文章编号:1004-0609(2008)08-1555-05
熔盐电解法制备铝锂中间合金
李继东,张明杰,张廷安,李 丹,张 卓
(东北大学 材料与冶金学院,沈阳 110004)
摘 要:研究在LiCl-LiF-Li2CO3纯锂盐体系中下沉式铝液阴极槽结构电解生成铝锂中间合金的工艺过程,尤其是以Li2CO3为原料代替LiCl的电解过程。采用熔盐电解监控仪测量电解过程中的反电动势、槽电压、电流等工艺参数及电解波形图,通过电位控制法调节Li2CO3的加料周期,同时根据得到的合金产品中的锂含量探讨影响电流效率的因素。结果表明,反电动势随电流密度增加而增大,加入2%的Li2CO3可使反电动势降低0.5 V;通过控制电位法测得加料周期为15 min;在680 ℃、电流密度为0.62 A/cm2的条件下持续电解3 h,最终制得了锂含量高达7.93%的铝锂合金,电流效率可达74.1%。
关键词:铝锂合金;熔盐电解;反电动势;电流效率
中图分类号:TF 827.2 文献标识码:A
Preparation of Al-Li master alloys by molten salt electrolysis
LI Ji-dong, ZHANG Ming-jie, ZHANG Ting-an, LI Dan, ZHANG Zhuo
(School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China)
Abstract: Al-Li master alloys were prepared using sinking-mode aluminum liquid as cathode and a mixture of LiCl-LiF-Li2CO3 as molten salt electrolyte in a laboratory cell. The electrolysis process with Li2CO3 as raw material instead of LiCl was studied in detail. The technological parameters such as back electromotive force, cell voltage, current and electrolysis waveform were measured by testing device of molten salt electrolysis. The period of Li2CO3 feeding was adjusted by the method of electric potential control. At the same time, the factor that affects current efficiency was discussed according to the lithium content of alloys obtained. The results indicate that back electromotive force increases with increasing current density and decreases by 0.5 V after adding 2% Li2CO3, the feeding period is 15 min measured by the method of electric potential control. Finally, the Al-Li alloy with the lithium content of 7.93%(mass fraction) can be obtained by electrolyzing for 3 h at the current density of 0.62 A/cm2 and at 680 ℃, and the current efficiency can reach 74.1%.
Key words: Al-Li alloy; molten salt electrolysis; back electromotive force; current density
