DOI:10.19476/j.ysxb.1004.0609.2018.04.24
羟基氧化铬合成及其在铬酸盐溶液中的除钒应用
陈 欣1, 2,郑诗礼2,张海林2,崔雯雯2,王舒磊2,李 平2,张 懿1, 2
(1. 天津大学 化工学院,精馏技术国家工程研究中心,天津 300350;
2. 中国科学院 过程工程研究所,湿法冶金清洁生产技术国家工程实验室,北京 100190)
摘 要:采用水热法合成纳米花状的β-CrOOH,并用于钒(V5+)的吸附分离。在碱性体系中,合成的β-CrOOH比表面积为174.882 m2/g,孔体积为0.602 cm3/g。在65 ℃、pH = 4时,β-CrOOH对V5+的最大吸附容量可达到32.66 mg/g。吸附机理表明,β-CrOOH在吸附过程中其表面会释放出羟基以形成配位不饱和Cr6+活性中心,溶液中的钒酸根(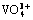 )单体通过钒氧双键(V=O)与不饱和的Cr6+活性中心结合,形成内球型配位,从而吸附钒。在铬酸盐的清洁生产应用中,将β-CrOOH置于铬铁矿无钙焙烧中和液(Na2CrO4-NaVO3-H2O)中用来选择性分离V5+离子。结果表明,适量的β-CrOOH能将铬中和液中91.57%的钒有效脱除掉,铬几乎无吸附,从而实现钒铬的有效分离。
)单体通过钒氧双键(V=O)与不饱和的Cr6+活性中心结合,形成内球型配位,从而吸附钒。在铬酸盐的清洁生产应用中,将β-CrOOH置于铬铁矿无钙焙烧中和液(Na2CrO4-NaVO3-H2O)中用来选择性分离V5+离子。结果表明,适量的β-CrOOH能将铬中和液中91.57%的钒有效脱除掉,铬几乎无吸附,从而实现钒铬的有效分离。
关键词:羟基氧化铬;分离;铬酸盐溶液;钒;水热法;选择性吸附
文章编号:1004-0609(2018)-04-0845-10 中图分类号:O614.61 文献标志码:A
铬盐是重要的无机化工基础原料,产品主要应用于冶金、制革、染料、金属表面处理、催化剂、医药等工业,与国民经济10%~15%的商品品种有关。无钙焙烧法[1]作为铬盐行业的主流生产方法,是以铬铁矿、纯碱为原料进行高温钠化氧化焙烧得到熟料,铬铁矿中Fe、Mg等碱性氧化物以不溶组分进入渣相,Cr、Al、V分别形成Na2CrO4,NaAlO2,NaVO3可溶性组分进入中和液中。针对中和液中的钒杂质,多采用钙化沉钒[2]脱除方法。就是在中和除铝的基础上,加入过量的CaO(理论添加量的10~13倍),使溶液中的钒以钒酸钙沉淀的形式被脱除;然后,再次加入Na2CO3除去多余的Ca2+,得到净化后的Na2CrO4溶液,用以进一步生产铬盐产品。此法虽能有效脱除铬中和液中90%左右的钒,但副产的含铬钒酸钙渣中钒含量低并含有高致癌性CaCrO4,堆存放置环境风险高,对生态环境危害极大的六价铬污染源亟待解决[3]。
吸附法除钒因其操作简单,处理效率高,成本低等优点,在化工冶金分离领域而被广泛使用。针对工业废水中钒的脱除,赵倩等[4]利用改性沸石可将工业废水中的钒浓度降低到1 mg/L,从而直接达到了排放标准;曹宏斌等[5]则采用离子交换树脂选择性脱除磷酸钠溶液中的钒。针对铬中和液中钒杂质的脱除问题,LI等[6]通过直接在铬盐溶液中原位合成γ-AlOOH同步选择性吸附铬溶液中的钒,从而达到脱除钒、铝的目的。杨保军等[3]针对溶液的低钒特点,进行了在中和溶液中加入Fe2(SO4)3生成钒酸铁的探索研究,但该法易引入铁等杂质。
已有研究报道了纳米结构CrOOH合成及在催化、涂料以及涂层材料等领域应用[7-11]。早在 1976年,CHRISTENSEN等[12]就通过水热法在不同的温度下合成了α-CrOOH、β-CrOOH和γ-CrOOH;2003年,ABECASSIS-WOLFOVICH等[13]采用均相沉淀法合成了3~9nm的超细CrOOH;2014年,LIANG等[14]采用氢化还原法在450 ℃和650 ℃的高温下分别合成了α-CrOOH和γ-CrOOH等。在应用方面,CrOOH被用来制备具有较高的近红外反射性能和优良黄绿色调的颜料和对合金具防腐蚀作用的镀层。
