文章编号:1004-0609(2016)-07-1480-07
TiF3对MgH2体系解氢热力学影响的第一性原理研究
袁 江1, 2,周惦武1,魏红伟2
(1. 湖南大学 汽车车身先进设计制造国家重点实验室,长沙 410082;
2. 张家界航空工业职业技术学院 航空维修系,张家界 427000)
摘 要:基于TiF3作为催化剂提高MgH2解氢性能的实验结果,采用基于密度泛函理论的Dmol3程序包,计算从MgH2体系中移走H原子所需能量及几何、电子结构的改变,探讨TiF3对MgH2体系解氢热力学影响及提高其解氢性能的机理。结果表明:Ti替代Mg,体系中Ti—H的成键作用比Mg-H强,Ti将MgH2中的H吸附到其周围,导致MgH2中Mg周围的H减少;创造Mg空位,随着温度的提高,体系中H—H之间的键长变短,两者情形均利于提高MgH2体系的热力学行为。创造Mg空位所需能量(6.65 eV)高于Ti替代Mg的能量(2.34 eV),表明低温下,Ti替代Mg对MgH2体系解氢更为有利,至此TiF3中的Ti替代MgH2中的Mg,利于加快化学反应的进行,使结构稳定的MgH2发生转变,生成结构不稳定的TiH2,这样体系解氢过程不是通过MgH2,而是转化为TiH2进行,因此TiF3可提高MgH2体系的解氢性能。
关键词:MgH2;TiF3;密度泛函理论;解氢性能
中图分类号:TG146.2 文献标志码:A
镁氢化合物(MgH2)理论储氢量高(质量分数7.6%),同时Mg资源丰富,质量轻,价格低廉,在燃氢飞机[1]、燃氢汽车和二次碱性电池等方面有广泛的应用前景。然而,MgH2较高的解氢温度与缓慢的解氢热力学等问题制约了其在工业上的实际应用[2]。为改善MgH2解氢热力学行为和降低其解氢温度,国内外学者开展了大量工作[3-5]。NURUL等[6]实验发现10% Nb3FeF6(质量分数,下同)添加可降低MgH2体系解氢温度100~162 ℃,其中反应过程中NbF5具有高效的催化作用。GRZECH等[7]通过对比MgH2和混合物MgH2+5%TiF3球磨实验结果,发现掺杂TiF3可细化MgH2颗粒尺寸,降低体系解氢温度。张慧等[8]研究了TiF3的掺杂均能够有效地改善LiBH4+MgH2复合体系的放氢性能,TiF3起催化作用。SONG等[9]计算了Ti合金化对MgH2体系稳定性变化的影响,发现Ti降低体系结构的稳定性。ISMAIL等[10]实验发现10% LaCl3添加可降低MgH2体系解氢温度和提高吸附动力性。JIN等[11]将金属氟化物FeF2,NiF2,TiF3,NbF5,CuF2,VF4,ZrF4,CrF2等作为催化剂添加到MgH2中,发现这些金属氟化物的添加,使MgH2显示出优异的解氢性能。可见,目前大多数工作集中在向MgH2体系内加入少量催化剂[3-14](如:Ni、Ti、V等3d过渡金属,Ge、Nb等非3d过渡金属,LaNi5、FeTi等金属间化合物,Nb2O5、Fe3O4等金属氧化物,NbF5、NiF2等金属氟化物)的实验研究较多,并发现这些催化剂在较高温度下,MgH2的解氢动力学性能得到一定程度改善,然而其解氢性能提高的理论机制却不清晰。本课题组前期初步探索了掺杂NiF2提高MgH2解氢性 能[12],迄今为止,国内外尚未见到这些氟化物(尤其TiF3)改善MgH2解氢性能理论机制方面的文献报道。
