文章编号:1004-0609(2013)04-1145-07
镁熔体在HFC-32/CO2气氛中的高温氧化行为
曾一文1, 2,吴国华2, 3
(1. 贺州学院 化学与生物工程学院,贺州 542899;
2. 上海交通大学 轻合金精密成型国家工程研究中心,上海 200240;
3. 上海交通大学 金属基复合材料国家重点实验室,上海 200240)
摘 要:用热重法测定镁熔体在HFC-32(二氟甲烷)/CO2气氛中的氧化动力学曲线,用XPS和XRD表征表面氧化膜中氧化产物的组成,讨论镁熔体在HFC-32/CO2气氛中的氧化机理。结果表明:镁熔体在HFC-32/CO2气氛中的氧化行为遵循抛物线规律,氧化膜的组成与HFC-32的浓度和温度有关;随着HFC-32浓度的增大,氧化膜中MgO的含量减小,MgF2的含量增大,氧化速率减小;随着温度的升高,氧化膜中MgO的含量增大,MgF2的含量减小,氧化速率增大。
关键词:镁熔体;氧化动力学;氧化机理;CH2F2(HFC-32)
中图分类号:TG146.2 文献标志码:A
Oxidation behavior of molten magnesium in atmospheres of
HFC-32/CO2 at high temperature
ZENG Yi-wen1, 2, WU Guo-hua2, 3
(1. School of Chemical and Biological Engineering, Hezhou University, Hezhou 542899, China;
2. National Engineering Research Center of Light Alloy Net Forming, Shanghai Jiao Tong University,
Shanghai 200240, China;
3. Key State Laboratory of Metal Matrix Composite, Shanghai Jiao Tong University, Shanghai 200240, China)
Abstract: The oxidation kinetic curves of molten magnesium in atmospheres of HFC-32 (methylene fluoride)/CO2 were measured by the weight gain method. The oxidation products contents of oxidation surface film were characterized by XPS and XRD. The oxidation mechanism of the magnesium in the mixed gas of HFC-32/CO2 was discussed. The results show that the oxidation behavior follows parabolic law and the film compositions change with the concentration of HFC-32 and the oxidation temperature. With the increase of the HFC-32 concentration in the mixed gas, the content of MgO in the oxide film decreases, the content of MgF2 increases, and the oxidation rate decreases. As the temperature increases, the content of MgO in the oxide film increases, the content of MgF2 decreases, and the oxidation rate increases.
Key words: molten magnesium; oxidation kinetics; oxidation mechanism; CH2F2(HFC-32)
基金项目:国家自然科学基金资助项目(51275295);广西高等学校优秀人才资助计划项目(RC2007035);贺州学院科研项目(2007KJ03);上海航天联合研究中心资助项目(USCAST2012-15)