本文作者着眼于取代铬盐中和液采用的钙化沉钒方法,合成了特殊形貌的β-CrOOH吸附剂,并将其应用到铬铁矿无钙焙烧中和液中钒杂质的选择性吸附分离方面。深入研究钒的吸附行为、动力学,得出β-CrOOH吸附钒的最佳条件,并探究其吸附机理。
1 实验
1.1 实验材料与试剂
实验所用的四水铬酸钠(Na2CrO4·4H2O)、四水甲酸钠(HCOONa·4H2O)、无水硫酸钠(Na2SO4)、氢氧化钠(NaOH)、PVP以及偏钒酸铵(NH4VO3)均为国药集团化学试剂有限公司生产的分析纯试剂。
1.2 实验仪器
数控超声清洗器(北京中晟铭科技有限公司)、DF-101S集热式恒温加热磁力搅拌器(北京兴德仪器设备有限公司)、DY-100不锈钢水热合成反应釜(北京兴德仪器设备有限公司),JXF-12均相反应器(烟台市招远松岭仪器设备有限公司)。G10型高速离心机(安新县白洋离心机厂制造)、DZF-6020真空干燥箱(上海一恒科技有限公司)。
1.3 β-CrOOH的制备方法
β-CrOOH典型的合成过程如下:将5mL 1 mol/L Na2CrO4、5mL 1.5 mol/L HCOONa、5 mL 2 mol/L Na2SO4溶液与2 g NaOH和2 g PVP混合并用水稀释至60 mL,并用数控超声清洗器在99 Hz功率下超声0.5 h得到充分分散的混合溶液。然后将溶液倒入到100 mL带有聚四氟乙烯内衬的不锈钢水热合成反应釜中,将反应釜拧紧放在均相反应器中在220 ℃下反应20 h。反应完毕后自然冷却至室温,并用离心机以8000 r/min的转速离心分离20 min得到沉淀,然后用去离子水将沉淀洗涤至检测不到硫酸根为止。最后将产物放在真空干燥箱中在90 ℃下干燥12 h得β-CrOOH。
1.4 β-CrOOH吸附钒实验
称取一定量的偏钒酸铵放入容量瓶加入高纯水并加热溶解,调制成所需的浓度和酸度之后备用。准确称取一定量合成好的β-CrOOH与偏钒酸铵溶液混合,在恒温水浴中吸附一定时间后检测溶液中V5+的含量并计算β-CrOOH对钒的吸附容量。然后通过改变溶液的pH值、温度、吸附时间以及钒的初始浓度来考察β-CrOOH对钒吸附容量的变化。最后根据实验结果得出最佳吸附条件。吸附容量用式(1)计算:
 (1)
(1)
式中:c0、ct分别为吸附前及时间为t时钒的浓度;w为β-CrOOH的质量;V为液体的体积。
1.5 测试与表征
采用D/Max-3B型X射线衍射仪(XRD)在工作电压为40 kV,工作电流为20 mA,扫描范围为5°~90°,扫描时间为3.24 min的条件下分析产品的物相;采用高分辨场发射透射电子显微镜(JEM-2100F)观察产品的微观结构;用Nicolet 5DX型傅里叶变换红外光谱仪分析样品的化学结构;用Quanta Chrome Instruments公司生产的AUTOSORB-1-MP型全自动比表面积和孔隙率分析仪检测样品的比表面积以及孔径分布;用美国 Thermo VG 公司生产的 ESCALAB250 多功能表面分析系统测量β-CrOOH吸附钒前后表面元素的变化;用ICP-OES检测溶液中钒的含量。
2 结果与讨论
2.1 β-CrOOH的表征与分析
通过对工艺条件的探讨,制备出了由纳米片自组装形成的纳米花状羟基氧化铬。图1(a)所示为产物的 XRD谱。由图1(a)可知,在2θ为27.68°、35.48°、61.07°以及81.08°位置出现了4个衍射峰,分别与β-CrOOH (JCPDS NO.20-0312)的(110)、(101)、(310)和(321)晶面对应。图1(b)所示为产物的能谱分析图。由图1(b)可以看出,产物中Cr元素质量分数为61.18%,O元素质量分数为38.82%,则O与Cr摩尔比为2.06,与β-CrOOH 中O与Cr的理论摩尔比相符,进一步说明生成的产物为β-CrOOH。此外,EDX谱中所出现Na峰和Cu峰分别由反应残留物以及碳膜铜网所致。
