
Thermodynamic properties of stable and metastable phases of Pt metal
PENG Hong-jian(彭红建)1, 2, XIE You-qing(谢佑卿)2, NIE Yao-zhuang(聂耀庄)2
1. School of Chemistry and Chemical Engineering, Central South University,
Changsha 410083, China;
2. School of Materials Science and Engineering, Central South University,
Changsha 410083, China
Received 7 March 2008; accepted 29 May 2008
Abstract: Isometric heat capacity cv and isobaric heat capacity cp of Pt with stable and metastable phases were calculated by using pure element systematic theory. These results are in excellent agreement with of SGTE (Scientific Group Thermodata Europe) database and JANAF (Joint Army-Navy-Air Force) experimental values. The calculation results of cv and cp of Pt metal in natural state are in good agreement with those calculated by FP(first-principles) method. It is found that the electron devotion to heat capacity is important to adjust in OA(one-atom) method while calculating heat capacity. The full information about thermodynamic properties of Pt metal with stable and metastable phases, such as entropy(S), enthalpy(H) and Gibbs energy(G) were calculated from 0 K to random temperature. The results are in good agreement with JANAF experimental value. In contrast to SGTE database, the thermodynamic properties from 0 K to 298.15 K are implemented.
Key words: Pt; heat capacity; thermodynamic property
1 Introduction
There are many methods[1-5], such as experimental evaluations and theoretic calculations, to get thermo- dynamic data of pure metals. In these methods, SGTE (Scientific Group Thermodata Europe) pure element database[6] contains thermodynamic data for around 78 elements in the stable and all metastable phases over 298.15 K. With 30 years of work, Prof. XIE has finally established the framework of systematic sciences of alloys(SSA) which is constituted by pure element systematic theory, physics and chemistry, and statistics thermodynamics of alloys. The core of systematic science of alloys is the one-atom(OA) theory of pure elements[7-12]. In order to keep the integrity of thermodynamic properties of SGTE pure element database, in this work, pure element systematic theory is adopted to calculate isometric heat capacity cv, isobaric heat capacity cp and thermodynamic properties of Pt metal and the results are compared with those of SGTE
database, JANAF(Joint Army-Navy-Air Force) and FP (first-principles) method.
2 Principles and methods
Generally, there is a relationship between isobaric heat capacity cp and isometric heat capacity cv:
cp=cv+TBVβ′2 (1)
where T is temperature; V is molar volume; B is isothermal bulk modulus; and β′ is the coefficient of volume thermal expansion. According to Debye- Grüneisen model[13], β′=3α.
 (2)
(2)

(3)
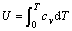 (4)
(4)
The relationship between constant K, Q and microscopic quantity has been conducted by MAI potential-energy function[8]:
 (5)
(5)
 (6)
(6)

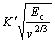 (7)
(7)
Once heat capacity is known, other thermodynamic properties, such as enthalpy(H), entropy(S) and Gibbs energy(G) can be calculated by Eq.(8):
 (8)
(8)
where U is the internal energy; j is the multiple of the half cutoff wavelength; θD is Debye temperature; R is gas constant; kB is Boltzman’s constant; r0 is average effective bond length of the crystal in equilibrium; m is atomic mass; n is the exponent of the MAI potential- energy function; Ec is cohesive energy; v is atomic volume; and h is Planck’s constant, respectively.
3 Temperature dependence of isometric heat capacity and isobar heat capacity
In order to keep the integrity of thermodynamic property for SGTE pure element database, isometric heat capacity cv and isobaric heat capacity cp of Pt metal with FCC, HCP, BCC structures and in liquid state were calculated by pure element systematic theory. Calculation results are shown in Fig.1. The results of SGTE database and experimental value of JANAF are provided for comparison. Calculation results by FP method[14] are shown in Fig.2.