收稿日期:2012-06-20;修订日期:2012-11-26
通信作者:吴国华,教授,博士;电话:021-54742630;传真:021-54742794;E-mail: ghwu@sjtu.edu.cn
镁的化学性质活泼,镁与氧之间的亲和力较高,易氧化,特别是在高温时,镁的氧化速度呈线性甚至指数增大,导致镁在熔炼过程中极易发生剧烈氧化燃烧现象。因此,镁熔炼过程通常采用气体保护法防止镁的恶性氧化。而具有良好保护效果的SF6 气体的温室效应是CO2 的23 900倍,需要寻找温室效应低的气体替代SF6,降低镁熔炼过程的环境负荷[1]。许多学者开展了这方面的研究[2]。陈虎魁等[3-7]研究了镁合金在HFC-134a(1,1,1,2-四氟乙烷,化学式为CF3CH2F)气氛中的氧化行为及机理。认为镁熔体与HFC-134a在高温时分解产生的F反应生成MgF2,使表面氧化膜更致密,镁合金在HFC-134a气氛中的氧化增量曲线为抛物线。聂书红等[8-9]证明在密封炉中,低浓度的HFC-134a气体对镁合金有很好的保护作用。XIONG等[10-11]和WANG等[12]研究了HFC-134a和SO2气体对镁合金的保护机理,认为镁熔体与保护气体反应生成致密度高的物质,阻止了氧通过表面膜与镁熔体继续反应,减缓了镁熔体的氧化程度。张心灵等[13-15]研究了HFC125/N2及其与SO2混合对镁合金的保护行为,得出低浓度的HFC125与SO2混合对镁熔体有较好的保护效果。HFC-134a和HFC125气体的温室效应分别是CO2 的1 340和2 800倍,而HFC-32(CH2F2)的温室效应是CO2 的650倍,约为HFC-134a的1/2,它具有与HFC-134a、HFC125相似的分子结构和性 质,对镁合金熔体也有较好的保护作用[16-17]。使用HFC-32气体保护镁合金,能更有效降低熔炼过程产生的温室效应。本文作者对镁熔体在HFC-32/CO2气氛中的氧化动力学进行研究,并通过XPS和XRD等方法对不同条件下形成的氧化膜成分进行表征,分析了HFC-32的浓度和熔炼温度对镁熔体氧化的影响,探 讨HFC-32/CO2气氛对镁熔体的保护机制。
1 实验
实验所用主要原料为商品纯镁,其化学组成(质量分数,%)如下:Al 0.002 9,Zn 0.01,Mn 0.002 5,Ni 0.000 1,Fe 0.000 8,Cu 0.000 4,Sn和Pb小于0.000 1,Mg余量。将纯镁切成28 mm×5 mm的圆饼状试样,并经320号砂纸研磨和乙醇清洗。实验所用的HFC-32气体由上海奥宏化学品有限公司提供,主要成分(体积分数)如下:HFC-32含量大于99.9%,HCI含量小于0.000 1%,H2O含量小于0.001%。电子分析天平(FA2004B)由上海平轩科学仪器有限公司提供。
采用热重法测定镁熔体在不同浓度HFC-32/CO2气氛及不同熔炼温度时的氧化动力学曲线。装置如图1所示。
电阻炉加热到设定温度后,以0.5 L/min的流量连续通入一定浓度的HFC-32与CO2混合气体约60 min后,将纯镁试样放入悬挂在天平下面的氧化镁坩埚中,并置于电阻炉内,当试样达到设定温度后,开始记录试样质量的变化,每10 min记录一次,直到实验完毕。
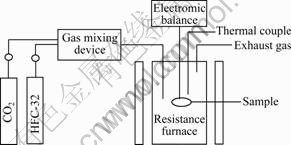
图1 氧化动力学实验装置图
Fig. 1 Schematic diagram of oxidation kinetics experiment
氧化实验后试样表面氧化产物和元素组成分别用X射线光电子能谱仪、X射线衍射仪和能谱仪进行分析。
2 结果与讨论
2.1 氧化动力学分析
不同温度下镁熔体在含1% HFC-32时的氧化增量曲线以及在700 ℃不同浓度(体积分数,下同)的HFC-32与CO2混合气体中的氧化增量曲线如图2所示。从图2(a)可以发现,随着温度升高镁熔体的氧化速率增大,镁熔体在l% HFC-32和CO2混合气体保护下,660~800 ℃温度范围内的氧化行为遵循抛物线规律。由图2(b)可以看出,随着混合气体中HFC-32浓度的增加,镁熔体的氧化速率逐渐减小,并且表现出不同的动力学规律。在含0.01% HFC-32与CO2的混合气体中,镁熔体的氧化增量最大,随时间呈线性增长。当混合气体中的HFC-32浓度增加到0.1%时,镁熔体的氧化增量减小,增量曲线随时间呈抛物线增长。当混合气体中的HFC-32浓度继续增加,氧化增量曲线均呈现抛物线规律变化,但增量随HFC-32浓度的增加而减小。
