinds of UV curable polyurethane acrylate oligomers (PUPA and PUCA) were synthesized via the addition reaction between isophorone diisocyanate (IPDI) and polyethylene glycol monoacrylate (PEA6) or polycaprolactone modified hydroxyethyl acrylate (PCLA2). The structures of PUPA and PUCA were characterized by Fourier transform infrared spectroscopy (FT-IR), 1H nuclear magnetic resonance (1H NMR), gel permeation chromatography (GPC) and differential scanning calorimeter (DSC), and the thermal stability and dynamic mechanical thermal properties of their cured films were measured by thermogravimetric analysis (TGA) and dynamic mechanical analysis (DMA), respectively. The viscosity of the oligomers and mechanical properties of the cured films were also studied. The results show that both oligomers have narrow molecular weight distribution. The viscosity of PUPA is 2.310 Pa·s at 25 °C, while that of PUCA is up to 3.980 Pa·s. The UV cured PUPA and PUCA films have homogeneous phase structure, and the PUCA film shows higher glass transition temperature and storage modulus. Furthermore, the PUCA film possesses better mechanical properties than PUPA, while the latter shows better alkali resistance.
Key words: UV curable oligomer; isophorone diisocyanate; polyurethane acrylate; hydroxyethyl acrylate
1 Introduction
UV curable acrylic functional monomers and oligomers have been widely used in various industrial fields such as coatings, printing inks, adhesives, dental composites, and photoresists, due to their outstanding advantages including instant drying, solvent-free formulations, and reduced energy consumption [1-4]. Polyurethane acrylate (PUA) is one of the most widely and diversely applied acrylic resins for UV curing because of its unique properties, such as excellent abrasion resistance, flexibility, hardness and solvent resistance [5-7]. Traditionally, PUA is synthesized by two-step process via the addition reaction between an excess of isocyanate and various polyol or polyamine, and then the remaining isocyanate functionalities are tipped with hydroxyethyl (meth)acrylate [8-11]. However, the synthesis process is complicated and requires a long reaction time. Therefore, to develop some effective methods to prepare PUA is a significant subject in UV curable chemistry field.
Polyethylene glycol monoacrylate (PEA6) and polycaprolactone modified hydroxyethyl acrylate (PCLA2) are two specific hydroxyethyl acrylates that contain ethylene oxide segments or caprolactone segments. Due to their low glass transition temperatures (Tg) and excellent plasticizing properties, their derivates have been widely used in screen printings, polymer electrolytes for lithium batteries, antifouling surfaces, and polyurethane dispersions [12-15]. Moreover, PEA6 and PCLA2 can endow the PUA a good flexibility and impact resistance. Therefore, it is very promising to prepare PUA with PEA6 and PCLA2.
In this work, two kinds of UV curable polyurethane acrylate oligomers (PUPA and PUCA) were synthesized via the addition reaction between isophorone diisocyanate (IPDI) and PEA6 or PCLA2. Then, the structures of oligomers and the properties of their cured films were characterized by Fourier transform infrared spectroscopy (FT-IR), 1H nuclear magnetic resonance (1H NMR), gel permeation chromatography (GPC), differential scanning calorimeter (DSC), viscosity test, thermogravimetric analysis (TGA) and dynamic mechanical analysis (DMA), mechanical test and chemical resistance test. Through the investigation, it is expected to develop two new PUA oligomers with excellent mechanical properties and definite the structure-property relationship.
2 Experimental
2.1 Materials
Polyethylene glycol monoacrylate (PEA6, Mn= 360 g/mol) was analytical reagent and purchased from Sigma-Aldrich Co., USA. Polycaprolactone modified hydroxyethyl acrylate (PCLA2, Mn=344 g/mol) was of industrial grade and obtained from Daicel Chemical Industries Ltd., Japan. Isophorone diisocyanate (IPDI) was of industrial grade and supplied by BASF Co., Germany. Dibutyltin dilaurate (DBTDL) was chemical pure and provided by GE Co. USA. Hydroquinone methylether (HQME) was chemical pure and obtained from Tianjin No. 1 Chemical Reagent Factory, China. 2-hydroxyl-2-methyl-l-phenyl-1-propanone (Darocur 1173) was of industrial grade and supplied by Ciba Co., Switzerland. PEA6 and PCLA2 were dried over 4 ? molecular sieves before use, and other reagents were used as received without further purification.