在JIN等[11]进行球磨实验过程中,发现存在两种产物即TiH2和MgF2,据此可推测,TiF3可使MgH2体系发生2TiF3+3MgH2→3MgF2+2TiH2+H2的化学反应,反应产物MgF2可能是MgH2中的一部分Mg与TiF3中的F化合形成的,这样MgH2中相应留下Mg空位,Mg空位有可能对体系的解氢性能产生影响[12];而产物TiH2可能是TiF3中Ti替代MgH2中的一部分Mg形成(Mg, Ti)Hx固溶体,再由固溶体接着转变而形成的,TiF3中Ti替代MgH2中Mg可能改变MgH2局部的几何参数和电子结构。由此看来,Ti替代MgH2中Mg、形成Mg空位均可能对MgH2体系的解氢热力学行为和解氢性能产生较大影响。基于上述分析与推测,球磨实验形成缺陷(如空位)、添加含Ti(如TiF3)元素的催化剂可显著提高MgH2解氢性能。为此,本文作者从TiF3作为催化剂提高MgH2解氢性能的实验结果出发,采用基于密度泛函理论的Dmol3程序包,考察了Ti替代MgH2中Mg、创造Mg空位对MgH2体系中Mg—H、Ti—H、H—H键长的变化,计算了从MgH2体系中移走H原子所需能量及几何、电子结构的改变,期望基于TiF3对MgH2体系解氢热力学影响的探讨,对TiF3提高MgH2体系解氢性能的机理有所了解,从而为设计高性能轻型镁基复合储氢合金提供理论指导。
1 计算模型与方法
为研究MgH2体系的几何、电子结构与能量的改变,本研究构造如图1的计算模型。该模型为48个原子组成的MgH2(2×2×2)(Mg16H32)超胞,其中MgH2晶体的空间群为P42/mnm(NO.136),晶格常数a=0.4501 nm,c=0.3010 nm,晶胞中各原子坐标为:+2Mg:(0,0,0),+4H:(0.304,0.304,0)[12]。为考察Ti替代MgH2中Mg、创造Mg空位对提高MgH2体系解氢性能的影响,本研究采取Ti替代超胞(Mg16H32)模型中心的Mg原子,将MgH2超胞中心Mg原子取走形成Mg空位,构造出相应的Mg15TiH32和Mg15H32模型,分别如图1(b)和1(c)所示。
本研究计算MgH2体系几何结构优化、能量与电子结构时,采用基于密度泛函理论的Dmol3程序包,采用LDA近似的PWC形式的电子交换关联能函 数[13],取全电子位势的势函数,采用DND基函数的电子波函数[14],布里渊区积分采用Monkhorst-Pack形式的特殊K点方法[15]。其中在MgH2中创造Mg空位,计算在高温下体系的稳定结构时,采用程序包中的Dynamics模块,选取NVT,采用GGA近似的BLYP形式的电子交换关联能函数,取全电子位势的势函数,采用带d轨道的双数值基(DNP)函数的电子波函数,取1.0fs时间步长,取0.01ps总模拟时间,采用Fine网格散点和Smearing energy进行能量快速收敛[13]。为获取体系最为稳定的位置,先进行几何结构优化,再进行能量计算。为保证Mg16H32超胞晶格常数固定不变,只优化晶胞和原子位置。结构优化时,计算精度设置为:应力≤0.004Ha,能量≤1.0×10-5Ha,位移≤0.0005 nm。
2 结果与分析
2.1 Ti替代对体系解氢的影响
为考察替代前后体系中Mg—H、Ti—H的键长变化,在几何结构优化后MgH2超胞(Mg16H32) 图2(a)模型基础上,分析八面体超胞中心Mg(即Mg(0),如图2(b)所示)原子与其第一近邻H(即H(A))原子与第二近邻H(即H(B))原子之间的键长变化,结果如图3所示。可见,中心Mg(0)—H(A)的键长为0.1964 nm,Mg(0)—H(B)的键长为0.1982 nm,Ti—H(A)键长分别为0.1835 nm,Ti—H(B)键长0.1855 nm。发现替代后,Ti—H的键长比替代前的Mg—H键长缩短,分别减少0.0129和0.0127 nm,这一定程度上显示:Ti原子与H原子之间有更强的亲和力,Ti—H的成键作用强于 Mg—H成键作用。
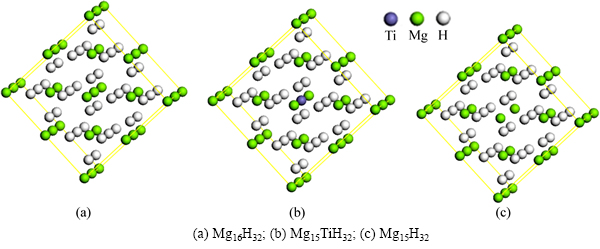
图1 3种计算模型