图2所示为不同温度下镁熔体在l% HFC-32中的氧化动力学曲线及700 ℃、不同浓度HFC-32的混合气体保护下氧化动力学曲线的拟合方程分别如表l和表2所示(Δm是单位面积试样质量的增加,单位是mg/cm2)。
镁熔体的氧化速率与温度的关系符合Arrhenius方程式[18],即
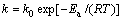 (1)
(1)
两边取对数得:
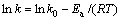 (2)
(2)
式中:k为氧化速率常数,mg·cm2/min,k0为频率因子;R为摩尔气体常数,R=8.314 J/(mol·K);Ea为反应的活
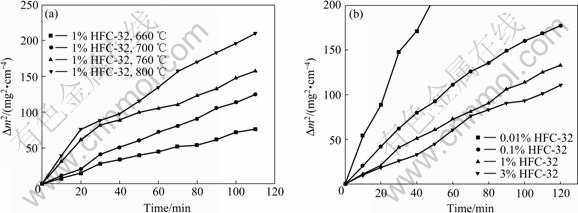
图2 不同条件下镁熔体的氧化增量曲线
Fig. 2 Curves of oxidation mass gain for molten magnesium under different conditions: (a) l% HFC-32 at different temperatures; (b) 700 ℃ at different HFC-32 concentrations (volume fraction)
表1 镁熔体在1%HFC-32/CO2气氛中不同温度时的氧化动力学曲线的拟合方程
Table 1 Fitting equations of oxidation kinetic curve from molten magnesium at different temperatures in 1% HFC-32/CO2 atmospheres

表2 镁熔体在700 ℃、不同浓度HFC-32/CO2气氛中的氧化动力学曲线的拟合方程
Table 2 Fitting equations of oxidation kinetics curve from magnesium at 700 ℃ in different concentrations HFC-32/CO2 atmospheres

化能,J;T为热力学温度,K。将表1所得不同温度下的速率常数代入式(2),并进行线性回归,得到镁在1%HFC-32与CO2的混合气体保护下发生反应的活化能为85.84 kJ/mol,速率常数与温度的关系如下:
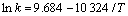 (3)
(3)
2.2 氧化产物分析
采用JSM7600F能谱仪对镁在700 ℃、不同HFC-32浓度及不同温度时表面所形成氧化膜进行了分析。其元素组成及含量(摩尔分数,%)分别如表3和表4所列。由表3可知,在700 ℃不同浓度的HFC-32/CO2气氛中,表面膜均由Mg、F、O和C四种元素组成,随着HFC-32含量的增大,O元素的含量逐渐减小,F元素的含量逐渐增大,即随着HFC-32含量的增大,更多的F将与Mg反应,使表面膜中MgF2的含量增大,表面膜的致密度增大,氧化速率减小。如表4所示,在不同温度下形成的表面膜的组成相同,随着温度的升高,Mg和O的含量升高,F的含量降低,说明氧化膜中氧化镁的含量增大,MgF2的含量减小,表面膜的致密度降低,氧化速率增大。
表3 镁熔体在700 ℃不同HFC-32浓度时所形成的氧化膜的元素组成及含量
Table 3 Element composition and content of surface films formed on molten magnesium at 700 ℃ and different concentrations of HFC-32

表4 镁熔体在1%HFC-32、不同温度时所形成氧化膜的元素组成及含量
Table 4 Element composition and content of surface films formed on molten magnesium at different temperatures in 1%HFC-32 atmosphere

