文章编号: 1004-0609(2004)10-1666-05
含Ca阻燃镁合金的高温氧化行为
樊建锋, 杨根仓, 程素玲, 谢 辉, 郝维新, 王 梅, 周尧和
(西北工业大学 凝固技术国家重点实验室, 西安 710072)
摘 要: Ca的加入大大提高了镁合金的燃点, 当Ca含量大于1.7%时, Mg-Ca合金能在1173K的高温下暴露于大气中30min而不燃烧。 SEM和XRD分析表明: 合金表面生成了主要由氧化钙和氧化镁组成的复杂氧化膜, 氧化膜内侧组织主要是Mg2Ca, 该氧化膜具有保护性, 但薄厚不一, 极不规则。 高温氧化动力学研究显示: 该合金在673K和873K氧化符合抛物线规律, 而在773K氧化遵循立方规律。 热力学计算结果表明: 在Mg-2.5Ca合金中从室温到1143K温度范围内Ca都比镁优先与氧结合生成CaO, 当Ca含量降低到一定程度时, 镁又与氧结合生成MgO, 所以形成了MgO+CaO的复合保护膜。
关键词: Ca; 镁合金; 氧化; 动力学 中图分类号: TG146.2
文献标识码: A
Oxidation behavior of ignition-proof magnesium-calcium alloys
FAN Jian-feng, YANG Gen-cang, CHENG Su-ling, XIE Hui, HAO Wei-xing, WANG Mei, ZHOU Yao-he
(State Key Laboratory of Solidification Processing, Northwestern Polytechnical University, Xian 710072, China)
Abstract: Ignition point of ignition-proof magnesium alloy was improved by the addition of calcium. When the content of Ca is larger than 1.7%, the alloy Mg-2.5Ca can avoid from ignition at 1173K for 30min. XRD and SEM analyses of oxide scale reveal that the protective oxide film formed on the surface of Mg-2.5Ca alloy is composed of MgO and CaO, and beneath the film is Mg2Ca. The film has protective function, but is inhomogeneous in structure. High-temperature kinetics measurements indicate that the oxidation dynamics curves measured at 673K and 873K follow parabolic law and the curve investigated at 773K follows the cubic law. Thermodynamic calculation reveals that from room temperature to 1143K, Ca can react with O to form CaO prior to Mg. When the content of Ca decreases to some degree, Mg can react with O to form MgO, resulting in the formation of MgO+CaO composite film.
Key words: Ca; magnesium alloys; oxidation; kinetics
镁合金是目前实际应用中最轻的金属结构材料, 它具有密度小、 比强度和密度高、 阻尼性和切削加工性好等优点, 因此, 在过去的几十年中铸造镁合金得到长足的发展[1-5]。 然而镁合金在熔炼和成型过程中极易发生氧化、 燃烧甚至爆炸, 这使得镁合金的进一步广泛应用受到了限制, 因此阻止其氧化燃烧一直是镁合金研究的一个重要课题。 目前较成熟的阻燃方法是熔剂保护法与气体(CO2, SO2和SF6)保护法。 然而, 这两种方法都有缺陷, 如易产生有毒气体污染环境和造成熔剂夹杂而损害合金性能, 此外, 熔炼、 浇注设备和工艺复杂, 加大了成本等[6-10]。 因此, 从20世纪50年代以来, 阻燃镁合金的研究受到极大重视。
所谓阻燃镁合金, 指通过向镁合金中添加合金元素, 使其在熔炼过程中自动生成保护性氧化膜, 这样将大大降低设备及工艺的复杂程度, 也不会对环境造成严重污染, 因而具有巨大的发展潜力。 随着汽车和电子工业对镁铸件的需求高速增长, 阻燃镁合金的研究尤显得突出。
