铬酸钠溶液酸化过程研究
刘桂华,张玉锋,周秋生,李小斌,彭志宏
(中南大学 冶金科学与工程学院,湖南 长沙,410083)
摘要:基于离子平衡和溶液电中性原理,对Na2O-CrO42-/Cr2O72--SO42--H2O系中的铬酸钠溶液酸化过程进行理论计算。理论计算结果表明:铬酸钠转化率随溶液pH降低而呈“S”形增大,且增大铬酸钠浓度或降低溶液温度有利于铬酸钠的转化。在酸化纯铬酸钠溶液和工业铬酸钠溶液时,铬酸钠转化率的变化规律与理论计算结果一致,溶液pH从7.8降低至5.5时,转化率从大约10%显著增加至大约93%,此时加入的酸主要促进CrO42-转化为Cr2O72-,与红外光谱变化对应的是880cm-1处CrO42-的Cr=O弯曲振动的特征峰逐渐变小,在780 cm-1和930 cm-1处Cr2O72-对应的Cr=O弯曲振动的特征峰逐渐增强;转化率还随Na2SO4浓度的升高而增大,在pH≤2.5时,由于硫酸氢钠的大量生成,转化率测定结果大于100%。
关键词:铬酸钠溶液;酸化;pH;转化率
中图分类号:TF791 文献标志码:A 文章编号:1672-7207(2012)04-1227-06
Acidification process of sodium chromate solution
LIU Gui-hua, ZHANG Yu-feng, ZHOU Qiu-sheng, LI Xiao-bin, PENG Zhi-hong
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: The conversion rate of chromate in the system Na2O-CrO42-/Cr2O72--SO42--H2O was calculated theoretically based on the principle of ionic balance and electro-neutrality. The results show that the conversion rate increases in the form of “S” curve when the pH of the chromate solution decreases, and increasing the concentration of Na2CrO4 in the solution or decreasing the temperature of the solution favors the conversion rate of chromate. The experiment results indicate that the measured variation trend of the conversion rate is fairly in agreement with the calculation results in the acidifying pure or industrial sodium chromate solution. The conversion rate rises sharply from about 10% to about 93% with the solution pH decrease from 7.8 to 5.5 and CrO42- transforms to Cr2O72- in the acidification process, corresponding to the gradual decline in the strength of Cr=O blending vibration of CrO42- at 880 cm-1 and the gradual increases in the strength of Cr=O blending vibration of Cr2O72- at 780 cm-1 and 930 cm-1. In addition, the conversion rate rises along with the increase of concentrations of Na2SO4, and the formation of NaHSO4 at pH≤2.5 leads to the conversion rate higher than 100%.