为了说明氧化膜中元素的结合方式,对1%HFC-32在700 ℃时形成的表面膜进行了XPS(AXIS UltraDLD, 日本岛津-Kratos 公司生产)和XRD(D/Max-2550 PC,日本 RIGAKU生产)分析,结果如图3所示。XPS分析得出氧化膜中含有Mg、F、O和C这4种元素,分别组成氧化镁和氟化镁及单质碳;XRD分析得出样品中含有MgF2、MgO、Mg和C这4相。综合以上分析结果可以看出,氧化膜主要由MgO、MgF2和单质C组成,但是这3种物质在氧化膜中的相对含量则随着混合气体中HFC-32的浓度和温度的变化而发生变化。

图3 700 ℃时镁熔体在1%HFC-32/CO2气氛中形成的氧化膜的XPS和XRD曲线
Fig. 3 XPS(a) and XRD(b) patterns of surface film formed on molten magnesium at 700 ℃ in 1%HFC-32/CO2 atmosphere
2.3 氧化过程的热力学分析
当镁熔体暴露在HFC-32/CO2气氛中时,镁熔体与气氛中的CO2以及HFC-32可能发生如下反应[19]:
Mg(l)+1/2CO2(g)=MgO(s)+C(s) (4)
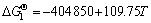 (4′)
(4′)
CH2F2(g)+1/2CO2(g)+Mg(l)=MgF2(s)+H2O(g)+3/2C(s) (5)
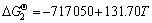 (5′)
(5′)
根据Van’tHoff等温方程式[18]即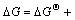
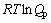 ,其中,Qp为化学反应的反应熵。对于式(4′)和(5′)可得
,其中,Qp为化学反应的反应熵。对于式(4′)和(5′)可得
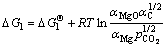 (6)
(6)
 (7)
(7)
由于Mg、MgF2、MgO和C均为纯物质,活度均为1,R=8.314 J/(mol·K),所以
 (8)
(8)
由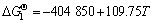 可得
可得
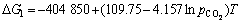 (8′)
(8′)
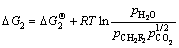 (9)
(9)
由 可得
可得
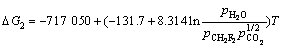 (9′)
(9′)
熔炼在常压下进行,炉内气压与大气压相等,即总压为101 kPa。在1%HFC-32/CO2的混合气体中,CO2的分压为99.99 kPa,HFC-32的分压为1.01 kPa,水的分压用该温度时水的饱和蒸汽压代替,可根据Antoine方程lg p=a–b/(t+c)[20]计算,其中,a=17.652 16,b=5 204.086,c=32.5。
反应(4)和(5)在熔炼条件下的吉布斯自由能如表5所示。
表5 反应(4)和(5)在不同温度下不同浓度HFC-32的吉布斯自由能
Table 5 Gibbs free energies of reactions (4) and (5) at different temperatures with different concentrations of HFC-32
和(5)在不同温度下不同浓度.jpg)
由表5可知,在上述条件下反应(4)和(5)的Gibbs自由能均为负值,所以从热力学方面考虑,在熔炼条件下,反应(4)和(5)均可发生。反应(5)的Gibbs自由能比反应(4)的Gibbs自由能更负,表明反应(5)比反应(4)更易进行,也就是说氧化膜中MgF2的含量应高于MgO的含量。这一热力学分析的结果与镁在HFC-32含量较高的混合气体中氧化膜的分析实验结果一致。
2.4 氧化机理分析
镁熔体氧化后生成的 MgO的致密度系数(Pilling-Bedworth ration, PBR) 小于1,而且随着温度升高,PBR值呈递减趋势,表明氧化膜在生长过程中,内部存在较大的拉应力,当温度升高时,拉应力会不断增大,最终将导致膜破裂,失去保护作用。MgF2的PBR值为1.32>1[21],MgF2的存在能增大表面膜的面积,减小拉应力,使表面膜致密,减小镁的氧化速率。