Fig. 1 Three kinds of supercell models:

图2 超胞模型
Fig. 2 Supercell model:
为进一步考察Ti替代Mg对MgH2解氢性能的影响,将MgH2八面体(见图2(b) )中心Mg(0)的第一近邻H(H(A))原子或第二近邻H(H(B))原子移走。进行结构优化后再分析Mg(0)—H(B)键长变化。发现未移走H(A)原子前,Mg(0)—H(B)键长为0.1964 nm,移走H(A)原子后,Mg(0)—H(B)的键长为0.1963 nm,比较发现Mg(0)—H(B)键长变化不大(见图3)。而Ti替代MgH2中的Mg后,Ti—H(B)之间的键长为0.1760 nm,与未移走H(A)原子(0.1790 nm)相比,明显变短。这表明Ti与H之间存在较强的成键作用,Ti类似“氢泵”,不断强烈吸引H原子到自身周围,不仅仅局限第一近邻H(A)原子,还可以是第二近邻H(B)原子,从而使Ti原子与H原子之间有形成氢化物TiHx团簇的趋势[12]。
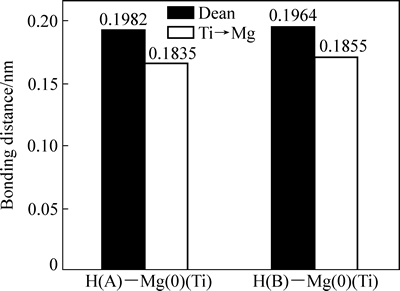
图3 H—Mg(Ti)原子之间的键长变化
Fig. 3 Bonding distance changes between H and Mg(Ti) atoms
从MgH2体系移走H原子所需要的能量,采用式(1)计算[l6]:
Ecoh=E(Mg16-xTixH32-y)-E(Mg16-xTixH32)+n/2E(H2) (1)
式中:x表示体系中Ti原子的个数;y和n表示体系中移走H原子的个数。计算移走H原子所需要能量:未替代Mg前,从MgH2体系中移走1个H原子(H(A))(x=0,y=1)所需要能量为1.54 eV,移走2个H原子(H(A)+H(B))(x=0,y=2)所需要能量为1.78 eV。而Ti替代Mg后,MgH2体系中移走1个H原子(H(A))(x=1,y=1)所需要能量为0.54 eV;而移走2个H原子(H(A)+H(B))(x=1,y=2)所需能量1.43 eV。计算结果表明: Ti替代MgH2体系中Mg后,移走H原子 (1个或者2个)所需能量均小于替代前移走H原子所需能量。而移走H原子所需的能量越小,表明体系解氢越容易。因此可见,Ti替代Mg有利于提高MgH2体系的解氢性能。
2.2 Ti替代Mg前后的态密度
Ti替代Mg前后的态密度如图4所示。替代前MgH2的态密度如图4(a)所示,可见,在费米能级(EF)右边附近存在一个能量间隙区间:+0.5 eV~+3.5 eV,费米能级(EF)以下的成键价带部分主要来自于Mg(s)的贡献,少量H(s)的贡献,在一定程度上,价带部分的Mg(s)与H(s)之间存在一定的杂化作用。杂化作用的存在,体系结构稳定,阻碍了MgH2体系的解氢动力学,解氢困难。而Ti替代Mg后MgH2的态密度如图4(b)所示,与如图4(a)比较发现,费米能级(EF)有明显的向低能级方向产生移动作用,同时存在两个能量间隙区间:-2.3 eV~-0.5 eV和+0.7 eV附近区间,其中+0.7 eV附近能量间隙区间是Ti替代Mg后新出现的,其主要原因是由于Ti替代作用而使在带隙中出现Ti的掺杂电子态。新的能量间隙区间(+0.7 eV附近)主要来自Ti(d)成键电子的贡献,同时在价带部分Ti(s),Ti(d)与H(s)之间存在强烈的杂化作用。由此发现,Ti替代Mg后,虽然Mg(s)与H(s)之间依然存在一定的杂化作用,但是新的能量间隙区间(+0.7 eV附近)使MgH2体系费米能级(EF)附近能隙变窄,并且同时向低能级方向产生移动。这样,对应MgH2体系的结构稳定性降低,使其解氢热力学行为得到改善。
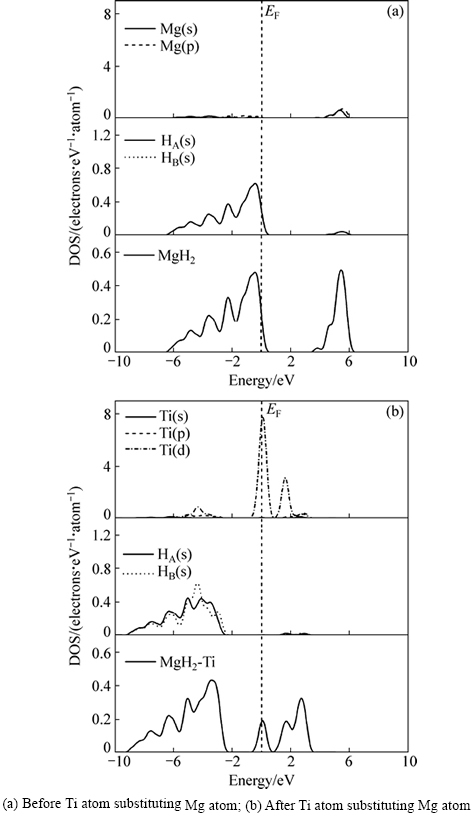
