


Trans. Nonferrous Met. Soc. China 22(2012) 1513-1516
Measurements of zinc oxide solubility in sodium hydroxide solution from 25 to 100 ℃
CHEN Ai-liang, XU Dong, CHEN Xing-yu, ZHANG Wen-yong, LIU Xu-heng
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 13 May 2011; accepted 30 September 2011
Abstract: The solubility of zinc oxide in sodium hydroxide solution was measured in a closed polytetrafluoroethylene vessel from 25 to 100 ℃. The ZnO solubility was determined by employing the method of isothermal solution saturation. The results show that only ZnO solid exists in the equilibrium state in the low concentration alkali regions, and the solubility of zinc oxide is almost invariable with temperature. With the increase of alkali concentration, equilibrium solid turns from ZnO to NaZn(OH)3 suddenly, this mutation is called invariant point, 3: 55ents on the X-ray intensities from Ti3-2aL ce dynamics of the compounds AlCo and AlNi []tweight. The alkali concentration of the invariant points increases with increasing temperature, but the solubility of NaZn(OH)3 decreases with increasing alkali concentration at the same temperature. At the same Na2O concentration, the higher the temperature is, the higher the solubility of NaZn(OH)3 is.
Key words: ZnO solubility; sodium hydroxide solution; Na2O-ZnO-H2O system; equilibrium phase diagram
1 Introduction
As sphalerite mines are becoming exhausted, researchers have diverted attention to zinc oxide ores. It is more necessary to extract zinc from zinc oxide ores [1-4]. There is a lot of gangue in the zinc oxide ores. Some metals, such as Mg, Ca and Fe, are leached if the ores are treated with acid leaching, and the cost is high. But the cost can be cut if zinc oxide ores are treated by alkali [5]. Moreover, ZnO can react with NaOH to produce ZnOH+,  and
and 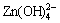 at pH= 6-14. When the alkaline concentration is higher (pH>12), NaZn(OH)3 or Na2Zn(OH)4 is produced as:
at pH= 6-14. When the alkaline concentration is higher (pH>12), NaZn(OH)3 or Na2Zn(OH)4 is produced as:
NaOH+ZnO+H2O=NaZn(OH)3
2NaOH +ZnO+H2O=Na2Zn(OH)4
NaZn(OH)3 or Na2Zn(OH)4 has certain solubility in the alkaline solution. Many experts have researched the leaching of zinc oxide ores with alkaline. ZHAO and STANFORTH [6] and FENG et al [7] obtained more than 85% Zn when the leaching operation was conducted with 5 mol/L NaOH above 95 ℃. In the previous studies, it was found that alkali leaching can obtain 73% Zn at 85 ℃ and 90% Zn at 110 ℃ with 4.5 mol/L NaOH solution [8,9].
It is increasingly popular to treat zinc oxide ores with alkali. For alkaline leaching, leaching temperature and alkali concentration have great effect on zinc extraction. So, it is necessary to investigate the solubility of ZnO in the NaOH solution from 25 to 100 ℃ [10,11]. But there are few researches on it except the report by URAZOV et al [12]. It was found that ZnO solubility was higher at 75 ℃ than that at 25 ℃ when Na2O concentration was below 25%. According to the results, zinc oxide or zinc hydroxide could be precipitated by decreasing temperature. Therefore, it would be an appropriate method to extract zinc from zinc oxide ores with alkali leaching. However, our previous studies showed that there was no ZnO precipitation by decreasing temperature. Thus, it is important to re-measure the ZnO solubility in NaOH solution from 25 to 100 ℃.
2 Experimental
2.1 Material
All the reagents used in this research are of analytical grade. Zn concentration was analyzed by EDTA titration and Na2O concentration was analyzed by neutralization titration with H+ in the solution. Solid was analyzed by chemical methods and characterized by X-ray powder diffraction analysis using Cu Kα radiation. The sodium zinc hydroxide solid obtained was verified by Schreinemaker’s method [13,14].
2.2 Equipment and procedure
The ZnO solubility was determined by employing the method of isothermal solution saturation [13]. Experiments were carried out in a closed polytetrafluoroethylene (PTFE) vessel in a water bath below 75 ℃, while at 100 ℃ it was in an oil bath. ZnO was added into 0.8 L alkaline solution. Different equilibrium time was taken at different alkali concentration and temperature. Thus, when the zinc content of the solution did not change for a long time (more than 12 h) and the solid was redundant (about more than 20 g) in the solid phase, the reaction of ZnO with alkali reached balance. The slurry was naturally separated through gravity sedimentation until the solution was clear.
Their content in the leaching solution was calculated by:
wm=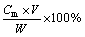
where, wm is the content of Na2O or ZnO in the leaching solution; Cm is the concentration of Na2O or ZnO in the leaching solution; V is the volume of the leaching solution and W is the mass of the leaching solution.