产物的形貌结果表明所得到的β-CrOOH是粒径大小十分均一的纳米花(见图2(a)),高倍透射电镜结果可进一步发现均一纳米花状的β-CrOOH是由许多超薄纳米晶片自组装形成(见图2(b))。在图2(c)中,最内层的两个明亮衍射环分别对应了β-CrOOH的(011)和(101)晶面,表明水热合成的β-CrOOH属于多晶结构。HRTEM图(见图2(d))中可发现清晰的晶格条纹,测量后确定晶面间距为0.2405 nm,与β-CrOOH(011)晶面相近,进一步验证了物相和SADE的测量结果。
为了测量β-CrOOH的比表面积和孔体积大小,将合成的β-CrOOH置于77 K的温度下检测其对N2气的吸脱附曲线(见图3)。从图3(a)中可以看出,水热法合成的β-CrOOH在低压区(p/p0<0.01)对N2气的吸附量垂直上升,这说明通过水热法得到的β-CrOOH具有少量的微孔;当压力升到高压区(p /p0=0.85)时,N2气的吸附量迅速上升,表明在此压力下吸附质N2气发生了毛细管聚集现象,这说明水热法得到的β-CrOOH具有大量的介孔。根据IUPAC分类,这种类型的等温线属于典型的第Ⅳ类型等温线[15]。图3(a)的BET结果显示:水热法得到的β-CrOOH比表面积为174.882 m2/g,孔体积为0.602 cm3/g。IUPAC将吸附剂的孔径分为3类:微孔(<2 nm)、介孔(2~50 nm)与大孔(>50 nm)。从图3(b)的DFT图中可以看出,水热法得到的β-CrOOH孔径分布范围较广,其孔径分布范围为0~65 nm,其中微孔体积为0.048 cm3/g,介孔体积为0.486 cm3/g,大孔体积为0.068 cm3/g,大部分孔径还是集中分布在介孔范围内。通过计算,β-CrOOH的平均孔径为13.71 nm。

图1 产物的XRD谱和EDX谱
Fig. 1 XRD pattern(a) and EDX spectrum(b) of precipitate
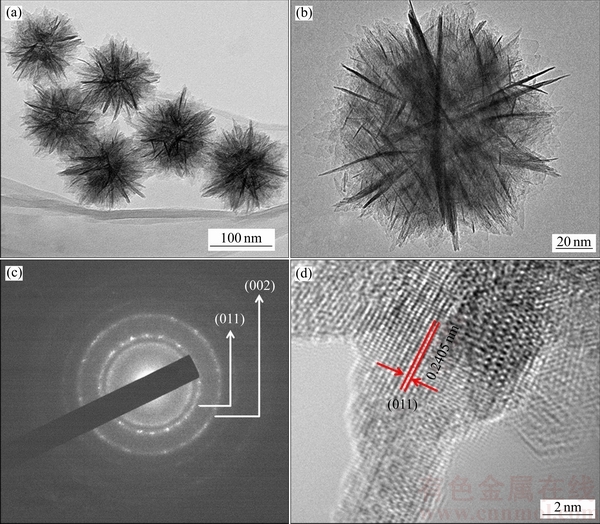
图2 β-CrOOH的TEM像、选区电子衍射图以及高分辨透射电镜图
Fig. 2 TEM images((a), (b)), SADE(c) and HRTEM images(d) of β-CrOOH structure
2.2 钒吸附行为
吸附温度、时间、pH值以及钒的初始浓度等吸附条件对β-CrOOH吸附能力具有至关重要的影响。为研究pH值对β-CrOOH吸附钒吸附容量的影响,将0.2 g的β-CrOOH与50 mL的220 mg/L(以单质V5+计)的NH4VO3溶液混合,在温度为65 ℃,不同pH值条件下搅拌吸附4 h,结果如图4(a)所示。由图4(a)可知,随着pH值升高,β-CrOOH对钒的吸附容量变化趋势是先增大再减小,在pH为3~5范围内,吸附能力达到32.66mg/g。导致这种现象的原因是当pH≤2时,溶液中的钒主要以VO2+的形式存在,而CrOOH表面带正电[16],对带正电的VO2+离子具有一定的排斥作用。因此,在pH=1时,CrOOH对钒的吸附容量较低为25.16 mg/g。当 2<pH<4 时,随着溶液pH值升高,溶液中的的量逐渐减少,β-CrOOH对钒的吸附容量也随着pH值的升高逐渐增大;当pH值为4时,此时溶液中的V5+都是以含氧阴离子的形式存在,吸附能力达到最大。随着pH进一步升高,溶液中OH-离子的浓度增大,与钒酸根产生竞争吸附从而导致了钒吸附容量降低;当pH=12时,β-CrOOH对钒的吸附容量降低到了6.21 mg/g。

图3 β-CrOOH的N2气吸脱附曲线以及用DFT方法计算的β-CrOOH孔径分布