图4 Ti替代Mg前后MgH2电子密度图
Fig. 4 DOS of MgH2 systems:
2.3 Mg空位对体系解氢的影响
JIN等[11]在金属氟化物TiF3添加MgH2体系的高效球磨实验结果发现,高效球磨过程中产生高密度缺陷,这些缺陷对提高MgH2体系解氢性能是十分有利的,同时实验过程中存在副产物MgF2,而MgF2的形成也可能与高效球磨MgH2过程中形成的Mg空位紧密相关。可见,有必要对Mg空位影响体系解氢的机理进行分析[17]。
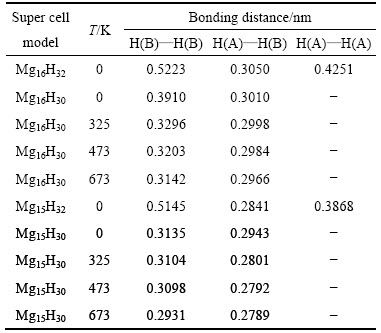
在MgH2体系中,创造1个Mg空位前后H—H键长前后变化的结果如表1所列。分析表1可知:体系温度在0 K时,在没有创造Mg空位的条件下,MgH2体系中H—H的最短键长为0.3050 nm;在创造Mg空位的条件下,MgH2体系中H—H的最短键长为0.2841 nm,与自由状态下H2分子中H—H的键长(0.0741 nm)相比,表明MgH2体系没有形成H2分子。但是,创造Mg空位的条件下,形成H2分子的倾向增强。进一步分析,创造Mg空位的条件下,温度为0 K时,体系移走2个H原子(H(A)和H(B)),H—H的最短键长为0.2943 nm,H2分子仍未形成;当温度升高到325 K时,H—H的最短键长为0.2801 nm;当温度为473 K时,H—H的最短键长为0.2792 nm;当温度为673 K时,H—H的最短键长为0.2789 nm。随着温度的提高,不管是否创造Mg空位,虽然H—H键长仍大于H2分子中H—H的键长,但键长却变短,形成H2分子的倾向增强。其中,创造Mg空位条件下,H—H键长更短,更加利于提高MgH2解氢热力学行为。
表1 创造1个Mg空位前后MgH2体系中H—H键长的变化情况
Table 1 Bonding distance changes of H—H atoms in MgH2 systems with and without Mg vacancy
为进一步证实创造Mg空位对MgH2体系解氢产生有益的作用,采用式(1)计算了从MgH2体系产生1个Mg空位并且同时移走2个H原子所需能量,发现计算结果-2.78 eV,为负值。而未形成Mg空位并且同时移走2个H原子所需能量为+1.78 eV,为正值。由此可看出,Mg空位的存在,可提高MgH2体系的解氢热力学行为。
2.4 TiF3掺杂体系解氢性能影响的机理
工业实际应用时,要求MgH2解氢温度越低越好,MgH2解氢所需的能量越小越好[18]。根据上述分析计算发现,Ti替代Mg和创造Mg空位对提高MgH2解氢热力学都是有利的。采用式(2)和式(3)分别计算Ti替代Mg所需要的能量和形成Mg空位所需要的能量:
ΔH1=[E(Mg15TiH32)+E(Mg)]-[E(Ti)+E(Mg16H32)] (2)
ΔH2=E(Mg15H32)+E(Mg)-E(Mg16H32) (3)
计算公式(2):计算Ti替代Mg所需要的能量ΔH1=2.34 eV;计算式(3):形成Mg空位需要的能量ΔH2=6.65 eV。比较ΔH1和ΔH2的能量大小,发现ΔH1明显小于ΔH2,表明在低温下,TiF3对MgH2体系解氢热力学而言,Ti替代Mg相对于创造Mg空位占有更大优势。