2.2 Synthesis of PUA oligomers
Two kinds of PUA oligomers (PUPA and PUCA) were synthesized through the addition reaction between IPDI and PEA6 or PCLA2, and their synthesis routes are shown in Fig. 1. IPDI and 0.5% (mass fraction) DBTDL were charged into a four-necked flask equipped with a magnetic force stirrer, a nitrogen gas inlet, a dropping funnel, a thermometer and a reflux condenser. Subsequently, a predetermined amount of PEA6 or PCLA2 with 0.5% (mass fraction) HQME was dropped into the flask to react with IPDI, then the temperature of the mixture was heated up to 60 °C. The molar ratio of the —NCO group of IPDI to the hydroxyl group in PEA6 (or PCLA2) was set at 1:1.01. The finish of reaction was determined by the disappearance of the peak at 2 260 cm-1 for —NCO group on the FT-IR spectrum. A stream of dry nitrogen gas was led into the flask and maintained throughout the reaction.
2.3 Preparation of UV cured PUA films
The oligomer (PUPA or PUCA) was first mixed with 3% (mass fraction) photoinitiator (Darocur 1173), and then the mixture was coated on a glass or tin plate by an applicator with a 100 μm gap. The sample was left for a flash-off period for 5 min at 50 °C to eliminate the air bubble and then cured for 45 s by exposing them to a UV lamp (365 nm, 2 kW, 30 mW/cm2) made by Shenzhen Nengia Automation Equipment Co., China. The cured film was stored in a desiccator at room temperature for further studies.
2.4 Measurements
2.4.1 FT-IR
The FT-IR spectra were recorded on a TENSOR27 FT-IR spectrometer (Bruker Co., Germany). The samples were coated on KBr pellets and the spectra were obtained over the range from 400 to 4 000 cm-1 at a resolution of 2 cm-1.
2.4.2 1H NMR
The 1H NMR spectra were recorded on an AVANCE 400 NMR spectrometer (Bruker Co., Germany) operated at 400 MHz using tetramethylsilane (TMS) as an internal standard, and CDCl3 as the solvent.
2.4.3 GPC
The GPC experiments were performed on Waters 1515 GPC instrument (Waters Co., USA) with THF as the mobile phase. The flow rate of THF was 1.0 mL/min. Narrow distribution linear polystyrenes were used as the standard to calibrate the apparatus, and the molecular mass of the oligomers was measured using universal calibration.
2.4.4 DSC
The DSC analysis were conducted on a DSC204 F1 instrument (Netzsch Co., Germany) under nitrogen atmosphere, at a heating rate of 10 °C/min from -80 °C to 100 °C.
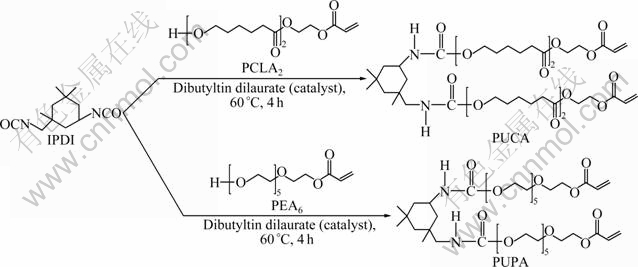
Fig. 1 Synthetic routes of PUPA and PUCA
2.4.5 DMA
The DMA measurements were performed on a DMA242C instrument (Netzsch Co., Germany) in tensile mode with rectangular sample geometry of 5 mm × 3 mm × 0.03 mm, at a heating rate of 3 °C/min from -50 to 100 °C and frequency of 1 Hz.
2.4.6 TGA
The TGA was performed on a TG209F1 instrument (Netzsch Co., Germany) under nitrogen atmosphere, at a heating rate of 10 °C/min from 30 °C to 600 °C.
2.4.7 Viscosity
The viscosities of the oligomers were measured by a NJD-1 rotational viscometer (Shanghai Jingke Co., China) according to ASTM D 3236—2004.
2.4.8 Mechanical properties
The mechanical properties of the cured films including pencil hardness (ASTM D 3363—2005), flexibility property (ASTM D 4145—2002), impact strength (ASTM D 2794—2004) and crosshatch adhesion (ASTM D 3359—2008) were measured.