基于以上热力学分析以及氧化膜成分分析结果,镁熔体在HFC-32与CO2混合气体中的高温氧化过程可以推测如下:当HFC-32浓度较低时,混合气体中含有较多的CO2,镁熔体首先与CO2发生反应,并在其表面生成一层含C的MgO。MgO的 PBR值<1,内部存在较大的拉应力,结构比较疏松,由于CO2本身并不能阻止镁熔体在高温时的氧化燃烧,说明氧化膜的C不能有效阻止镁熔体和CO2的扩散,导致镁熔体和CO2可以通过孔隙直接迁移传质,氧化反应将持续快速进行。当HFC-32浓度较高时,从热力学角度考虑,HFC-32与镁熔体的反应趋势大于CO2与镁熔体的反应趋势,镁表面应首先形成MgF2,但是由于HFC-32与高温镁熔体的反应需要复杂的分解过程,因此,MgO首先在镁表面形成。MgO形成以后,镁表面CO2含量降低,而HFC-32含量相对升高,HFC-32与镁熔体发生反应的速度加快,导致HFC-32与穿过多孔性MgO膜向外扩散的镁蒸气发生反应,在镁表面产生MgF2,此时,在镁表面形成了由MgO、C和MgF2所组成的复合膜。当复合膜完全覆盖镁表面以后,氧化主要通过反应物离子穿过氧化膜的扩散进行。由于Mg2+穿过氧化膜向外扩散的速率远大于O2-和 F-向内扩散的速率,故氧化膜的生长主要通过扩散出来的Mg2+在氧化膜表面与O2-和F-反应这种外氧化的方式进行。由于MgF2致密度系数较大,而且生成的复合膜中MgF2的含量高于MgO的含量,加上C有一定的致密作用[21],所以复合膜具有较高的致密度,该致密的复合膜降低了Mg2+的扩散速率,从而使得镁熔体的氧化速度减小。
当混合气体中HFC-32浓度较低时,氧化膜主要由MgO组成,MgF2很少,复合膜结构比较疏散,此时,氧化过程由化学反应控制,符合线性规律。化学反应速率方程如下:
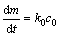 (10)
(10)
将式(10)积分后可得:
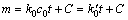 (11)
(11)
式中:m表示反应中镁熔体氧化的质量增量;c0是混合气体中CO2的浓度;k0是速率常数。积分常数C为直线的截距,当t=0时,试样表面已有氧化膜的增量。式(11)表明试样的氧化增量与时间呈线性关系,说明镁熔体在HFC-32浓度较低的混合气体中氧化时,氧化增量随时间呈直线增长。
当混合气体中HFC-32浓度较高时,在镁熔体表面形成了一层主要由MgF2组成的致密氧化膜,反应物通过该氧化膜的扩散成为氧化过程的控制步骤。根据Fick扩散定律[7],镁熔体在氧化膜中的扩散通量(J)为
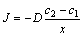 (12)
(12)
式中:D是Mg2+在氧化膜中的扩散系数;c2和c1分别表示Mg2+在混合气体/氧化膜和氧化膜/金属界面上的浓度;x表示氧化膜厚度。假设在混合气体/氧化膜和氧化膜/金属界面上达到热力学平衡以及整个氧化层中达到热力学平衡,此时c2、c1应是常数。由扩散过程控制氧化速率,故
 (13)
(13)
对式(13)进行积分,得到
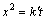 (14)
(14)
式中: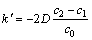 。式(12)表明,在HFC-32浓度
。式(12)表明,在HFC-32浓度
较高的混合气体中,镁试样单位表面积的氧化增量与时间呈抛物线规律。这一结论与动力学实验结果一致。
3 结论
1) 镁熔体在不同浓度的HFC-32混合气体中熔炼,遵循不同的氧化动力学规律。在较低HFC-32浓度的混合气体中,氧化行为遵循线性规律;在较高HFC-32浓度的混合气体中,氧化行为遵循抛物线规律。
2) 镁熔体在HFC-32与CO2混合气体中的氧化速度随着混合气体中HFC-32浓度的增加和熔炼温度的降低而减小。
3) 氧化膜主要由MgF2、MgO和C组成,这3种物质在氧化膜中的相对含量则随着混合气体中HFC-32的浓度和温度的变化而变化。
REFERENCES
[1] JOHN F, SWINTON K. Environmental challenges facing the magnesium industry-SF6 replacement[C]// Proceedings of the 60th Annual World Magnesium Conference. Germany: International Magnesium Association, 2003. 10-16.