根据TiF3加入对于MgH2解氢的实验结果[11, 19]显示存在反应产物MgF2;文献[20]的实验结果显示存在反应产物δ-TiH2;Ti强烈吸引H原子到自身周围,形成氢化物团簇TiHx相一致。由此可见,上述关于TiF3的加入,推测MgH2体系发生的化学反应2TiF3+ 3MgH2→3MgF2+2TiH2+H2是合理的。进一步分析可知,MgH2的负合金形成热的实验值为(-76.15±9.2) kJ/mol[15],结果为负值,说明MgH2的相结构稳定,体系不易解氢。而Ti替代Mg后,形成的反应物TiH2,TiH2负合金形成热的实验值为-54.81 kJ/mol[12],低于MgH2的负合金形成热,表明TiH2的相结构不稳定,体系比MgH2体系解氢容易。这样,TiF3加入MgH2体系中,MgH2体系解氢过程不是通过MgH2,而是转化为TiH2来进行,TiF3起到催化作用。因此,TiF3可提高MgH2体系的解氢热力学。
3 结论
1) Ti替代Mg和创造Mg空位,均利于提高MgH2体系的热力学行为,其中低温下,Ti替代Mg对MgH2体系解氢更为有利。
2) TiF3中的Ti替代MgH2中Mg,利于加快化学反应2TiF3+3MgH2→3MgF2+2TiH2+H2向右进行,使结构稳定的MgH2发生转变,生成结构不稳定的TiH2,这样体系解氢过程不是通过MgH2,而是转化为TiH2进行,TiF3起到催化作用。因此,TiF3提高了MgH2体系的解氢热力学。
REFERENCES
[1] KNOTEK V, VOJTECH D. Electrochemical hydriding performance of Mg-TM-Mm (TM=transition metals, Mm=mischmetal) alloys for hydrogen storage[J]. Transactions of Nonferrous Metals Society of China, 2013, 27(7): 2047-2059.
[2] 丁向前, 朱云峰, 卫灵君, 宦清清, 李李泉. 催化剂对LiAlH4+MgH2体系放氢性能的影响及催化机理[J]. 中国有色金属学报, 2013, 25(5): 1356-1361.
DING Xiang-qian, ZHU Yun-feng, WEI Ling-jun, HUAN Qing-qing, LI Li-quan. Effect of catalysts on dehydrogenation of LiAlH4+MgH2 composite and catalytic mechanism[J]. The Chinese Journal of Nonferrous Metals, 2013, 25(5): 1356-1361.
[3] 张 健, 朱 璞, 毛 聪, 周惦武. 碳材料掺杂对镁基氢化物释氢性能的影响及其微观机理[J]. 中国有色金属学报, 2015, 25(9): 2464-2470.
ZHANG Jian, ZHU Pu, MAO Cong, ZHOU Dian-wu. Influence and micro-mechanism of carbon materials doping on dehydrogenation properties of magnesium based hydride[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(9): 2464-2470.
[4] 王家盛, 韩树民, 李 媛, 沈 娜, 张 伟. MgH2+20%(wt) MgTiO3复合材料的吸/放氢性能[J]. 物理化学学报, 2014, 30(12): 2323-2327.
WANG Jia-sheng, HAN Shu-min, LI Yuan, SHEN Na, ZHANG Wei. Hydriding/dehydriding properties of an MgH2+20%(wt) MgTiO3 composite[J]. Acta Phys Chim Sin, 2014, 30(12): 2323-2327.