3 Results and discussion
3.1 Phase diagram of Na2O-ZnO-H2O system at 25 and 75 ℃
The equilibrium data at 25 and 75 ℃ are plotted in Fig. 1. Points A and D represent solids ZnO and NaZn(OH)3, respectively (as shown in Fig. 2 ). Points O, B and C are all located on the saturated liquid line. The solubility of ZnO in pure water at 30 ℃ is only 0.0016 mg/mL and can be considered to be 0. So, point O on the ordinate axis of the diagram represents the solubility of ZnO in pure water at 25 ℃. Curves OB and BC represent the compositions of saturated ternary solutions that are in equilibrium with solids ZnO (A) and NaZn(OH)3 (D)(containing 22.3% Na2O and 58.3% ZnO), respectively. Point B is the intersection point of curves OB and BC and is called the invariant point. Two ingredients points for a sample are one point in the solid above curve OB and one point in liquor on the curve OB. Two different coupling lines of these two points would cross at point A (with 100% ZnO). Similarly, different coupling lines of two ingredients of solid above curve BC and liquor on curve BC would cross near point D, which indicates that only one identical equilibrium solid point exists when Na2O concentration is more than 28%.
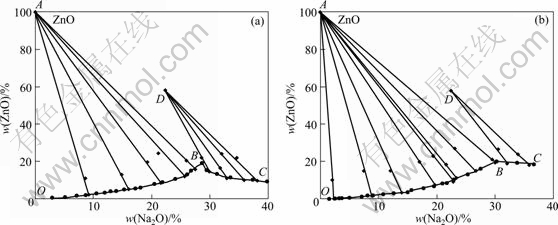
Fig. 1 Equilibrium diagram of Na2O-ZnO-H2O system at 25 ℃ (a) and 75℃ (b)
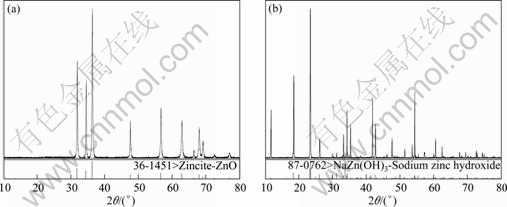
Fig. 2 XRD patterns of equilibrium solid phase of line OB (a) and line BC (b)
The straight line connected ZnO or NaZn(OH)3 (D) with water (origin) does not cut its corresponding saturation curve, which indicates that double salt is incongruently soluble in the ternary system at 25 ℃. The dots of the straight line are the contents of equilibrium wet solid phase. Moreover, the higher the ZnO content of liquor is, the closer it is to B point.
There are the same results in the system at 75 ℃.
3.2 Solubility
The solubility of zinc oxide is determined at different Na2O concentration at 25, 50, 75 and 100 ℃, respectively, and the results of the ternary phase diagrams of Na2O-ZnO-H2O system are shown in Fig. 3. The solubility of zinc oxide in 5% Na2O solution increases slowly, and then increases faster in 5%-28% Na2O solution at 25 ℃, in 5%-29% Na2O solution at 50 ℃, in 5%-30% Na2O solution at 75 ℃ and in 5%-34% Na2O solution at 100 ℃, respectively. The change tendency of the four solubilities of zinc oxide (Line OB) is almost similar at the four different temperatures. However, the four maximum values of zinc oxide solubility are different. After the solubility reaches the maximum, with further increase of alkali concentration, the solubility of NaZn(OH)3 begins to decrease. The higher the temperature is, the higher the solubility of NaZn(OH)3 is. It is similar to the solubility of zinc oxide in the KOH solution [15]. Thus, according to Bayer process of alumina, ZnO in the alkaline solution on the left part of equilibrium curve cannot be obtained but NaZn(OH)3 in the alkaline solution on the right part of equilibrium curve can be obtained. Fortunately, ZnO can be obtained by diluting.
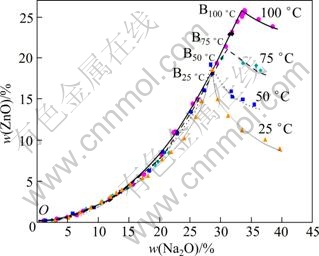
Fig. 3 Solubility of zinc in Na2O-ZnO-H2O system
However, the above results are different from the research by URAZOV et al [12], as shown in Fig. 4. The solubility of zinc oxide increases obviously with the increase of temperature and Na2O concentration. The above results are similar to our previous work.
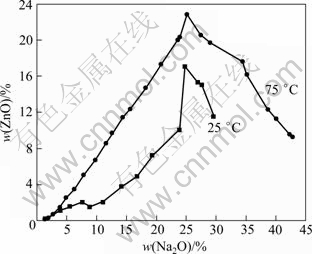
Fig. 4 Solubility of zinc oxide in Na2O-ZnO-H2O system by URAZOV et al
4 Conclusions
1) The equilibrium phase diagram of Na2O- ZnO-H2O system in the sodium hydroxide solution was revalued from 25 to 100 ℃. The solubility of zinc oxide is different from the report by URAZOV et al while it is similar to that in the KOH solution. It is instructive to treat zinc oxide ores with sodium hydroxide.