1942年日本专利[9]报道在镁合金熔剂中添加Ca可以更好地阻止熔炼过程中的燃烧。 日本Kyushu国家工业研究所的Sakamoto等[11]测定了含1%~5%Ca的镁合金的燃点, 结果表明1%的Ca就能提高燃点250℃, 而且只要不发生机械破坏, 燃烧就难以产生, 而对于含5%Ca的镁合金, 即使在970℃的大气中暴露60min, 其氧化膜仍很薄, 表面光滑均匀。 此后关于含Ca镁合金阻燃性能的文章虽然时有报道, 但却很少涉及到含Ca镁合金在近熔点温度范围内的阻燃机理和氧化动力学研究。 本文作者考察了Ca对镁合金高温氧化行为的影响, 详细研究了含Ca镁合金的表面膜的结构及其形成表面保护膜的氧化动力学过程, 希望为含Ca镁合金的工业化应用做理论探索。
1 实验
所有合金都由工业纯Mg(99.9%)和纯Ca(99.5%)在电阻炉中及保护性气氛(CO2-0.5%SF6)下熔配而成。 材料成分用感应式耦合等离子原子发射光谱法(简称ICP-AES)测定。 对切割尺寸为20mm×20mm×5mm的试样进行燃点测试实验, 测试前试样需进行机械抛光和丙酮浸泡降解处理。 图1所示为燃点测试设备示意图。 用XPert Pro MPD型X射线薄膜衍射法测定氧化膜的相成分, 衍射所用靶材为CuKα靶。 微观组织形貌分析在带有能谱仪的扫描电镜(SEM & EDS)上进行。 大气条件下的恒温氧化动力学实验在Universal V2.4F型的热重分析仪(Thermogravimetric Analyzer, 简称TGA)上进行, 该热重分析仪的最大称量为2g, 最小分度值为1 μg。 为了尽可能减少镁合金表面在试验前的氧化, 试样在机械抛光和丙酮降解后立即放入热重分析仪中进行试验。
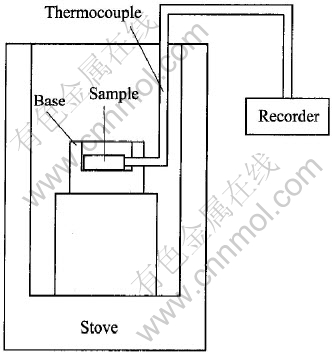
图1 燃点测试设备示意图
Fig.1 Schematic diagram of ignition points testing device
2 结果及分析
由于镁和氧之间极高的化学亲和力, 用传统的在大气下进行打磨与抛光的方法很难制备出完全洁净的金属表面, 因此本试验中所用的试样表面都有一层厚约25的初始氧化膜[12]。
2.1 燃点测试
燃点测试时典型的温度-时间曲线如图2所示。 升温速率为4K/min。 实验结果显示镁合金的燃烧热足以使其温度—时间曲线的斜率产生剧烈变化, 这也从侧面证明了本实验所用的燃点测试方法的可靠性。 这里将镁合金温度—时间曲线上拐点处的温度定义为镁合金的燃点。
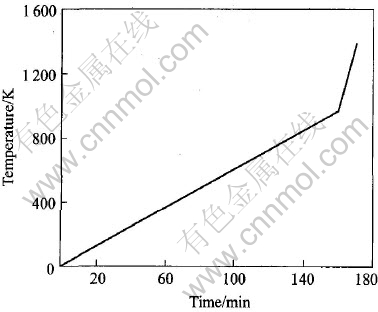
图2 镁合金的典型温度—时间曲线
Fig.2 Typical temperature vs time curve of magnesium alloys
大气下的燃点测试结果见表1。 表中“no ignition”指在1173K保温而不燃烧。 加入0.31%的Ca就能使燃点提高约120K, 但在含Ca量小于1%的范围内, 燃点稳定于1000K左右, 变化不大。 当Ca含量大于1%后, 随Ca含量增加, 燃点快速提升, 当含量大于1.7%后, 可于1173K保温而不燃烧。
2.2 氧化膜结构
图3所示为Mg-2.5Ca在1173K保温30min所得到的氧化膜表面及横截面形貌图, 其中图3(a)所示为宏观形貌, 图3(b)、 (c)、 (d)所示为扫描电镜下的微观形貌。 可以看出(图3(a)、 (c))合金表面覆盖了一层凹凸不平的膜, 薄厚不一, 形状极不规则, 而氧化表面的微观组织为颗粒状(图3(b)), 颗粒沿磨痕方向致密排列, 其平均直径约为1.5μm。 能谱分析表明图3(c)表面膜中灰色部分含Ca较多, 白色部分几乎不含Ca, 只有O和Mg。 图3(d)显示表面膜层下的一些地方含有大量的共晶相。 结合X射线薄膜衍射结果(图4), Mg-Ca合金的表面氧化膜主要由MgO、 CaO和Mg2Ca组成, 由于Ca是表面活性元素, 高温氧化过程中大量的Ca偏聚到表面, 形成致密的保护膜后, 剩余的Ca就与Mg生成共晶团, 附着于表面膜下。
表1 不同成分镁合金的燃点
Table 1 Ignition points of magnesium alloys with different compositions

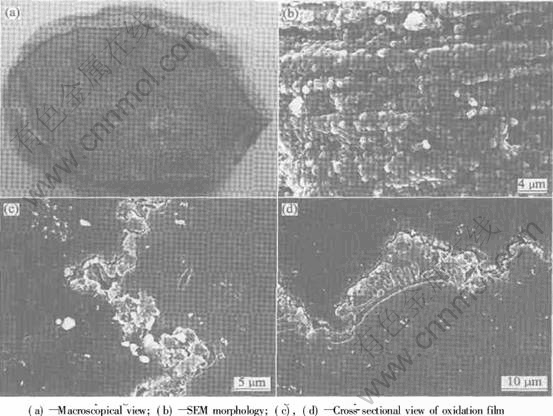
图3 在1173K保温30min后Mg-2.5Ca合金氧化表面形貌
Fig.3 SEM morphologies of oxidation surface of Mg-2.5Ca alloy oxidized at 1173K for 30min