Fig. 3 Nitrogen adsorption-desorption isotherms(a) of synthesized β-CrOOH(b) pore size distribution profiles of β-CrOOH derived by using density functional theory

图4 初始pH、温度、时间以及V的浓度对β-CrOOH吸附V的影响
Fig. 4 Effects of initial pH(a), temperature(b), time(c) and V concentration(d) on adsorption of V ions onto β-CrOOH
为研究温度对β-CrOOH吸附钒吸附容量的影响,将0.2 g β-CrOOH与50 mL的220 mg/L(以单质V5+计)的NH4VO3溶液混合,固定pH值(pH=4)和吸附时间(t=4 h),在不同温度条件下进行吸附。结果如图4(b)所示,当温度低于65 ℃时,β-CrOOH对钒的吸附容量随着温度升高呈线性增加。这是因为升高温度,溶液中的钒酸根离子的运动更加剧烈,从而增大了钒酸根与β-CrOOH表面活性位点的接触的机会;同时升高温度β-CrOOH的表面还会创造更多的活性位点,从而增加了钒的吸附容量。当温度高于65 ℃时,β-CrOOH对钒的吸附容量随着温度的升高而增加的越来越缓慢,这是因为β-CrOOH的表面活性位点数量有限,温度再升高活性位点的数量增加的越来越少,从而导致β-CrOOH对钒的吸附容量增加得越来越缓慢。
为考察时间对钒吸附容量的影响规律,将0.4 g β-CrOOH与100 mL 220 mg/L(以单质V5+计)的NH4VO3溶液混合,固定pH值(pH=4)和吸附温度(θ= 65 ℃),在不同时间条件下进行吸附,结果如图4(c)所示。从图4(c)中可以看出,随着时间的延长,β-CrOOH对钒的吸附容量逐渐增大,当时间达到4 h时吸附容量达到32.66 mg/g时,随着时间的进一步延长,吸附容量不再发生变化。这说明β-CrOOH吸附钒4 h就可达到吸附平衡。
为考察钒的初始浓度对β-CrOOH吸附钒的吸附容量影响,将0.2 g β-CrOOH与不同浓度的钒溶液混合,在pH=4,温度为65 ℃下吸附4 h,结果如图4(d)所示。从图4(d)可看出,当溶液中钒的浓度小于2.2× 10-4时,随着浓度的升高,吸附容量快速增加,原因是低浓度产生的浓度驱动力较大,从而导致吸附容量增加。当钒浓度高于2.2×10-4时,钒浓度升高对β-CrOOH吸附容量影响不大,表明β-CrOOH对钒的吸附接近饱和。
2.3 β-CrOOH吸附钒动力学
吸附动力学是用来描述吸附剂对金属离子的吸附速率,本实验中采用拟一级与拟二级反应动力学模型对β-CrOOH吸附钒的时间与吸附量之间的关系进行拟合,确定β-CrOOH吸附钒动力学特性。所述方程如下:
拟一级动力学模型,
 (2)
(2)
拟二级动力学模型,
 (3)
(3)
式中:k1为一级反应常数,h-1;k2为二级反应常数,g/(mg·h);t为时间,h;Qm为最大吸附容量,g/mg;Q为平衡时的吸附容量,mg/g,拟合得出的各动力学参数,如表1所示。
由表1中可知,β-CrOOH吸附钒更加符合拟二级动力学模型。由拟二级动力学模型获得的理论平衡吸附量为34.28 mg/g,与实验所得的平衡吸附量32.66 mg/g吻合较好,表明吸附是动力学控制的主要步骤。
2.4 β-CrOOH吸附钒等温线
常见的吸附等温线有Langmuir和Freundlich等温线吸附模型,Langmuir吸附模型假设吸附是单分子层吸附,吸附剂的表面均匀分布着能量相同的活性中心,只要一个活性位点被占据,在同一点就不会再发生吸附[17]。Freundlich等温吸附模型是假设吸附剂对吸附质的吸附为非均一的多分子层吸附,也就是多层吸附,并且随着吸附质浓度的增加,吸附量也不断的增加[18]。
表1 β-CrOOH吸附钒拟一级和拟二级动力学参数