锂是自然界中最轻的金属元素,密度仅为0.534 g/cm3,向纯铝中掺入1%(质量分数)的锂可使合金密度降低3%,弹性模量增加约6%,用其代替常规的铝合金,可使构件质量减轻10%~15%,刚度提高15%~20%[1?3]。因此,铝锂合金作为一种低密度、高弹性模量、高强度和高比刚度的理想结构材料而广泛应用于航空航天及国防太空等领域[4?5]。
目前,生产铝锂合金的方法多为对掺法,即向纯铝中加入金属锂熔炼,配制铸成一定浓度的铝锂合金。熔盐电解法是以铝液作阴极,电解生成的金属锂在阴极中析出并扩散在铝液中,一步直接合金化。与对掺法生产铝锂合金相比,熔盐电解法具有浓度均匀、锂的烧损率小、生产连续且易控制和低成本等优点,因而引起了人们的广泛研究[6?11]。同时电解一步合金化也应用于其它铝基合金的制备过程,如熔盐电解法制取铝锶合金、铝钪合金等[12?13]。以Li2CO3为原料代替LiCl进行电解不仅减轻了阳极气体对环境的污染,更降低了能耗和生产成本。本文作者采用连续脉冲-计算机法研究在LiF-LiCl电解质体系中,以Li2CO3为原料,以石墨做阳极,铝液作阴极,熔盐电解法制取铝锂合金的工艺过程,同时着重对电解过程的影响因素如反电动势、电流效率和合金浓度等进行了深入探讨。
1 实验
1.1 实验装置
实验中所用电解槽结构如图1所示,为使侧壁绝缘,将底部开孔的刚玉内衬套在石墨坩埚中,以带有瓷保护套管的石墨做阳极,覆盖住底孔的铝液作为阴极,温度由DWT?702精密温度自动控温装置控制,精度在±0.5 ℃,控温范围在0~1 000 ℃。
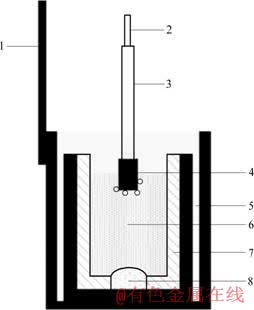
图1 电解槽简图
Fig.1 Configuration of electrolytic bath: 1— Iron outer with guide rod; 2—Anode guide rod; 3—Ceramic protection sleeve; 4—Graphite anode; 5—Graphite crucible; 6—Electrolyte; 7—Insulated sleeve; 8—Aluminum liquid
1.2 电解测试装置—熔盐电解监控仪
采用自行研制的熔盐电解监控仪测量电解过程中的反电动势,其原理与文献[14?15]相似,在连续脉冲示波器法的基础上,使计算机兼备了示波器的功能。通过计算机的测量能精确显示出熔盐电解过程中的温度、反电动势、槽电压、电流等主要参数,明确指示哪种化合物分解析出,特别适用于电解槽中合金的制备。
1.3 试剂
实验中所用氯化锂为北京化工厂生产的分析纯无水LiCl,LiF和Li2CO3均为上海试剂三厂生产的分析纯试剂,所有的药品均在300 ℃的恒温电阻箱中烘干2 h后封存待用。
2 结果与讨论
2.1 电解质的选择及温度的确定
要从电解质中电解析出锂与阴极铝液形成铝锂合金,所选支持电解质应有较高的分解电压。由于Li+的析出电位较负,很难找到更负的阳离子熔盐作为锂电解的支持电解质。同时,为了在电解过程中不引入其他杂质,故选用LiCl-LiF纯锂盐作为电解质,这样在电解过程中仅有Li+放电析出,保证了合金纯度。加入Li2CO3代替LiCl作为电解原料,电解生成CO2基本无污染且降低了槽电压,减少了能耗。由于该体系的氧化物溶解能力有限,所以模仿铝电解中氧化铝的勤加料、少加料原则控制Li2CO3的含量,在本研究中选用LiCl60%-LiF40%作为基础电解质体系。由于LiCl易挥发,电解期间要注意不断补充电解质。由图2所示的LiCl-LiF的二元相图可知,该电解质配比下的初晶温度在650 ℃左右,同时考虑到电解温度不可低于铝的熔点660℃,但是过高的温度又使得电解质挥发严重及电流效率降低,因此在本研究中将电解温度定为680 ℃。
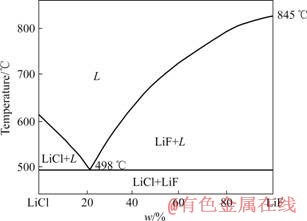
图2 LiCl-LiF熔盐体系相图
Fig.2 Phase diagram of LiCl-LiF system
2.2 电流密度对反电动势的影响
2.2.1 LiCl-LiF体系中的反电动势