2.4.9 Chemical resistance and solvent resistance
The chemical resistance of cured films was checked according to ASTM D 1647—2009. Glass panels coated with PUA were dipped into 3% HCl, 5% NaOH, 10% NaCl solutions, and the changes in the appearance were monitored after 7 d. The solvent resistance of cured films was determined by the double rub method with ethanol as solvent according to ASTM D 5402—2006. The results were reported as the minimum number of double rubs at which the films were observed to fail or else, 250, which was the maximum number of double rubs.
3 Results and discussion
3.1 Structure characterization of PUA oligomers
The structures of PUPA and PUCA oligomers were characterized by FT-IR, 1H NMR and GPC. Figure 2 shows the FT-IR spectra of mixture of IPDI and PEA6 (a), PUPA (b), mixture of IPDI and PCLA2 (c) and PUCA (d). As shown in Figs. 2(b) and (d), the appearance of the strong characteristic peaks, such as the stretching vibrations of —NH— at 3 390 cm-1, the stretching vibrations of C=O of carbamate groups at 1 720 cm-1, and the deformation vibrations of —NH— at 1 520 cm-1 confirms the formation of PUA. Furthermore, the spectra of PUPA and PUCA do not show the characteristic peak of —NCO at 2 260 cm-1 nor the stretching vibration peak of —OH at 3 580 cm-1, indicating that all —NCO groups have reacted with the hydroxyl groups in PEA6 or PCLA2 to form PUA. The appearance of absorption peaks at 1 635 cm-1 (C=C),
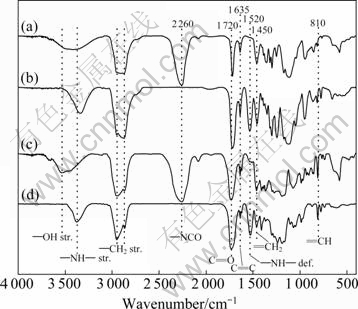
Fig. 2 FT-IR spectra of mixture of IPDI and PEA6 (a), PUPA (b), mixture of IPDI and PCLA2 (c) and PUCA (d)
1 450 cm-1 (=CH2) and 810 cm-1 (=CH) indicates that the C=C bonds of acrylate have incorporated into the polyurethane chains.
Figure 3 shows the 1H NMR spectra of PUPA (a) and PUCA (b), and the assignation of peaks is also remarked. For the PUPA (a) and PUCA (b), most of the peaks are in accordance with those of PUA made from polyethylene glycol or polycaprolactone [16-18]. Three characteristic peaks at (6.4-6.5)×10-6, (6.1-6.2)×10-6 and (5.8-5.9)×10-6 are clearly observed, which prove the existence of acrylic groups (CH2=CH—COO—) in the molecular structure of PUPA and PUCA. The peak at (7.2-7.3)×10-6 is assigned to the carbamate groups (—NH—COO—), indicating that the structure of —NH— is formed through the reaction between —NCO of the IPDI and —OH groups of the PEA6 or PCLA2. Moreover, the 1H NMR spectra can be used to calculate the reaction degree by comparing the integral area of the protons of the —NH— groups and the protons of the CH2OCH— groups [17]. According to 1H NMR spectra (Fig. 3), it is calculated that the molar ratio of carbamate groups to acrylic groups in PUPA and PUCA is nearly 1:1. The results indicate that the reaction between IPDI and PEA6 or PCLA2 is accomplished.
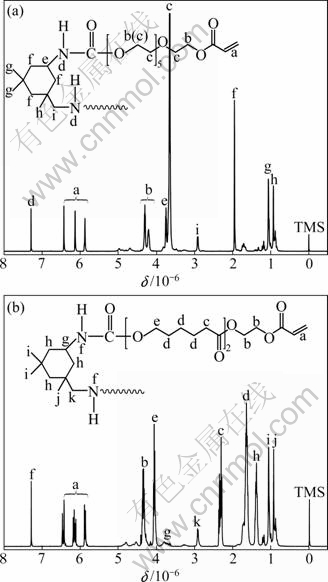
Fig. 3 1H NMR spectra of PUPA (a) and PUCA (b)
Figure 4 shows the GPC curves of PUPA and PUCA, and the analysis results are summarized in Table 1. As can be seen, the molecular mass for PUPA and PUCA measured by conventional calibration are in good agreement with the theoretical values. Moreover, PUPA shows lower elution time compared to PUCA, indicating that PUPA possesses higher molecular mass than PUCA. The monodisperse model and relatively narrow polydispersity of the GPC plots also confirm that the synthesized PUPA and PUCA have high conversion rate. According to the analyses above, it can be concluded that PUPA and PUCA are synthesized successfully.