[2] 庞 松, 吴国华, 孙 明, 戴吉春, 张 扬, 丁文江. 镁合金熔炼保护气体研究现状与展望[J]. 铸造, 2011, 60(3): 259-264.
PANG Song, WU Guo-hua, SUN Ming, DAI Ji-chun, ZHANG Yang, DING Wen-jiang. Present status and prospect on protective gas used in melting of magnesium alloy[J]. Foundry, 2011, 60(3): 259-264.
[3] 陈虎魁, 刘建睿, 黄卫东. 稀释气体对镁熔体在HFC-134a气氛中高温氧化行为的影响[J]. 稀有金属材料与工程, 2008, 37(11): 1913-19187.
CHEN Hu-kui, LIU Jian-rui, HUANG Wei-dong. Influence of diluent gas on the oxidation behavior of molten magnesium in atmospheres containing HFC-134a[J]. Rare Metal Materials and Engineering, 2008, 37(11): 1913-1918.
[4] CHEN Hu-kui, LIU Jian-rui, HUANG Wei-dong. The protective surface film formed on molten ZK60 magnesium alloy in 1,1,1,2-tetrafluoroethane/air atmospheres[J]. Corrosion Science, 2010, 52: 3639-3645.
[5] CHEN Hu-kui. Effect of melt temperature on the oxidation behavior of AZ91D magnesium alloy in 1,1,1,2-tetrafluoroethane air atmospheres[J]. Materials Characterization, 2010, 61: 894-898.
[6] CHEN Hu-kui, LIU Jian-rui, HUANG Wei-dong. Characterization of the protective surface films formed on molten magnesium in air HFC-134a atmospheres[J]. Materials Characterization, 2007, 58: 51-58.
[7] 陈虎魁, 刘建睿, 黄卫东. 镁熔体在空气/HFC-134a气氛中的高温氧化特性及机理分析[J]. 金属学报, 2007, 43(6): 625-630.
CHEN Hu-kui, LIU Jian-rui, HUANG Wei-dong. High temperature oxidation characteristics and mechanism of molten magnesium in the atmosphere of air containing HFC-134a[J]. Acta Metallurgica Sinica, 2007, 43(6): 625-630.
[8] 聂书红, 熊守美. 密封熔炼炉中空气分压对镁合金保护效果的影响[J]. 材料保护, 2007, 40(3): 14-17.
NIE Shu-hong, XIONG Shou-mei. Effect of partial pressure of air in HFC134a/N2/air atmosphere on protection of magnesium alloy in a sealed smelting furnace[J]. Materials Protection, 2007, 40(3): 14-17.
[9] 聂书红, 熊守美. 在密封熔炼炉含0.01%HFC134a的氮气中熔炼AZ91D镁合金[J]. 中国有色金属学报, 2006, 16(3): 436-441.
NIE Shu-hong, XIONG Shou-mei. Melting AZ91D magnesium alloy in sealed furnace with protective atmosphere of N2 containing 0.01%HFC134a[J]. The Chinese Journal of Nonferrous Metals, 2006, 16(3): 436-441.
[10] XIONG Shou-mei, WANG Xian-fei. Protection behavior of fluorine-containing cover gases on molten magnesium alloys[J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 1228-1234.
[11] XIONG Shou-mei, WANG Xian-fei. Protection behavior of fluorine-containing cover gases on molten magnesium alloys[J]. Transactions of Nonferrous Metals Society of China, 2010, 20(7): 1228-1234.