Key words: sodium chromate solution; acidification; pH; conversion rate
铬盐产品是一类用途广泛的无机化工原料[1]。我国是铬盐生产大国[2],采用碱法(无钙法[3]或有钙法[4])处理铬铁矿生产重铬酸钠等铬盐产品,铬盐产量约占世界总产量的35%。在铬盐生产过程中,必须通过加酸使铬酸钠转变为重铬酸钠,这一过程称之为酸化。酸化工艺条件不仅影响副产品硫酸钠的分离和设备的腐蚀程度,而且还影响铬酸根离子的结构及其分配,继而直接影响铬盐产品质量[5]。由于酸化体系复杂、影响因素多,且酸化过程的理论研究鲜见报道,因而目前企业主要基于生产实践经验优化工艺参数和控制转化率,常出现产品颜色不稳定的现象。丁翼[6]基于离子平衡理论,并假设HSO4-为中性,加酸量与铬盐量相等,提出了基于HSO4-电离常数的转化率关系式,但理论计算值与生产值有明显差异。郭庆华[7]基于pH试纸与pH计测得的pH存在差值,提出了生产实践中用pH试纸控制转化率的技术原型。Palmer等[8-10]通过电化学测定或活度系数计算研究了Na2CrO4纯体系中CrO42-,HCrO4-和Cr2O72-的水解平衡常数及其分布区域,但没有研究转化率和溶液结构。基于此,本文作者根据离子平衡和溶液电中性原理,计算和分析酸化过程中的转化率,以纯铬酸钠溶液和生产中的铬酸钠碱性液为对象,实验测定了铬酸钠溶液酸化过程中转化率的变化规律,并结合红外光谱分析,对铬酸钠溶液酸化过程进行了理论研究,旨在为工业生产提供理论依据。
1 铬酸钠溶液酸化的理论计算
以最常用的硫酸酸化生产重铬酸钠为例,理论分析转化率的影响规律。酸化目的是使铬酸根离子转化为重铬酸根离子,转化率(转化率为重铬酸根离子与碱性液中总铬酸根离子物质的量之比)是衡量酸化效率的关键参数。硫酸酸化反应方程式如下:
2Na2CrO4+H2SO4=Na2Cr2O7+Na2SO4+H2O (1)
尽管在酸化过程中,铬酸根离子存在形态存在多种,本文仅考虑常见的3种铬酸根离子:CrO42-,HCrO4-和Cr2O72-。在酸化时,溶液中3种铬酸根离子存在可逆反应,离子方程式为:
 (2)
(2)
铬酸氢根的离解常数为:
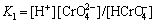 (3)
(3)
重铬酸根离子的水解平衡常数为:
 (4)
(4)
在实际酸化过程中,溶液体系非常复杂,酸化终点pH一般控制在2.5~3之间。为简化计算,本文假设酸化初始pH为9左右,且不考虑HSO4-和OH-,碱性液中铬酸钠浓度(以Na2Cr2O7·2H2O计)为c mol/L。基于溶液显电中性,那么,溶液中电荷平衡如下:
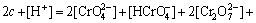
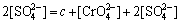
得:
 (5)
(5)
理论转化率x:
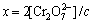 (6)
(6)
根据反应方程式(1)得到:
 (7)
(7)
联立方程式(1)~(7)得:

 (8)
(8)
由式(8)可以看出:转化率与溶液pH、铬酸钠溶液浓度、HCrO4-的离解常数以及Cr2O72-的水解常数 有关。
1.1 转化率与铬酸钠溶液浓度之间的关系
铬酸钠溶液浓度的高低直接影响设备利用率和产出率,丁翼[5]认为转化率与铬酸钠溶液浓度无关,只和酸与铬酸盐间的物质的量比有关。本文根据式(8)计算了25 ℃不同浓度下,理论转化率与pH之间的关系,如图1所示。

图1 铬酸钠溶液质量浓度对理论转化率的影响
Fig.1 Influence of concentration of sodium chromate solution on theoretical conversion rate
由图1可知:在铬酸钠浓度不变时,随着pH的降低,在pH=9.2~7.8间转化率变化大约10%,此阶段加入的酸主要用于中和溶液中的游离碱;而pH从7.8降低至5.5时,转化率由大约10%显著增加至大约93%,此时加入的酸主要促进铬酸根离子转化为重铬酸根离子,对酸的加入存在明显的缓冲能力;当pH从5.5降低至1.0时,转化率变化了大约7%。从图1还可以看出:在pH≥5时,铬酸钠溶液酸化过程中转化率随着铬酸钠浓度的增加而升高。因为随着铬酸钠溶液浓度的增加,溶液的密度和黏度升高[11],可能有利于聚合反应2HCrO4-?Cr2O72-+H2O的发生;在pH≤5时,转化率随着铬酸钠浓度的降低基本保持不变。