所谓反“电动势”是指在电解过程中的实际分解电压,它由理论分解电压、阳极过电压和阴极过电压组成。
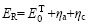
在680 ℃电解LiF-LiCl体系,改变电流密度,测得反电动势如图3中曲线A所示,反电动势随阳极电流密度增加而增加。当电流为零时,此时虽无电流通过,但阴阳极之间仍有一定电位差,约1.5~1.6 V之间,此时相当于电池向外反馈出的电动势,这种现象在测量铝电解中也曾发现[15],它反映了炭阳极上吸附氧时对铝的电位。在电解初期要预先电解15 min,使得吸附在炭电极上的氧气消耗并使炭电极完全湿润。
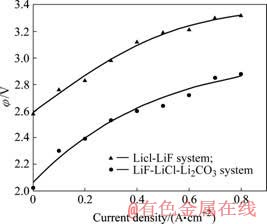
图3 电流密度与反电动势的关系
Fig.3 Relationships between current and back EMF (60%LiCl- 40%LiF, t = 680 ℃)
2.2.2 临界电流密度
持续加大电流密度,当阳极电流密度加大到1.0 A/cm2以上便发生类似于铝电解槽中的阳极效应,此时槽电压急剧升高,电流突然降到很小,无气泡析出,阳极周边出现弧光放电现象且槽内发出嗡嗡声响,因此本实验中电解的临界电流密度为1.0 A/cm2。临界电流密度大小主要与氯化物的浓度有关,随着LiCl电解的不断消耗和挥发使得体系中的氯化物的浓度降低,电解质液面不断降低,深入液面下的阳极面积减少导致阳极电流密度接近临界电流密度值。当体系中氯化物的浓度降低到一定程度时发生氟离子放电,此时电解无法正常进行。为了避免阳极效应的频繁发生,电解过程中需定期补充电解质尤其是LiCl,保证液面高度和极距大小。
2.2.3 LiF-LiCl-Li2CO3系中的反电动势
当以LiF-LiCl作为支持电解质并向体系添加Li2CO3时,电流密度对反电动势的影响如图3中曲线B所示。
当向LiF-LiCl体系中添加Li2CO3之后,在相同的电流密度下,反电动势降低了0.5 V左右。这说明此时在阳极上已不再是Cl?放电而是由新加入的Li2CO3带入的O2?放电了。由于O2?析出电位低于Cl?的析出电位,因此反电动势则随之降低。
2.3 电位控制法调节电解过程中Li2CO3的下料
在电解温度为680 ℃、阳极电流密度为0.4 A/cm2条件下,电解15 min稳定后,每次按比例加入2%的Li2CO3,测得时间与反电动势变化的加料周期曲线如图4所示。
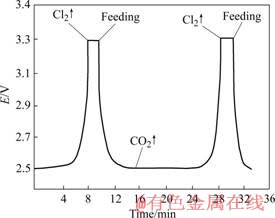
图4 加料周期曲线
Fig.4 Period curve of material feeding
为了保证Li2CO3正常电解,防止加入Li2CO3量过多溶解不了而使电解质发粘或电解质中Li2CO3的浓度不足而发生其它离子放电,因此,实验中采用了电位控制法监控反电动势的变化,根据勤加料、少加料的原则及时补充Li2CO3。当Li2CO3分解生成Li2O和CO2时,由于O2?的析出电位低于Cl?的析出电位,故在阳极上放出的是CO2。

此时反电动势下降到低位。随着Li2CO3被电解逐渐消耗,O2?的浓度不断降低,由于在恒电流条件下电解,则电极上反应速度是恒定的。当O2?减少到一定程度后,其扩散到电极表面的速度不足以供给电极反应时,则电位稍正的Cl?放电析出Cl2,反电动势随电极电位升高并逐渐回升至加料前的最高值,此时需补充加料Li2CO3。Cl2反电动势的规律变化表明了一个加料周期。在本实验条件下,加料周期约为15 min。
2.4 不同条件下电解铝锂合金
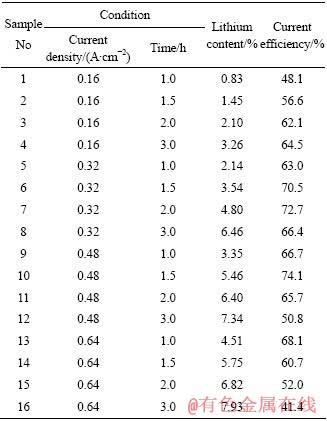
在680 ℃下,称取30 g的铝块作阴极,炭阳极下入电解质中1 cm深,使其面积达到12.5 cm2,分别在不同的电流和电解时间的条件下制取一定浓度的铝锂合金并且根据式(2)求出各自的电流效率,实验结果如表1所列。

表1 不同实验条件下电解铝锂合金的实验结果
Table1 Results of preparation of Al-Li alloy by electrolysis under different conditions
1) 电流效率的研究