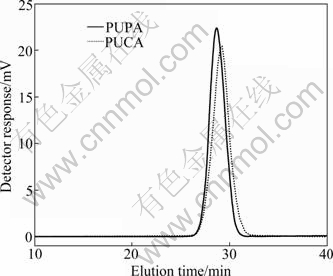
Fig. 4 GPC curves of PUPA and PUCA
Table 1 GPC data of PUPA and PUCA

3.2 Glass transition temperature of PUA oligomers
Generally, Tg of polymer mainly depends on intermolecular interaction and the chain flexibility [15, 18]. Figure 5 shows the DSC curves of PUPA and PUCA oligomers. It can be seen that PUPA has lower Tg value (-20.1 °C) than PUCA (-13.2 °C). This is because of less ratios of ester groups in PUPA than PUCA, which leads to lower polarity and intermolecular interaction in PUPA. Besides, PUPA with ethylene oxide segments has higher flexibility than PUCA with caprolactone segments.
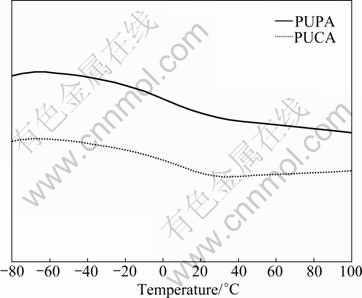
Fig. 5 DSC curves of PUPA and PUCA
3.3 Viscosity of PUA oligomers
The viscosity of oligomer is one of the most important parameters, and it directly affects the flow property, the rate of air release and the rate of photopolymerization. Figure 6 shows the viscosity of PUPA and PUCA versus temperature. It can be seen that the PUPA exhibits lower viscosity and its value is 2.310 Pa·s at 25 °C, while the viscosity of PUCA is up to 3.980 Pa·s. The viscosity of PUPA and PUCA decreases sharply with the increase of temperature. This is because the molecular mobility increases and intermolecular entanglement reduces with the increase of temperature. Meanwhile, the free volume increases with the increase of temperature, thus the inter/intramolecular hydrogen bond force resulted from carbamate groups in PUA reduces accordingly. Moreover, the difference of viscosity of PUPA and PUCA decreases gradually with the increase of temperature. This is because PUCA has higher Tg than PUPA, and the according results can be calculated by the semi-empirical Willams-Landel-Ferry (WLF) equation [19]. The results reveal that the viscosity of PUA oligomers are affected by their structure and the inter/intermolecular hydrogen bond force.
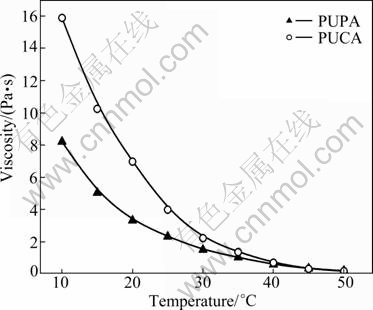
Fig. 6 Viscosity of PUPA and PUCA at different temperatures
3.4 DMA of UV cured PUA films
The DMA is used to investigate the dynamic mechanical thermal properties of the UV cured PUA films. The temperature at the peak of loss factor (tan δ) is defined as the glass transition temperature (Tg). The extrapolated onset of the drop of storage modulus (E′) is defined as the softening point (Ts). The Ts/Tg ratio expresses the width of a tan δ peak, and higher Ts/Tg ratio leads to narrower tan δ peak and the obtained film is more homogeneous [20]. Figure 7 shows the temperature dependence versus loss factor (tan δ) of the UV cured PUA films. It can be seen that the Tg of PUPA films is lower than that of PUCA films, as listed in Table 2, indicating that PUPA film with ethylene oxide segments has higher flexibility than that of PUCA with polycaprolactone segments. Moreover, it is known that the magnitude of the tan δ value can be used to estimate the damping behavior of a material [21-23]. The tan δ value of PUPA is higher than that of PUCA (Table 2), and this indicates higher damping property of PUPA film. This can be attributed to the higher flexibility of the ethylene oxide segments in the glass transition temperature region.