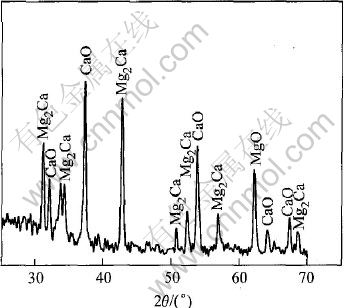
图4 镁合金表面的X射线衍射谱
Fig.4 X-ray diffraction pattern of oxides on Mg-2.5Ca alloy oxidized for 2.5h at 1173K in air
2.3 高温氧化动力学
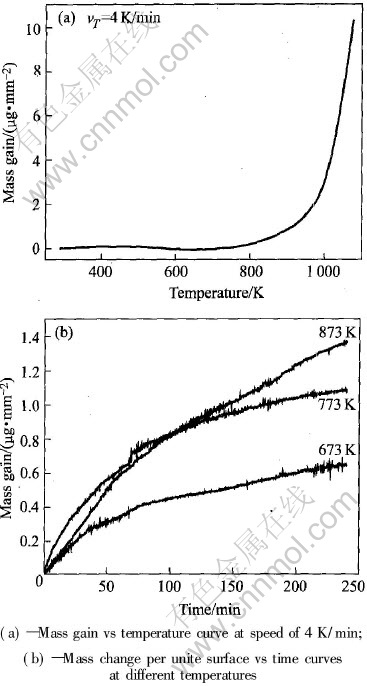
图5 Mg-Ca表面氧化动力学曲线
Fig.5 Dynamic curves of oxidation on surface of Mg-Ca alloy
图5(a)所示是以4K/min的速度缓慢升温的质量增加—温度曲线图。 在0~800K范围内, 增量很小, 800K时增量开始增加, 到900K以上, 氧化增量快速上升。 参照Mg-Ca合金相图, Mg-2.5Ca合金的液相线温度约为900K, 可知Mg-2.5Ca合金在低温时氧化缓慢, 而在近熔点温度范围内氧化开始加剧, 所以研究近熔点温度的氧化行为对提出防止合金氧化的措施有重要意义。 图5(b)是Mg-2.5Ca合金在673K、 773K和873K温度下单位表面积的质量改变与保温时间的关系。 通过非线性拟合, 可以得到氧化动力学曲线的拟合方程。 当温度为673K和873K时, 氧化符合抛物线规律, 其方程分别为
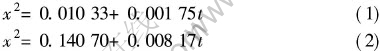
式中 t为时间, x为单位表面积上的氧化增量。
温度为773K时, 合金的氧化遵循立方规律:
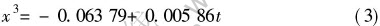
按抛物线规律生长的氧化膜是理想的具有保护效果的氧化膜, 其氧化过程受原子扩散控制, 关于抛物线规律的形成机理, Wagner[13]已有非常详细的论述。 将式(1)、 (2)、 (3)写成通式:

当n〈2时, 表示氧化的扩散阻滞并非完全随膜厚度的增加而成正比增大, 膜的应力、 空洞、 缺陷和晶界可能是造成扩散偏离平方抛物线关系的原因。 当n>2时, 表明扩散阻滞作用比膜增厚所产生的阻滞更为严重。 金属氧化物或锈层的掺杂、 离子扩散速度和致密阻挡层的形成都是可能的原因。 本研究中n>2, 说明Mg-2.5Ca合金的表面膜能够阻止合金的进一步氧化, 且其氧化过程受扩散阻滞控制。 纯镁的高温氧化动力学曲线呈直线形式, 其氧化速率与时间无关, 氧化过程完全由界面反应控制[14]。
2.4 氧化热力学
氧化过程中Mg-Ca合金表面可能发生的化学反应如下:
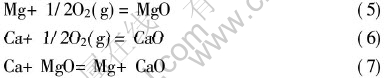
由于氧化反应从室温时就已开始, 并且所生成的氧化膜在合金熔化前就需起到保护作用, 故上式中的金属作固态处理。 室温下式(7)的Gibbs自由能为[3]

即在标准状态下(100kPa, 298K)ΔG7总小于零, 表明CaO比MgO优先生成。 在实际中Gibbs自由能差尚需用下式校正:
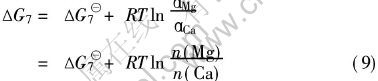
在Mg-2.5Ca合金中, 镁和钙的质量分数分别为0.975和0.025, 代入式(9), 得
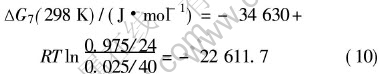
若令ΔG7=0, 可得T=1143.6K, 这说明在Mg-2.5Ca合金中从室温到1143K温度范围内Ca都比镁优先与氧结合生成CaO, 当Ca含量低到一定程度时, 镁又与氧结合生成MgO, 所以形成了如前所述的MgO+CaO的复合保护膜。
3 结论
1) Ca的加入大大提高了镁合金的燃点, 当Ca含量大于1.7%时, Mg-Ca合金可在1173K下保温30min而不燃烧。
2) Mg-2.5Ca合金表面生成了由氧化钙和氧化镁组成的复杂氧化膜, 该氧化膜非常致密, 能够阻止镁合金的进一步氧化甚至燃烧。
3) 在673K和873K时, Mg-2.5Ca合金的高温氧化动力学曲线遵循抛物线规律, 而在773K下则符合立方规律。
REFERENCES
[1] 吕振宜, 翟春泉. 压铸镁合金的应用现状及发展趋势 [J]. 铸造, 1998, 12: 50-53.
L zheng-yi, ZHAI Chun-quan. Application and developing tendency of die casting magnesium alloys[J]. Foundry, 1998, 12: 50-53.
[2] Brown R. Magnesium automotive meeting[J]. Light Metal Age, 1992, 50(5):18-29.
[3] 曾小勤. 阻燃镁合金及其阻燃机理研究[D]. 上海: 上海交通大学, 2000.