Table 1 Adsorption rate constant obtained from pseudo-first-order model and pseudo-second-order model of V ions on CrOOH

Langmuir方程表达式为
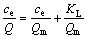 (4)
(4)
Freundlich方程表达式为
 (5)
(5)
式中:Q为吸附剂的吸附容量,mg/g;Qm为吸附剂的最大吸附容量,mg/g;ce为吸附平衡时溶液中V5+的浓度,mg/L;KL为Langmuir吸附平衡常数,L/mg;KF为Freundlich吸附系数;n为Freundlich常数。
根据式(4)、(5)分别对实验数据进行线性拟合,结果如表2所示。
表2 β-CrOOH吸附钒的Langumir和Freudlich吸附等温线拟合参数
Table 2 Langmuir and Freundlich parameters for V5+ ion adsorption onto β-CrOOH

从表2中可以看出,β-CrOOH吸附钒更加符合Langmuir等温线模型。这就说明,β-CrOOH吸附钒是单分子层吸附,且被吸附的离子之间无相互作用。在Freundlich等温线模型中,一般认为0.1<1/n<0.5是易于吸附,1/n>2时则难于吸附,由表2中可以看出,1/n=0.479,小于0.5,表明β-CrOOH对钒的吸附容易进行。
2.5 β-CrOOH吸附钒的机理研究
图5所示为β-CrOOH与β-CrOOH吸附V5+之后的FT-IR光谱图。从β-CrOOH的红外光谱图中可以看出,1625 cm-1的吸收峰代表水分子弯曲振动,1379 cm-1的吸收峰为—OH的变形振动,1089 cm-1处的吸收峰为(HO)—Cr=O的非对称弯曲振动,而793 cm-1和547 cm-1处的吸收峰为Cr=O的弯曲振动和伸缩振 动[19];通过与β-CrOOH的红外光谱对比,发现β-CrOOH吸附V5+之后在1040 cm-1出现了一个吸收峰,单独的钒酸根单体拥有一个V=O键和3个V—O键,其红外吸收峰的位置分别在1016~1040 cm-1、840~940 cm-1。因此,β-CrOOH-V在1040cm-1出现的吸收峰表明V=O键与β-CrOOH结合,形成内球型配位[20]。
为进一步研究β-CrOOH吸附钒的机理,采用XPS分析技术观测了β-CrOOH吸附钒前后表面各元素的变化,结果如图6所示。由图6可知,Cr在结合能577.3 eV 和587.3 eV出现了两个峰,表明Cr在β-CrOOH中的化学价态仍为Cr(Ⅲ),未发生化学价态变化[21]。当β-CrOOH吸附钒之后,V 2p1/2(结合能为517.1 eV)和V 2p3/2(结合能为524.8 eV)出现,表明表面吸附现象发生。无论吸附前后,均可观察到O 1s特征峰,(结合能为530.4~533.4 eV)。

图5 β-CrOOH与吸附V5+之后的β-CrOOH红外图谱
Fig. 5 FT-IR spectra of CrOOH andβ-CrOOH after V5+ adsorption
一般来说,O 1s峰一般由O2-(530.4~530.6 eV),OH-(531.5~532.0 eV)以及自由水(532.8~533.4 eV) 3个峰组成。采用Guass函数拟合O 1s峰,结果如图7和表2所示。β-CrOOH在吸附钒前,表面O2-的含量为22.35%,OH-的含量为77.65%。吸附钒之后表面O2-含量增加到了51.02%,而OH-含量却减少到了48%。比较吸附前后元素质量比可知,在β-CrOOH在吸附钒之前,m(Cr):m(O):m(V)为1:2.373:0,而在吸附钒之后,m(Cr):m(O):m(V)为1:2.96:0.08,表明β-CrOOH吸附钒之后,表面O元素的含量增加。