[12] WANG Xian-fei, XIONG Shou-mei. Protection behavior of SO2-containing cover gases to molten magnesium alloy[J]. Transactions of Nonferrous Metals Society of China, 2011, 21(4): 807-813.
[13] 张心灵, 游国强, 查吉利, 龙思远. 空气对流对HFC125/N2气氛下镁熔体表面膜的影响[J]. 稀有金属材料与工程, 2011, 40(8): 1496-1499.
ZHANG Xin-ling, YOU Guo-qiang, ZHA Ji-li, LONG Si-yuan. Effect of air convection on surface film of magnesium alloy melt under HFC125/N2 atmosphere[J]. Rare Metal Materials and Engineering, 2011, 40(8): 1496-1499.
[14] 张心灵, 游国强, 龙思远, 赵福全, 徐荣峰. HFC-125/N2混合气体对熔态AZ91D镁合金的保护行为研究[J]. 铸造, 2010, 59: 936-939.
ZHANG Xin-ling, YOU Guo-qiang, LONG Si-yuan, ZHAO Fu-quan, XU Rong-feng. Protection behavior of HFC-125 and N2 mixture gases for AZ91D magnesium alloys melt[J]. Foundry, 2010, 59: 936-939.
[15] 张心灵, 游国强, 龙思远, 宋东福. HFC125/N2中添加SO2对镁合金熔体保护效果的影响[J]. 热加工工艺, 2010, 39(17): 62-63, 67.
ZHANG Xin-ling, YOU Guo-qiang, LONG Si-yuan, SONG Dong-fu. Influence of adding SO2 in HFC125/N2 on protective effect of magnesium alloy melt[J]. Hot Working Technology, 2010 , 39(17): 62-63, 67.
[16] 曾一文, 彭立明, 毛协民, 丁文江. 二氟甲烷在镁合金熔炼保护的研究[J]. 特种铸造及有色合金, 2005, 16(7): 406-407.
ZENG Yi-wen, PENG Li-ming, MAO Xie-min, DING Wen-jiang. Protection of molten magnesium alloys by a mixing gas containing difluoromethane[J]. Special Casting & Nonferrous Alloys, 2005, 16(7): 406-407.
[17] 曾一文, 毛明现, 黄志强. 镁合金在HFC-32气氛中熔炼的研究[J]. 铸造, 2006, 55(8): 776-779.
ZENG Yi-wen, MAO Ming-xian, HUANG Zhi-qiang. Research on magnesium melting protected by cover gas mixtures containing HFC-32[J]. Foundary, 2006, 55(8): 776-779.
[18] 大连理工大学无机化学教研室编. 无机化学[M]. 5版. 北京: 高等教育出版社, 2006.
Inorganic Chemistry Department of Dalian University of Technology. Inorganic chemistry[M]. 5th ed. Beijing: Higher Education Press, 2006.
[19] J A迪安, 魏俊发. 兰氏化学手册[M]. 北京: 科学出版社, 2003.
DEAN J A, WEI Jun-fa. Lange’s handbook of chemistry[M]. Beijing: Science Press, 2003.
[20] 刘 晖, 张洪涛, 肖 红. 水饱和蒸汽压的Antonic方程常数的比较[J]. 内蒙古石油化工, 2009, 13: 32-34.
LU Hui, ZHANG Hong-tao, XIAO Hong. Comparison the constants of Antonic equation in the saturated vapor pressure of water[J]. Inner Mongolia Petrochemical, 2009, 13: 32-34.
[21] 李华伦, 商宝禄. 镁及其合金在含SF6气氛中的高温氧化特性[J]. 西北工业大学学报, 1984, 2(1): 77-89.
LI Hua-lun, SHANG Bao-lu. The oxidation of magnesium and its alloys in atmosphere containing SF6 at high temperature[J]. Journal of Northwestern Polytechnical University, 1984, 2(1): 77-89.
(编辑 龙怀中)