[5] 李志宝, 孙立贤, 张莹洁, 徐 芬, 褚海亮, 邹勇进, 张焕芝, 周怀营, 张 箭. MgH2储氢热力学研究进展[J]. 中国科学: 化学, 2014, 44(6): 964-972.
LI Zhi-bao, SUN Li-xian, ZHANG Ying-jie, XU Fen, CHU Hai-liang, ZOU Yong-jin, ZHANG Huan-zhi, ZHOU Huai-ying, ZHANG Jian. Progress on hydrogen storage thermodynamic of MgH2[J]. Scientia Sinica Chimica, 2014, 44(6): 964-972.
[6] NURUL N, ITAM S, NURUL S M, MOHAMMAD I. The effect of Na3FeF6 catalyst on the hydrogen storage properties of MgH2[J]. Dalton Transactions, 2016, 3(5): 1077-1082.
[7] GRZECH A, LAFONT U, MAGUSIN P C M M. Microscopic Study of TiF3 as hydrogen storage catalyst for MgH2[J]. Journal of Physical Chemistry C, 2012, 116(49): 26027-26035.
[8] 张 慧, 周奕汐, 孙立贤, 曹 忠, 徐 芬, 刘淑生, 张 箭, 宋莉芳, 司晓亮, 焦成丽, 王 爽, 李志宝, 刘 双, 李 芬. Fe2O3与TiF3添加剂对LiBH4-MgH2复合氢化物体系的协同催化作用[J]. 高等学校化学学报, 2012, 33(4): 781-785.
ZHANG Hui, ZHOU Yi-xi, SUN LI-xian, CAO Zhong, XU Fen, LIU Shu-sheng, ZHANG Jian, SONG Li-fang, SI Xiao-liang, JIAO Cheng-li, WANG Shuang, LI Zhi-bao, LIU Shuang, LI Fen. Synergistic catalysis of Fe2O3 and TiF3 additives on the LiBH4-MgH2 composite[J]. Chemical Journal of Chinese Universities, 2012, 33(4): 781-785.
[9] SONG Y, GUO Z X, YANG R. Influence of selected alloying elements on the stability of magnesium dihydride for hydrogen storage applications MgH2[J]. Physical Review Letters B, 2014, 12(3): 132106-132109.
[10] ISMAIL M. Effect of LaCl3 addition on the hydrogen storage properties of MgH2[J]. Energy, 2015, 79(1): 177-182.
[11] JIN S A, SHIM J H, CHO Y W, YI K W. Dehydrogenation and hydrogenation characteristics of MgH2 with transition metal fluorides[J]. Journal of Power Sources, 2007, 172(1/2): 859-862.
[12] 周惦武, 张 健, 刘金水. Ni掺杂MgH2体系解氢性能的机理[J]. 中国有色金属学报, 2009, 19(2): 315-321.
ZHOU Dian-wu, ZHANG Jian, LIU Jin-shui. Mechanism of dehydrogenating properties of Ni doped MgH2 systems[J]. The Chinese Journal of Nonferrous Metals, 2009, 19(2): 315-321.
[13] SUDHA P G, ASVINI M A T, RAJESWARA P R, IYAKUTTI K. First principles study of structural, electronic and mechanical properties of transition metal hydrides (TMH, TM=Mo, Tc, Ru)[J]. Transactions of Nonferrous Metals Society of China, 2013, 23(9): 2700-2707.
[14] ABDELLATIEF M, CAMPOSTRINI R, LEONI M, SCARDI P. Effects of SnO2 on hydrogen desorption of MgH2[J]. International Journal of Hydrogen Energy, 2013, 38(3): 4664-4669.
[15] 袁 江, 周惦武, 彭 平, 侯德政. LiNH2-X(X=Mg, Al, Ti, Nb)体系解氢性能的第一原理计算[J]. 化学学报, 2011, 69(14): 1715-1720.
YUAN Jiang, ZHOU Dian-wu, PENG Ping, HUO De-zheng. First-principles calculation of dehydrogenating properties of LiNH2-X (X=Mg,Al,Ti,Nb) systems[J]. Journal of the Chinese Chemical Society, 2011, 69(14): 1715-1720.