ZENG Xiao-qin. Study on ignition proof magnesium alloy and its ignition proof principles[D]. Shanghai: Shanghai Jiaotong University, 2000.
[4] Magers D. Global review of magnesium parts in automobiles[J]. Light Metal Age, 1997, 55(7): 60-72.
[5] Czerwinski F. Correlating the microstructure and tensile properties of a thixomolded AZ91D magnesium alloy[J]. Acta Matter, 1994, 49: 1225-1235.
[6] Luo A E. Review of cast magnesium alloy for elevated temperature applications[J]. Journal of Materials Science, 1994, 29: 5259-5271.
[7] Fisher P A. Production, Properties and Industrial Uses of Magnesium and Its Alloys[M]. Foundary Technology, ASM, 1982. 251-267.
[8] 黄晓锋, 周 宏, 何镇明. 镁合金的防燃研究及其进展[J]. 中国有色金属学报, 2000, 10: 271-274.
HUANG Xiao-feng, ZHOU Hong, HE Zhen-ming. Research and development on ignition proof magnesium alloys[J]. The Chinense Journal of Nonferrous Metals, 2000, 10: 271-274.
[9] Sakamoto M. Suppression of ignition and burning of molten Mg alloys by Ca bearing stable oxide film[J]. Journal of Materials Science Letters, 1997, 16(12): 1048-1050.
[10] Pekguleryuz M O, Avdesian M M. Effects of element from No.Ⅳ or Ⅴ on the mechanics properties of magnesium alloys [J]. Light Metals, 1992, 42(12): 679-696.
[11] Sakamoto M. Mechanism of non-combustibility and ignition of Ca bearing Mg melt[A]. Proceedings of the Fifth Asian Foundry Congress[C]. 1997. 380-389.
[12] Czerwinski F. The oxidation behaviour of an AZ91D magnesium alloy at high temperatures [J]. Acta Materialia, 2002, 50: 2639-2654.
[13] Wagner C. Diffusion and High Temperature Oxidation of Metals in Atom Movements[J]. Am Soc Metals, Cleveland, 1951. 153-168.
[14] 胡 忠, 张启勋, 高以熹. 铝镁合金铸造工艺及质量控制[M]. 北京: 航空工业出版社, 1990.
HU Zhong, ZHANG Qi-xun, GAO Yi-xi. Casting Technology and Quality Controlling of Aluminum and Magnesium Alloys [M]. Beijing: Aerospace Industry Press, 1990.
基金项目: 国家自然科学基金资助项目(50271055); 航空基金资助项目(01G53048); 教育部博士点基金资助项目(20020699018)
收稿日期: 2004-03-09; 修订日期: 2004-05-13
作者简介: 樊建锋(1977-), 男, 博士研究生.
通讯作者: 樊建锋; 电话: 029-87776759; 传真: 029-88492374; E-mail: fanjianfeng77@sina.com
(编辑 吴家泉)