1.2 转化率与温度之间的关系
基于不同温度45 ℃,35 ℃,25 ℃和15 ℃下铬酸氢根的离解常数K1及重铬酸根离子的水解常数K2,根据式(8)计算了在酸化液铬质量浓度为240 g/L时,酸化率与pH之间的关系,如图2所示。由图2可以看出:在相同pH条件下,铬酸钠溶液酸化过程中转化率随着温度的升高而减少。根据反应2HCrO4-? Cr2O72-+H2O,由文献[12]可知:该反应的反应热ΔfH°298=-16 kJ/mol,说明该反应是放热反应,因此,升高温度不利于酸化反应的进行。

图2 反应温度对理论酸化率的影响
Fig.2 Influence of reaction temperature on theoretical conversion rate
2 实验原料、仪器与方法
2.1 铬酸钠溶液的制备及酸化
用蒸馏水溶解分析纯的Na2CrO4配制铬酸钠质量浓度为240 g/L的铬酸钠溶液,并通过往溶液中加入98%的分析纯H2SO4调节溶液的pH进行酸化。酸化过程中pH由pHS-25型pH计(上海雷磁仪器厂)进行溶液pH的测定,铬酸钠溶液浓度按国标[13]进行测定。
2.2 红外光谱的采集
采用FT-IR红外光谱仪(Nicolet 6700)测定,采取KBr压片,溶液涂覆的方法采集红外图谱。扫描次数为32,光谱分辨率为4 cm-1。压片涂覆法测量铬酸钠以及重铬酸钠溶液时可靠性及重复性良好[14]。在检测溶液的红外光谱前,应先测空白样并将其设为背景。整个实验过程在氮气的保护作用下进行。
3 实验结果与讨论
3.1 铬酸钠溶液酸化过程中溶液转化率与pH的关系
采用分析纯的铬酸钠配制铬酸钠溶液,加入浓H2SO4调节溶液pH,在25 ℃下测定分析不同pH对应的酸化率,并结合理论转化率研究了转化率与溶液之间pH之间的关系,结果如图3所示。
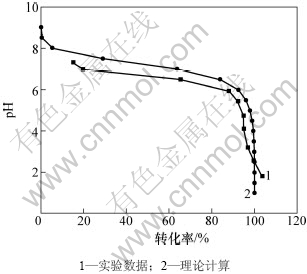
图3 实验转化率和理论转化率的关系
Fig.3 Relationship between theoretical conversion rate and experimental one
由图3可以看出,理论计算和实验值均随着pH的降低,转化率先小幅度增大,然后大幅增大,最后又小幅增大,二者的转化率随pH变化的规律基本是一致的。但在pH≤2.5时,实验测定的转化率大于100%,与理论计算值存在差异,这主要是因为理论计算时假设pH≥2.5;酸化时部分硫酸根生成硫酸氢钠,使一部分酸变成无效酸,致使转化率过高;而且理论转化率的计算没有考虑溶液中电解质平均活度系数。
另外,对质量浓度为240 g/L不同pH的铬酸钠溶液进行红外光谱研究,结果如图4所示。
由图4可以看出:随着H2SO4的加入,溶液pH降低,880 cm-1处CrO42-的Cr=O弯曲振动的特征 峰[15]逐渐变小,而780 cm-1和930 cm-1处Cr2O72-对应的Cr=O弯曲振动的特征峰逐渐增强,说明随着pH降低,铬酸钠溶液中CrO42-的浓度逐渐减小,Cr2O72-的浓度逐渐增加,其间发生了CrO42-向Cr2O72-的转化,与图3的实验数据基本一致。
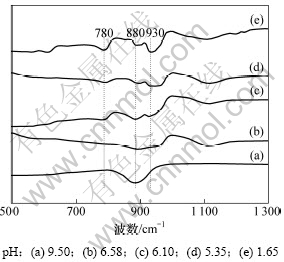
图4 酸化过程中铬酸钠溶液的红外谱图
Fig.4 IR spectra of sodium chromate solutions during acidification process
3.2 铬酸钠浓度对转化率的影响
采用分析纯的铬酸钠配制铬酸钠溶液,加入浓H2SO4调节溶液pH,在25 ℃下测定不同铬酸钠溶液质量浓度66,175和273 g/L的转化率与酸化液pH之间的关系,结果如图5所示。

图5 铬酸钠溶液质量浓度对酸化率的影响
Fig.5 Influence of concentration of sodium chromate solution on conversion rate