The temperature dependence of storage modulus (E′) of UV cured films is shown in Fig. 8. It can be seen that the E′ of PUCA is higher than that of PUPA, indicating that the cured PUCA film has higher stiffness. Furthermore, the cured PUPA and PUCA films have single tan δ peak, indicating that their films have homogeneous phase structure. Meanwhile, because of the existence of intensive phase mixing between soft segments and hard segments, the high Ts/Tg ratio of PUPA and PUCA is achieved by incorporating low molecular mass polyether or polyester segments.
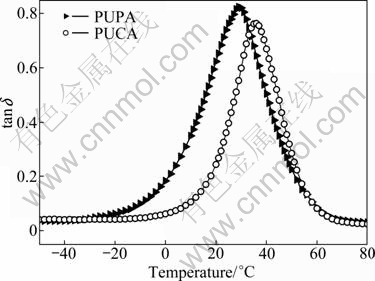
Fig. 7 Dependence of tan δ on temperature of UV cured PUPA and PUCA films
Table 2 Dynamical mechanical data of UV cured films

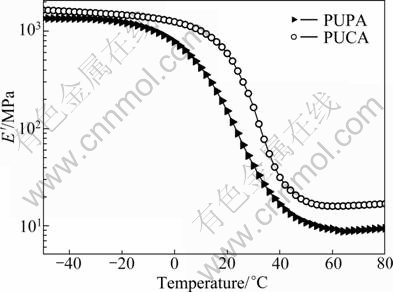
Fig. 8 Dependence of E′ on temperature of UV cured PUPA and PUCA films
3.5 Thermal stability of UV cured PUA films
The TGA and DTG curves of UV cured PUA films are shown in Figs. 9(a) and (b), respectively. The initial decomposition temperature of PUA films is evaluated by the temperature of 5% mass loss (T-5%), the char residue at 600 °C is obtained from the TG curves, and the temperature of the maximum mass loss rate (Tmax) of PUA films is obtained from the DTG curves. As can be seen, the thermal decomposition processes of PUPA and PUCA have two stages. The PUPA starts to decompose at 272 °C, and the first stage is at 220-380 °C and its Tmax1 is 346 °C. The second stage is at 380-480 °C and its Tmax2 is 390 °C. As for PUCA, its TGA curve has a more apparent two decomposition stages, and it starts to decompose at 270 °C, which is quite close to the T-5% of PUPA. The second decomposition stage of PUCA is at 395-510 °C and its Tmax2 is 429 °C. Generally, the first decomposition stage of PUA results from the degradation of urethane segments, and the subsequent stage is due to the degradation of soft segments from the polyether or polyester [24-26]. As can be seen in Fig. 9, PUCA films show higher thermal decomposition temperature in the second stage, and this can be explained by the fact that the caprolactone segments in PUCA have better thermal stability than the ethylene oxide segments in PUPA.
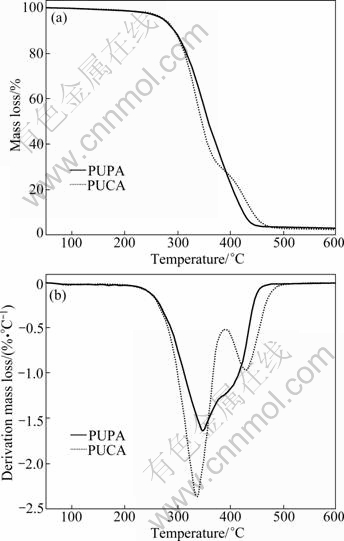
Fig. 9 TGA and DTG curves of UV cured PUA films: (a) TGA curve; (b) DTG curve
3.6 Mechanical properties and chemical resistance of UV cured PUA films
The properties of the UV cured PUPA and PUCA films were measured, and the results are given in Table 3. It can be seen that both UV cured films possess superior flexibility. Furthermore, the UV cured PUCA film has higher pencil hardness and impact strength than PUPA film. The higher hardness of PUCA film is due to the higher stiffness of polycaprolactone segments in the polymeric backbones. The impact strength of films depends mainly on the absorption and conversion of impact energy, and the flexibility of molecule chains [18, 22]. Therefore, PUCA film exhibits higher impact strength, due to its higher storage modulus.