从表1可知,在阳极电流密度为0.16 A/cm2时,电流效率随时间的延长而提高;在电流密度为0.48 A/cm2时,电解1.5 h后电流效率达到最大值74.1%,而后延长电解时间,电流效率有所降低。电解时间与电流效率的关系如图5所示。
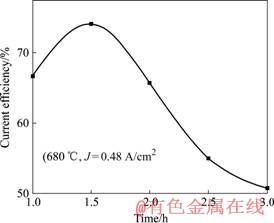
图5 电解时间与电流效率的关系
Fig.5 Relationship between electrolysis time and current efficiency
从数据的变化中可以看出电流效率、合金浓度和电解时间三方的关系。一定电流密度下,在电解的初期,电流效率有所增加,而随着电解时间的延长,合金中锂浓度越来越高,当合金浓度接近5.0%~5.4%时,电流效率随时间的延长而逐渐降低。此时,金属锂的析出速度大于锂向铝阴极扩散速度,金属锂便积累在铝液表面。由于铝液沉在电解槽底部,而金属锂的密度很轻,因此大量积累在铝液上表面的锂来不及扩散进铝液便上浮被氯气氧化或燃烧损耗,还有一部分又溶进电解质中,这些都会造成锂的损失导致电流效率降低。
从实验结果中还可以看出,在一定电解时间条件下,改变阳极电流密度也会影响电流效率的变化。电流密度与电流效率的关系如图6所示。
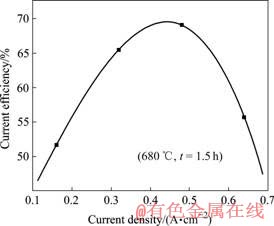
图6 电流密度与电流效率的关系
Fig.6 Relationship between current density and current efficiency
最初增加电流密度,反应速度加快,电流效率不断提高;达到一定程度以后继续加大电流密度,电流效率逐渐降低。因为此时随着阳极电流密度持续增加,阳极气体排放量增大,使电解质的循环搅动增强,由于此结构电解槽的阴阳两极没有被隔开,阴极电解析出的部分金属锂易被带到阳极区而氧化,导致二次反应的发生,从而引起电流效率的降低。
在提高电流效率问题上,依然有许多工作要做,如改进电解槽结构使阴阳两极分隔开来、加大极距、合金中锂含量不宜太高、避免发生阳极效应等方面。
2) 电解时间与合金浓度
由公式(2)和实验结果可以看出,提高电流密度、延长电解时间都可以增加合金中的锂含量。本实验中,锂的质量分数最高可达7.93%。
3 结论
1) 电解LiCl-LiF体系,反电动势随阳极电流密度增加而增大,临界电流密度经测量为1.0 A/cm2。加入2%的Li2CO3可使反电动势降低0.5 V左右,通过控制电位法测得加料周期为15 min。
2) 电流效率受电解时间和电流密度的影响最高可达74.1%而后逐渐降低。
3) 在680 ℃,电流密度为0.64 A/cm2的条件下,电解3 h可制取锂的质量含量高达7.93%铝锂合金。以Li2CO3为原料代替LiCl进行电解减轻了阳极气体对环境的污染,更降低了能耗和生产成本。因此,电解氧化物是今后电解制备铝基合金的发展方向。
REFERENCES
[1] 周昌荣, 潘青林, 朱朝明, 何运斌, 尹志民. 新型铝锂合金的研究和发展[J]. 材料导报, 2004, 18(5): 30?32.
ZHOU Chang-rong, PAN Qing-lin, ZHU Chao-ming, HE Yun-bin, YIN Zhi-min. Development and study of new types aluminum-lithium alloys[J]. Material Review, 2004, 18(5): 30?32.
[2] 黄兰萍, 郑子樵, 黄永平. 2197铝锂-合金的组织和性能[J]. 中国有色金属学报, 2004, 14(12): 2066?2072.
HUANG Lan-ping, DENG Zi-qiao, HUANG Yong-ping. Microstructure and properties of 2197 Al-Li alloy[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(12): 2066?2072.
[3] 尹登峰, 郑子樵. 铝锂合金研究开发的历史与现状[J]. 材料导报, 2003, 17(2): 18?20.