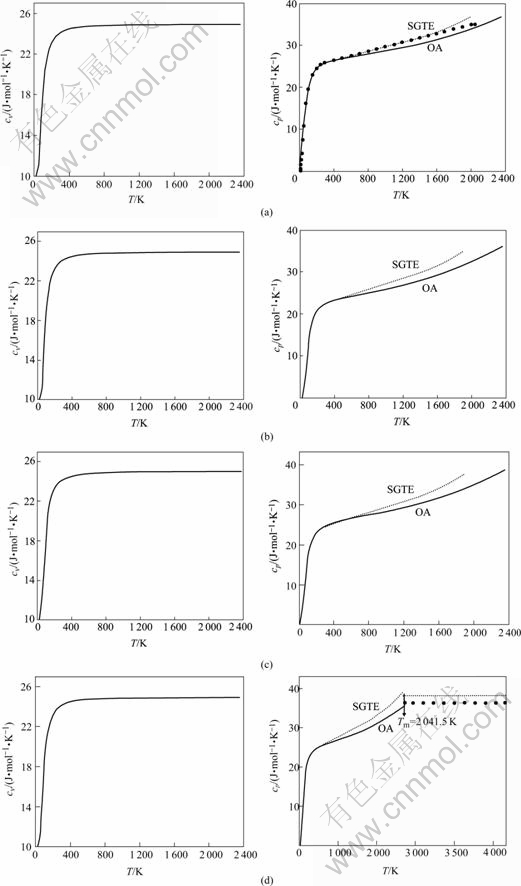
Fig.1 Curves of cv—T and cp—T of Pt metal with FCC (a), HCP (b), BCC (c) structures and in liquid state (d) calculated by OA method (●—Experimental value[15])

Fig.2 Curves of cv—T and cp—T of Pt metal in natural state calculated by FP method (●—Experimental value[15])
From Figs.1 and 2, isometric heat capacity cv in natural state calculated by OA method is in good agreement with calculation results by FP method[15]. Isobaric heat capacities cp in natural state and liquid state are in good agreement with JANAF experimental values, results of SGTE database, and calculation results by FP method. At the same time, it reinforces the results from 0 to 298.15 K for SGTE database. Even though FP method cannot calculate heat capacity of metastable phases, it can show contributions to heat capacity cv(e) made by electrons. This indicates that OA method needs to be revised while calculating heat capacity. Contributions to heat capacity cv(e) made by electrons cannot be neglected.
4 Temperature dependence of entropy, enthalpy and Gibbs energy
When the thermodynamic property of FCC-Pt at 0 K is taken as referred standard, that is, Sf=0, Hf=564 kJ/mol[16], temperature dependence of the thermo- dynamic properties of Pt metal with FCC, HCP, BCC structures and in liquid state are shown in Figs.3 and 4 accordingly. From these figures, compared with SGTE database, it reinforces thermodynamic properties from 0 to 298.15 K at low temperatures, making the information of pure metal more integral. At the same time, the thermodynamic properties of metastable phases can be calculated and the values of its own melting point can be obtained, respectively.

Fig.3 Curves of S—T and H—T of Pt metal with FCC (a), HCP (b), BCC (c) structures and in liquid state (●—Experimental value[15])
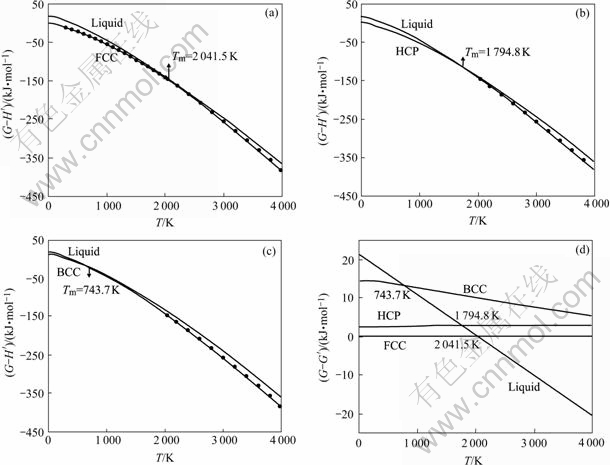
Fig.4 Curves of G—T and total Gibbs energy (d) of Pt metal with FCC (a), HCP (b), BCC (c) structures and in liquid state (●—Experimental value[15])
5 Conclusions
1) Isometric heat capacity cv and isobaric heat capacity cp of Pt metal with stable and metastable phases were calculated by using pure element systematic theory. The results are in good agreement with those calculated by FP method and JANAF experimental values.
2) Specific heat calculated by OA method needs to be revised. Contributions to specific heat made by electrons can not be neglected.