根据FT-IR和XPS数据结果可以推断出,在β-CrOOH 吸附钒的过程中,β-CrOOH表面释放出氢氧根来形成不饱和的Cr6+活性中心,从而导致β-CrOOH表面的OH-(531.5~532.0 eV)含量下降,O2-(530.4~530.6 eV)的含量上升。而溶液中的离子通过V=O与不饱和的Cr6+活性中心结合,形成内球型配位,从而吸附钒,并导致β-CrOOH吸附钒之后表面O元素的含量上升。
2.6 β-CrOOH脱除铬中和液中的钒
为考察β-CrOOH对钒铬的竞争吸附,将0.2g的 β-CrOOH放置到50 mL钒铬浓度各为500 mg/L的混合溶液中,在温度为65 ℃,pH=4的条件下吸附4 h。通过检测溶液中钒铬的浓度,计算出β-CrOOH对钒铬的吸附容量分别为31.79 mg/g和 1.74 mg/g。将吸附完之后的β-CrOOH放置到200 mL浓度为3 mol/L的NaOH溶液中,在80 ℃下搅拌3 h,然后测量溶液中钒铬的浓度并计算钒铬的解吸率。计算结果表明,钒的解吸率达到了92.25%,而铬的解吸率达到了100%。

图6 β-CrOOH吸附钒前后表面的XPS全扫描图
Fig. 6 XPS spectra of β-CrOOH before(a) and after((b)-(d)) V adsorption

图7 CrOOH吸附V前后O 1s峰解析谱图
Fig. 7 O 1s spectra of CrOOH before and after V adsorption
表3 β-CrOOH吸附钒前后O 1s峰的化学结构分析
Table 3 Functional group analysis of O 1s peak for β-CrOOH and β-CrOOH after V adsorption
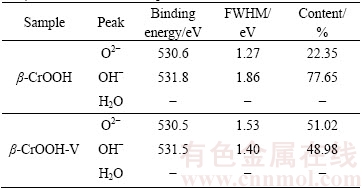
表4 β-CrOOH吸附钒前后表面元素组成
Table 4 Element of surface for β-CrOOH and β-CrOOH after V adsorption

在典型的铬铁矿无钙焙烧中和液(Na2CrO4- NaVO3-H2O)中,铬的浓度为80~100 g/L(以Cr计),钒的浓度为0.6~1.0 g/L(以V计)。为了模拟真实的铬铁矿无钙焙烧中和液体系,在实验室中准确配制铬浓度为90 g/L(以Cr计),钒浓度为0.8 g/L(以V计)的钒铬混合溶液,并将pH值调到4后备用。
准确称取一定量的纳米花状的β-CrOOH放置到20 mL配制的铬铁矿无钙焙烧中和液(Na2CrO4- NaVO3-H2O)体系中,在温度65 ℃的条件吸附4 h。吸附完毕后过滤,并用1 L 65 ℃的热水冲洗滤渣。将洗液与滤液混合,并通过测量溶液中钒的浓度来计算β-CrOOH在高铬低钒体系中对钒的脱除效率。然后将吸附完毕的β-CrOOH放置到80 ℃、3 mol/L的NaOH溶液中搅拌3 h,检测解吸液中铬的浓度,从而计算β-CrOOH对铬的脱除率。计算结果如表5所示。
表5 β-CrOOH用量对Na2CrO4-NaVO3 体系中V5+和Cr6+的脱除率的影响
Table 5 Effect of β-CrOOH on removal of V5+ in Na2CrO4- NaVO3 system

从表5中可以看出,β-CrOOH在真实的铬铁矿无钙焙烧中和液(Na2CrO4-NaVO3-H2O)中对钒具有很好的脱除效果。并且随着吸附剂β-CrOOH添加量的增加,V5+的脱除效果也越来越好,当β-CrOOH的添加量增加到2 g时,中和液中V5+的脱除率达到了91.57%,而铬的损失率为0.42%。在铬盐生产中,传统的除钒方法是通过加入过量的CaO(理论添加量的10~13倍)生成钒酸钙沉淀,此法能将90%左右的钒脱除掉,但铬的损失率却达到了20%[2]。由此可以看出,利用β-CrOOH可在不引进Ca2+的同时,将铬铁矿无钙焙烧中和液中的V5+有效脱除掉,并且铬损失率可以控制在0.42%左右,从而实现钒铬的绿色分离。
3 结论