Table 3 Properties of UV cured PUA films
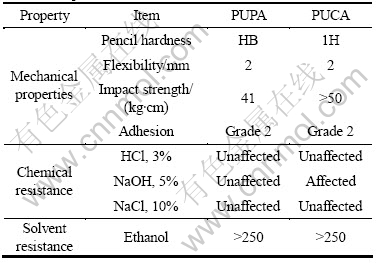
The chemical resistance and solvent resistance of the UV cured PUPA and PUCA films are also listed in Table 3. It can be seen that both films are almost unaffected under the environment of the HCl and NaCl. PUPA film is hardly unaffected upon their exposure to NaOH, while PUCA film will lose gloss after 3 d. It is due to the alkali hydrolysable of ester groups in polycaprolactone segments. Furthermore, both cured films show no variation up to 250 double rubs in the case of ethanol resistance, indicating that the UV cured PUPA and PUCA films show excellent solvent resistance.
4 Conclusions
1) Two kinds of UV curable polyurethane acrylate oligomers are successfully synthesized. Both oligomers possess relatively narrow molecular mass distribution.
2) PUPA has lower Tg value (-20.1 °C) than PUCA (-13.2 °C). The viscosity of PUPA is 2.310 Pa·s at 25 °C, while the viscosity of PUCA is up to 3.980 Pa·s.
3) The UV cured PUPA and PUCA films have homogeneous phase structure. PUCA film shows higher glass transition temperature and storage modulus, while PUPA film has higher damping property.
4) Both films have good thermal stability, and their films have superior flexibility and solvent resistance. Furthermore, PUCA film has better impact strength and pencil hardness than PUPA, but PUPA shows better resistant to alkali resistance.
References
[1] CHIANG T H, HSIEH T E. A study of monomer’s effect on adhesion strength of UV-curable resins [J]. International Journal of Adhesion and Adhesives, 2006, 26(7): 520-531.
[2] SRIVASTAVA A, AGARWAL D, MISTRY S, SINGH F. UV curable polyurethane acrylate coatings for metal surfaces [J]. Pigment & Resin Technology, 2008, 37(4): 217-223.
[3] EL-MOLLA M. Synthesis of polyurethane acrylate oligomers as aqueous UV-curable binder for inks of ink jet in textile printing and pigment dyeing [J]. Dyes and Pigments, 2007, 74(2): 371-379.
[4] OPREA S, VLAD S, STANCIU A, MACOVEANU M. Epoxy urethane acrylate [J]. European Polymer Journal, 2000, 36(2): 373-378.
[5] ZHANG Jing, XIAO Pu, SHI Su-qing, NIE Jun. Synthesis and photopolymerization kinetics of multifunctional aromatic urethane acrylates containing tertiary amine group [J]. Polymers for Advanced Technologies, 2009, 20(1): 16-20.
[6] WANG F, HU J Q, TU W P. Study on microstructure of UV-curable polyurethane acrylate films [J]. Progress in Organic Coatings, 2008, 62(3): 245-250.
[7] LIN Y H, LIAO K H, CHOU N K, WANG S S, CHU S H, HSIEH K H. UV-curable low-surface-energy fluorinated poly(urethane-acrylate)s for biomedical applications [J]. European Polymer Journal, 2008, 44(9): 2927-2937.
[8] ZHANG Tong, WU Wen-jian, WANG Xiao-jie, MU Yu-ping. Effect of average functionality on properties of UV-curable waterborne polyurethane-acrylate [J]. Progress in Organic Coatings, 2010, 68(3): 201-207.
[9] LI Zhi-hua, HUANG Yao-peng, REN Dong-yan, ZHENG Zi-qiao. Structural characteristics and properties of polyurethane modified TDE-85/MeTHPA epoxy resin with interpenetrating polymer networks [J]. Journal of Central South University of Technology, 2008, 15(3): 305-308.
[10] ASHA S K, THIRUMAL M, KAVITHA A, PILLAIC K S. Synthesis and curing studies of PPG based telechelic urethane methacrylic macromonomers [J]. European Polymer Journal, 2005, 41(1): 23-33.
[11] HE Yong, ZHOU Meng-bo, WU Bo, JIANG Zhan-lin, NIE Jun. Synthesis and properties of novel polyurethane acrylate containing 3-(2-hydroxyethyl) isocyanurate segment [J]. Progress in Organic Coatings, 2010, 67(3): 264-268.