由图5可以看出:在pH≥5时,铬酸钠溶液酸化过程中转化率随着铬酸钠溶液质量浓度的降低而减少。如在pH=6.26时,175 g/L铬酸钠溶液的转化率为76.19%,而66 g/L铬酸钠溶液的转化率为67.77%;在pH=7.3时,铬酸钠质量浓度为273,175和66 g/L时,相应的转化率分别为18.25%,17.51%和8.98%;在pH≤5时,转化率随浓度降低差别不明显,这与理论计算结论一致。
为了进一步验证在pH≥5时,转化率与铬酸钠溶液浓度有关,在pH=6.76,铬酸钠质量浓度分别为535,428,321,214和107 g/L的红外光谱结果如图6 所示。
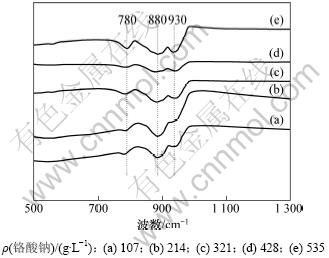
图6 不同质量浓度铬酸钠溶液的红外谱图
Fig.6 IR spectra of sodium chromate solutions with different concentrations
由图6可以看出:在pH≥5时,随着铬酸钠溶液质量浓度的升高,溶液880 cm-1处CrO42-对应的Cr=O弯曲振动逐渐变小,而780 cm-1和930 cm-1处Cr2O72-对应的Cr=O弯曲振动的特征峰逐渐增强。说明随着质量浓度的升高,铬酸钠溶液中CrO42-的质量浓度逐渐减小,Cr2O72-的质量浓度逐渐增加。这表明随着铬酸钠溶液质量浓度的升高,转化率不断增大。
3.3 硫酸钠对转化率的影响
Na2SO4是酸化过程中伴随生成的副产品,基于 H2SO4的电离平衡以及电荷平衡,计算18 ℃下,纯 H2SO4体系中 Na2SO4和 NaHSO4质量分数随溶液pH的变化规律,结果如图7所示。
由图7可以看出:NaHSO4质量分数都随着溶液pH的降低而增高。在pH从2.5降低至0时,溶液中Na2SO4(SO42-)质量分数从86.34%减少至1.96%,NaHSO4(HSO4-)质量分数从13.65%增加至98.04%,使一部分酸变成无效酸,致使转化率大于100%。
根据上述结论,在45 ℃的水浴中,分别向铬酸钠溶液中加入0,10,30 g/L Na2SO4,研究 Na2SO4对转化率的影响规律,实验结果如图8所示。
由图8可以看出:随着溶液中Na2SO4质量浓度的升高,在相同pH条件下转化率增大,但在pH≥5.5时,Na2SO4质量浓度对表观转化率影响不明显;而在pH≤5.5时,随着Na2SO4质量浓度的增加,表观转化率明显增高,如当pH=4.7时,外加Na2SO4质量浓度分别为0,10,30 g/L时,溶液中转化率分别为94.79%,97.44%和106.12%;而当pH=2.08,外加Na2SO4质量浓度分别为10 g/L和30 g/L时,铬酸钠转化率分别为105.08%和112.81%。这主要是因为低pH时Na2SO4含量增加使得NaHSO4生成量增加,进而使转化率增高。同时也可以解释某厂无钙法生产铬盐时转化率比传统有钙法生产时的转化率高的原因,即无钙法碱性液中Na2CO3浓度比有钙法碱性液中Na2CO3浓度高,进而无钙法中酸化液Na2SO4浓度比有钙法的高,致使无钙法表观转化率达114%~116%,而有钙法的表观转化率只为102%~104%。

图7 溶液pH对Na2SO4和NaHSO4质量分数的影响
Fig.7 Influence of pH value on distribution of Na2SO4 and NaHSO4

图8 铬酸钠溶液中Na2SO4质量浓度对转化率的影响
Fig.8 Influence of concentration of Na2SO4 on conversion rate
4 结论
(1) 铬酸钠的转化率随溶液pH的降低呈“S”形增大,且增大铬酸钠浓度和降低酸化温度有利于铬酸钠的转化。
(2) 实验测定的纯铬酸钠溶液和工厂铬酸钠溶液酸化过程中的转化率实验值与理论计算结果一致,且铬酸钠的转化率随溶液中杂质Na2SO4浓度的增加而升高,当pH<2.5时,铬酸钠的转化率将大于100%。
(3) 在铬酸钠溶液酸化过程中,CrO42-不断转变为Cr2O72-,对应于880 cm-1处CrO42-的Cr=O弯曲振动的特征峰强度逐渐变小,而780 cm-1和930 cm-1处Cr2O72-对应的Cr=O弯曲振动的特征峰强度逐渐增强。
参考文献:
[1] 李荫昌. 中国红矾钠生产现状与市场展望[J]. 无机盐工业, 2004, 36(3): 19-21.