YIN Deng-feng, ZHENG Zi-qiao. History and current status of aluminum-lithium alloys research and development[J]. Material Review, 2003, 17(2): 18?20.
[4] FRIDLYANDER I N. Current-technology aluminum alloys for aerospace applications[J]. Metal Science and Heat Treatment, 2001, 7(7): 7?8.
[5] GUPTA R K, NIRAJ N, NAGASIREESHA G, SHARMA S C. Development and characterization of Al-Li alloys[J]. Mater Sci & Eng A, 2006, 420(2): 228?234.
[6] 徐君莉, 石忠宁, 邱竹贤. 熔盐电解法制取Al-Li母合金[J]. 矿冶工程, 2004, 24(3): 56?57.
XU Jun-li, SHI Zhong-ning, QIU Zhu-xian. Preparation of Al-Li master alloy by molten salt electrolysis[J]. Mining and Metallurgical Engineering, 2004, 24(3): 56?57.
[7] 戴兴福, 张明杰, 谭亚菊. 熔盐电解法制取高浓度的铝锂合金[J]. 轻金属, 1998, 12(1): 38?40.
DAI Xing-fu, ZHANG Ming-jie, TAN Ya-ju. Preparation of Al-Li alloy with high concentration by molten salt electrolysis[J]. Light Metals, 1998, 12(1): 38?40.
[8] SATO Y, SAITO S, ARAIKE E, SUZUKI T, YAMAMURA T. Electrode behavior for the electrolytic production of Al-Li alloy by using molten chlorides[J]. Journal of Japan Institute of Light Metals, 1993, 43(1): 33?39.
[9] SATO Y, KARIYA H, SUZUKI T, SAITO S, YAMAMURA T. High efficient electrolytic production of Al-Li alloy by using Li2CO3 as a source of lithium[J]. Journal of Japan Institute of Light Metals, 1998, 48(3): 138?142.
[10] 张明杰, 李继东, 王耀武. Li2CO3在KCl-LiCl熔体中的电化学行为[J]. 东北大学学报: 自然科学版, 2005, 26(6): 570?573.
ZHANG Ming-jie, LI Ji-dong, WANG Yao-wu. Electrochemical behavior of lithium carbonate in KCl-LiCl melt[J]. Journal of Northeast University: Natural Science, 2005, 26(6): 570?573.
[11] COX A, MORRIS J W. Improving energy efficiency of electrowinning of lithium[J]. Light Metal, 1998, 11(6): 1295?1298.
[12] YANG Shao-hua, GAO Bing-liang, WANG Zhao-wen, SHI Zhong-ning, BAN Yun-gang, KAN Hong-min, CAO Xiao-zhou, QIU Zhu-xian. Preparation of Al-Sc alloys by molten salts electrolysis[C]// Powell A. Innovation in Eleotrometallurgy. Orland: TMS, 2007: 1?4.
[13] 李继东, 张明杰, 张廷安, 杨少华. 熔盐电解法生产Al-Sr合金中反电动势和电流效率的研究[J]. 稀有金属, 2007, 31(4): 577?580.
LI Ji-dong, ZHANG Ming-jie, ZHANG Ting-an, YANG Shao-hua. Research on the EMF and current efficiency in the process of Al-Sr alloy preparation by molten salt electrolysis[J]. Chinese Journal of Rare Metals, 2007, 31(4): 577?580.
[14] 张明杰, 王兆文. 熔盐电化学原理与应用[M]. 北京: 化学工业出版社, 2006: 162?163.
ZHANG Ming-jie, WANG Zhao-wen. Principle and application of electrochemistry of fused salts[M]. Beijing: Chemical Industry Press, 2006: 162?163.
[15] QIU Z X, ZHANG M J. Studies on anode effect in molten salts electrolysis[J]. Electrochemical Acta, 1987, 16(4): 607?609.
基金项目:国家自然科学基金重点资助项目(50574024)
收稿日期:2007-11-30;修订日期:2008-04-15
通讯作者:李继东,博士研究生;电话:13840413898;E-mail: lijidong1980@tom.com
(编辑 何学锋)