[12] TAKAHASHI T, WATANABE H, MIYAGAWA N, TAKAHARA S, YAMAOKA T. Application of photopolymer to core-hair type microgels with various hair length [J]. Polymers for Advanced Technologies, 2002, 13(1): 33-39.
[13] KIM C S, KIM B H, KIM K. Synthesis and characterization of polyether urethane acrylate-LiCF3SO3-based polymer electrolytes by UV-curing in lithium batteries [J]. Journal of Power Sources, 1999, 84(1): 12-23.
[14] IGUERB O, BERTRAND P. Graft photopolymerization of polyethylene glycol monoacrylate (PEGA) on poly(methyl methacrylate) (PMMA) films to prevent BSA adsorption [J]. Surface and Interface Analysis, 2008, 40(3/4): 386-390.
[15] TASIC S, BOZIC B, DUNJIC B. Synthesis of new hyperbranched urethane-acrylates and their evaluation in UV-curable coatings [J]. Progress in Organic Coatings, 2004, 51(4): 321-328.
[16] ASHA S K, THIRUMAL M, KAVITHA A, PILLAI C K S. Synthesis and curing studies of PPG based telechelic urethane methacrylic macromonomers [J]. European Polymer Journal, 2005, 41(1): 23-33.
[17] HE Yong, ZHOU Meng-bo, WU Bo, JIANG Zhang-lin, NIE Jun. Synthesis and properties of novel polyurethane acrylate containing 3-(2-hydroxyethyl) isocyanurate segment [J]. Progress in Organic Coatings, 2010, 67(3): 264-268.
[18] CHAN-CHAN L H, SOLIS-CORREA R, VARGAS-CORONADO R F, CERVANTES-UC J M, CAUICH-RODRIGUEZ J V, QUINTANA P, BARTOLO-P?REZ P. Degradation studies on segmented polyurethanes prepared with HMDI, PCL and different chain extenders [J]. Acta Biomaterialia, 2010, 6(6): 2035-2044.
[19] BARBEAU P H, GERARD J F, MAGNY B, PASCAULT J P. Effect of the diisocyanate on the structure and properties of polyurethane acrylate prepolymers [J]. Journal of Polymer Science Part B: Polymer Physics, 2000, 38(21): 2750-2768.
[20] KIM B, LEE K, JO N. Basic structure-property behavior of UV curable polyurethane acrylates [J]. Journal of Polymer Science Part A: Polymer Chemistry, 2000, 34(11): 2095-2102.
[21] TEY J N, SOUTAR A M, MHAISALKAR S G, YU H, HEW K M. Mechanical properties of UV-curable polyurethane acrylate used in packaging of MEMS devices [J]. Thin Solid Films, 2006, 504(1): 384-390.
[22] YOO H J, LEE Y H, KWON J Y, KIM H D. Comparison of the properties of UV-cured polyurethane acrylates containing different diisocyanates and low molecular weight diols [J]. Fibers and Polymers, 2001, 2(3): 122-128.
[23] ATHAWALE V D, KULKARNI M A. Polyester polyols for waterborne polyurethanes and hybrid dispersions [J]. Progress in Organic Coatings, 2010, 67(1): 44-54.
[24] GITE V V, MAHULIKAR P P, HUNDIWALE D G. Preparation and properties of polyurethane coatings based on acrylic polyols and trimer of isophorone diisocyanate [J]. Progress in Organic Coatings, 2010, 68(4): 307-312.
[25] LU M G, LEE J Y, SHIM M J, KIM S W. Thermal degradation of film cast from aqueous polyurethane dispersions [J]. Journal of Applied Polymer Science, 2002, 85(12): 2552-2558.
[26] SEO J C, JANG E S, SONG J H, CHOI S, KHAN S B, HAN K. Preparation and properties of poly(urethane acrylate) films for ultraviolet-curable coatings [J]. Journal of Applied Polymer Science, 2010, 118(4): 2454-2460.
(Edited by HE Yun-bin)
Foundation item: Project(2007168303) supported by Guangdong-Hong Kong Technology Cooperation Funding
Received date: 2011-06-11; Accepted date: 2011-07-07
Corresponding author: ZENG Xing-rong, Professor, PhD; Tel: +86-20-87114248; E-mail: psxrzeng@gmail.com