LI Yin-chang. The Chinese production status and market prospect of sodium dichromate[J]. Inorganic Chemical Industry, 2004, 36(3): 19-21.
[2] 李兆业. 铬盐行业的状况及发展建议[J]. 无机盐工业, 2006, 38(4): 1-5.
LI Zhao-ye. The current status and development suggestion of chromium salts industry[J]. Inorganic Chemical Industry, 2006, 38(4): 1-5.
[3] Meussdoerffer J N, Niederpreum H, Nieder V H G. Disintegration of chromes: US, 4500350[P]. 1977-02-07.
[4] Mcketta J, Kubicek D L. Sodium chromate and sodium dichromate production capacities[J]. Encyclopedia of Chemical Process & Design, 1979, 8: 303-323.
[5] 丁翼. 重铬酸钠生产酸化工艺探讨[J]. 无机盐工业,1987, 16(6): 6-8.
DING Yi. Technology discussion of the acidification process of sodium chromate solution[J]. Inorganic Chemicals Industry, 1987, 16(6): 6-8.
[6] 丁翼. 铬化合物生产与应用[M]. 北京: 化学工业出版社, 2003: 108-109.
DING Yi. Chromium compounds production and applications[M]. Beijing: Chemical Industry Press, 2003: 108-109.
[7] 郭庆华. 铬酸钠酸化率与溶液pH值[J]. 无机盐工业,1997(4): 36-37.
GUO Qing-hua. Acidification and pH value of the sodium chromate solution[J]. Inorganic Chemicals Industry, 1997(4): 36-37.
[8] Palmer D A, Wesolowski D, Mesmer R E. A potentiometric investigation of the hydrolysis of chromate(VI) ion in NaCl media to 175 ℃[J]. Journal of Solution Chemistry, 1987, 16: 443-463.
[9] Sasaki Y. Equilibrium Studies on Polyanions. 9. The first steps of acidification of chromate ion in 3 M Na(ClO4) Medium at 25 ℃ degrees[J]. Acta Chemica Scandinavica, 1962, 16(3): 719-734.
[10] Tong J Y, King E L. A spectrophotometric investigation of the equilibria existing in acidic solutions of chromium(VI)[J]. Journal of the American Chemical Society, 1953, 75: 6180-6186.
[11] WANG Ji-yan, WANG Fu-an, ZHANG Peng. Densities and viscosities of chromium trioxide + potassium chromate +potassium dichromate + water from (298.15 to 333.15) K[J]. Chemical and Engineering Data, 2008, 53: 648-653.
[12] Ball J W, Nordstrom D K. Critical evaluation and selection of standard state thermodynamic properties for chromium metal and its aqueous ions, hydrolysis species, oxides, and hydroxides[J]. Journal of Chemical and Engineering Data, 1998, 43(6): 895-918.
[13] GB/T 15555.7—1995, 固体废物-六价铬的测定: 硫酸亚铁按滴定法[S].
GB/T 15555.7—1995, Solid Waste, Determination of Chromium (Ⅵ): Titrimetric Method[S].
[14] 李小斌, 王丹琴, 梁爽, 等. 铝酸钠溶液的电导率与结构的关系[J]. 高等学校化学学报, 2010, 31(8): 1651-1655.
LI Xiao-bin, WANG Dan-qin, LIANG Shuang, et al. Relationship between electric conductivity and ion structure of sodium aluminate solution[J]. Chemical Journal of Chinese Universities, 2010, 31(8): 1651-1655.
[15] 中天一雄. 无机和配位化合物的红外和拉曼光谱[M]. 黄德如, 汪仁庆, 译. 北京: 化学工业出版社, 1992: 151-183.
Nakamoto K. Infrared and raman spectra of inorganic and coordination compounds[M]. HUANG De-ru, WANG Ren-qing, trans. Beijing: Chemical Industry Press, 1992: 151-183.
(编辑 赵俊)
收稿日期:2011-04-12;修回日期:2011-07-02
基金项目:湖南省科技重大专项计划项目(2009FJ1009)
通信作者:周秋生(1972-),男,湖南涟源人,博士, 教授,从事铬清洁生产研究;电话:0731-88830453;E-mail:qszhou@csu.edu.cn