[16] HAN L Y, XIAO X Z, FAN X L, LI Y. Enhanced dehydrogenation performances and mechanism of LiBH4/Mg17Al12-hydride composite[J]. Transactions of Nonferrous Metals Society of China, 2014, 24(1): 157-164.
[17] DAI J H, SONG Y, YAN R. Effects of substituting La with M (M=Sm,Nd,Pr) on electrochemical hydrogen storage characteristics of A2B7-type electrode alloys[J]. Transactions of Nonferrous Metals Society of China, 2014, 12(24): 4012-4022.
[18] 周广有, 郑时有, 方 方. Ti掺杂的MgH2和Mg2NiH4的放氢性能[J]. 化学学报, 2008, 66(9): 1037-1041.
ZHOU Guang-you, ZHENG Shi-you, FANG Fang. Hydrogen desorption performance of Ti-Mediated MgH2 and Mg2NiH4[J]. Journal of the Chinese Chemical Society, 2008, 66(9): 1037-1041.
[19] BORA P L, AHMAD R, SINGH A. Remarkable enhancement in hydrogen storage on free-standing Ti3B and BC3 supported Ti3 clusters[J]. International Journal of Hydrogen Energy, 2014, 40(2): 1054-1061.
[20] JIA Y, SUN C, CHENG L, WAHAB M A, CUI J, JIN Z, MIN Z, YAO X D. Destabilization of Mg-H bonding through nano-interfacial confinement by unsaturated carbon for hydrogen desorption from MgH2[J]. Physical Chemistry Chemical Physics, 2013, 15(16): 5814-5820.
First-principles investigation of TiF3 solution hydrogen thermodynamic effects on MgH2
YUAN Jiang1, 2, ZHOU Dian-wu1, WEI Hong-wei2
(1. State Key Laboratory of Advanced Design and Manufacturing for Vehicle Body,
Hunan University, Changsha 410082, China;
2. Zhangjiajie Aviation Industry Vocational Technical College of
Aviation Maintenance Department, Zhangjiajie 427000, China)
Abstract: Based on experimental results in which the dehydrogenating properties of MgH2 systems was improved by the addition of TiF3 as the catalyst, the energy to remove H atoms, geometry and electronic structure of MgH2 systems were calculated by using Dmol3 program based on the density functional theory. Solutions for MgH2 hydrogen doping TiF3 thermodynamic effects were studied.The results show that the role of Ti—H bond is stronger than the effect of Mg—H bond, thus the H atoms around Mg atoms in MgH2 phase diffuse to the Ti atom around, resulting in reduction of the H atoms around Mg. The formation of Mg vacancy can improve the thermodynamic behavior of MgH2 system. At low temperatures, the Ti atoms of TiF3 can substitution some Mg atoms of MgH2 system, which occupy the larger advantage for the formation of Mg vacancies. The dehydrogenating properties of MgH2 systems is improved with TiF3 because of the Ti atoms of TiF3 substitution the Mg atoms of MgH2 system formed TiH2, the reaction of 2TiF3+3MgH2→3MgF2+2TiH2+H2 during mill process is accelerated, and a stable structure MgH2 shift, generating unstable structure TiH2, this system is not the solution process by MgH2, but converts to TiH2, so TiF3 can improve the performance of hydrogen.
Key words: MgH2; TiF3; density functional theory; dehydrogenating property
Foundation item: Projects(2012AA111802) supported by National High Technology Research and Development Program 863; Project(71075003) supported by the State Key Laboratory of Advanced Design and Manufacturing for Vehicle Body, Hunan University, Changsha, China; Project(ZJJKT2014-008) supported by Zhangjiajie Aviation Industry Vocational Technical College, Zhangjiajie, China
Received date: 2015-03-13; Accepted date: 2016-04-05
Corresponding author: YUAN Jiang; Tel: +86-13789349423; E-mail: 57121076@qq.com
(编辑 王 超)
基金项目:国家高技术研究发展计划重点资助项目(2012AA111802);湖南大学汽车车身国家重点实验室自主课题(71075003);张家界航空工业职业技术学院资助项目(ZJJKT2014-008)
收稿日期:2015-03-13;修订日期:2016-04-05
通信作者:袁 江,副教授;电话:13789349423;E-mail:57121076@ qq.com