3) The complete information about thermodynamic properties of Pt metal, such as entropy S, enthalpy H and Gibbs energy(G) from 0 K to any temperature can be calculated and the results are in good agreement with JANAF experimental values. Compared with SGTE database, it reinforces thermodynamic properties from 0 K to 298.15 K.
References
[1] SAUNDERS N, MIODOWIK A P, DINSDALE A T. Metastable lattice stabilities for the elements [J]. Calphad-Computer Coupling of Phase Diagrams and Thermochemistry, 1988, 12: 351-374.
[2] WANG Y, AURTAROLO S, JIANG C, ARROYAVE R, WANG T, CEDER G, CHEN L Q, LIU Z K. Ab initio lattice stablity in comparison with CALPHAD lattice stability [J]. CALPHAD, 2004, 28(6): 79-90.
[3] ZHANG Z J. Calculation of the properties of some metals and alloys [J]. Journal of Physics: Condens Matter, 1998, 10(4): 495-499.
[4] LI Xiao-bo, XIE You-qing, PENG Hong-jian, TAO Hui-jing. Low temperature thermodynamic study of the stable and metastable phase of silver [J]. Acta Metallurgica Sinica Letters, 2007, 20(5): 355-362.
[5] LI Xiao-bo, XIE You-qing, NIE Yao-zhuang, PENG Hong-jian. Low temperature thermodynamic study of the stable and metastable phase of vanadium [J]. Chinese Science Bulletin, 2007, 52(22): 3041-3046.
[6] DINSDALE A T. SGTE data for pure elements [J]. CALPHAD, 1991, 15(4): 317-425.
[7] XIE You-qing. The framework of metallic materials systematic science [J]. Mater Rev, 2001, 15(4): 12-15. (in Chinese)
[8] XIE You-qing, MA Liu-ying. The theoretical lattice parameter of valence electron structure of crystal [J]. J Cent South Inst of Mining and Metall, 1985, 8(1): 1-10. (in Chinese)
[9] XIE You-qing. A new potential function with many-atom interactions in solid [J]. Science in China (Series A), 1993, 36(1): 90-99.
[10] XIE You-qing. One atom self-consistency method for determining electronic structure of crystal [J]. Chinese Science Bulletin, 1992, 37(16): 1529-1532.
[11] TAO Hui-jing, XIE You-qing, PENG Hong-jian, YU Fang-xin, LIU Rui-feng, LI Xiao-bo. Temperature dependences of atom states and physical properties of fcc, metastable hcp- and bcc-Cu [J]. Acta Metallurgia Sinica, 2006, 42(6): 565-571.
[12] TAO Hui-jing, XIE You-qing, PENG Hong-jian, YU Fang-xin, LIU Rui-feng, LI Xiao-bo, NIE Yao-zhuang. Thermodynamic property of fcc-Au and liquid-Au [J]. J Cent South Univ: Science and Technology, 2006, 37(16): 1036-1042.
[13] TAO Hui-jing, XIE You-qing, PENG Hong-jian, YU Fang-xin, LIU Rui-feng, LI Xiao-bo, NIE Yao-zhuang. Thermodynamic property of pure elemental Cu [J]. The Chinese Journal of Nonferrous Metals, 2006, 16(7): 1207-1213. (in Chinese)
[14] NIE Yao-zhuang, XIE You-qing. Ab initio thermodynamics of the hcp metal Mg, Ti, and Zr [J]. Physical Review, 2007, B75: 174117- 174124.
[15] CHASE M W. NIST-JANAF thermochemical tables (Part I) [M]. 4th ed. Gaithersburg: National Institute of Standards and Technology, 1998: 1125-1136.
[16] American Institute of Physics. American Institute of Physics Handbook [M]. 3rd ed. New York: McGraw-Hill Book Company, 1972.
Foundation item: Project(50471058) supported by the National Natural Science Foundation of China; Project(08JJ3099) supported by the Natural Science Foundation of Hunan Province, China; Project supported by the State Key Laboratory of Powder Metallurgy, China
Corresponding author: PENG Hong-jian; Tel: +86-731-8879287; E-mail: phj108@163.com
DOI: 10.1016/S1003-6326(08)60290-3
(Edited by YANG Bing)