2) The change tendency of the solubility of zinc oxide is similar at 25, 50, 75 and 100 ℃, but the four maximum values of zinc oxide solubility are different, and that of NaZn(OH)3 decreases with increasing alkali concentration. At the same Na2O concentration, the higher the temperature is, the higher the solubility of NaZn(OH)3 is.
References
[1] IKENOBU S. Method for processing siliceous zinc ores [J]. Metals and Materials Society, 2000, 13(5): 427-435
[2] NAVIDI KASHANI A H, RASHCHI F. Separation of oxidized zinc minerals from tailings: Influence of flotation reagents [J]. Minerals Engineering, 2008, 21: 967-972.
[3] SHIRIN E, FERESHTEH R, SADRNEZHAAD S K. Hydrometallurgical treatment of tailings with high zinc content [J]. Hydrometallurgy, 2006, 82: 54-62.
[4] QIN W Q, LI W Z, LAN Z Y, QIU G Z. Simulated small-scale pilot plant heap leaching of low-grade oxide [J]. Minerals Engineering, 2007, 20: 694-700.
[5] FRENAY J. Leaching of oxidized zinc ore in various media [J]. Hydrometallurgy, 1985, 15: 243-253.
[6] ZHAO Y C, STANFORTH R. Production of Zn powder by alkaline treatment of smithsonite Zn–Pb ores [J]. Hydrometallurgy, 2000, 56: 237-249.
[7] FENG L Y, YANG X W, SHEN Q F, XU M L, JIN B J. Pelletizing and alkaline leaching of powdery low grade zinc oxide ores [J]. Hydrometallurgy, 2007, 89: 305-310.
[8] CHEN A L, ZHAO Z W, JIA X J, LONG S, HUO G S, CHEN X Y. Alkaline leaching Zn and its concomitant metals from refractory hemimorphite zinc oxide ore [J]. Hydrometallurgy, 2009, 97: 228-232.
[9] ZHAO Z W, LONG S, CHEN A L, HUO G S, JIA X J, CHEN X Y. Mechanochemical leaching of refractory zinc silicate (hemimorphite) in alkaline solution [J]. Hydrometallurgy, 2009, 99: 255-258.
[10] B??EZETH P, PALMER D A, WESOLOWSKI D J, XIAO C B. New measurements of the solubility of zinc oxide from 150 to 350 ℃ [J]. Journal of Solution Chemistry, 2002, 31: 947-973.
[11] de WET J R, SINGLETON J D. Development of a viable process for the recovery of zinc from oxide ores [J]. Journal of the South African Institute of Mining and Metallurgy, 2008, 108: 253-259.
[12] URAZOV K K, RIPSONZ B M, RAFCHEKHOV V C. Solubility of zinc oxide in aqueous solution [J]. Non-Ferrous Metals, 1956, 7: 37-42.
[13] DU C H, ZHENG S L, ZHANG Y. Phase equilibria in the K2O–Al2O3–H2O system at 40 ℃ [J]. Fluid Phase Equilibria, 2005, 238: 239-241.
[14] LAUORSE R A, KORS E D. The solubility of zincite in basic hydrometallurgy solvents [J]. The American Mineralogist, 1963, 48: 642-648.
[15] DYSON W H, SCHREIER L A, SHOLETTE W P, SALKIND A J. Physical-chemical studies of KOH-ZnO electrolytes [J]. J Electrochem Soc, 1968, 115(6): 566-569.
25~100 ℃下ZnO在NaOH溶液中溶解度测定
陈爱良,徐 冬,陈星宇,张文勇,刘旭恒
中南大学 冶金科学与工程学院,长沙 410083
摘 要:采用等温溶解饱和度法,在25~100 ℃下于密闭的聚四氟乙烯容器内测定ZnO在NaOH溶液中的溶解度。结果表明:在低浓度NaOH溶液中,平衡体系中只有ZnO一种固相存在,此时氧化锌的溶解度基本上不随温度改变。随着NaOH浓度增高,平衡固相由ZnO突然转变为NaZn(OH)3。这个突变点叫不变点。不变点的NaOH浓度随着温度的升高而增加,但在同一温度下NaZn(OH)3的溶解度随NaOH浓度增加而减少。在同Na2O浓度下,温度越高,NaZn(OH)3的溶解度越高。
关键词:ZnO溶解度;NaOH溶液,Na2O-ZnO-H2O体系;平衡相图
(Edited by FANG Jing-hua)
Foundation item: Project (2007CB613603) supported by the National Basic Research Program of China
Corresponding author: ZHAO Zhong-wei; Tel: +86-731-88830476; E-mail: zhaozw@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(11)61349-6