1) 利用水热法在碱性体系中合成了纳米花状的β-CrOOH,并将其应用到溶液中钒的脱除,考察了溶液的pH值、温度、时间以及钒的初始浓度对β-CrOOH吸附钒能力的影响。研究表明,在温度为65 ℃,在pH=4条件下,当吸附达到平衡时,β-CrOOH对V5+的最大吸附容量可达到32.66 mg/g。
2) β-CrOOH吸附钒的动力学更加符合拟二级动力学模型,其线性相关系数达到了0.981;β-CrOOH吸附钒的行为基本符合Langmuir等温线模型,表明β-CrOOH吸附钒为单分子层吸附。
3) 通过β-CrOOH吸附钒前后FT-IR和XPS分析,在β-CrOOH 吸附钒的过程中,β-CrOOH表面释放出氢氧根来形成不饱和的Cr6+活性中心,而溶液中的离子通过V=O与不饱和的Cr6+活性中心结合,形成内球型配位,从而达到吸附钒效果。
4) 将制得的β-CrOOH应用到铬铁矿无钙焙烧中和液中选择性吸附钒,对钒的脱除效果达到91.57%,避免了典型的铬酸盐生产过程中(钙化沉钒法)高致癌CaCrO4残留物的产生,从而实现了铬酸盐溶液中V5+离子的绿色脱除。
REFERENCES
[1] 李小斌, 齐天贵, 彭志宏, 刘桂华, 周秋生. 铬铁矿氧化焙烧动力学[J]. 中国有色金属学报, 2010, 20(9): 1822-1828.
LI Xiao bin, QI Tian gui, PENG Zhi hong, LIU Gui hua, ZHOU Qiu sheng. Kinetics of chromite ore in oxidation roasting process[J]. The Chinese Journal of Nonferrous Metals, 2010, 20(9): 1822-1828.
[2] 赵东峰, 田 侣, 丁瑞锋, 刘桂华, 周秋生, 李小斌, 彭志宏. 铬酸钠碱性液中加石灰除钒[J]. 中国有色金属学报, 2011, 21(12): 3162-3168.
ZHAO Dong-feng, TIAN Lu, DING Rui-feng, LIU Gui-hua, ZHOU Qiu-sheng, LI Xiao-bin, PENG Zhi-hong. Vanadate removal from alkaline sodium chromate solution by adding lime[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(12): 3162-3168.
[3] 杨得军, 王少娜, 陈晓芳, 郑诗礼, 李世厚. 铬盐无钙焙烧工艺铬酸钠中性液铁盐除钒[J]. 中国有色金属学报, 2014, 24(1): 279-285.
YANG De-jun, WANG Shao-na, CHEN Xiao-fang, ZHENG Shi-li, LI Shi-hou. Removing vanadium from sodium chromate neutral liquid by non-calcium roasting technology with chromium salt[J] . The Chinese Journal of Nonferrous Metals, 2014, 24(1): 279-285.
[4] 赵 倩, 张 戈. 改性沸石静态吸附钒的实验研究[J]. 实验室研究与探索, 2010, 29(7): 31-33.
ZHAO Qian, ZHANG Ge. Experimental study on adsorption about Vanadium using modified zeolite[J]. Research and Exploration in Laboratory, 2010, 29(7): 31-32.
[5] 沈 妮, 林 晓, 曹宏斌, 郭 奋, 张 懿. 离子交换树脂脱除高浓磷酸钠溶液中的钒(Ⅴ)和铬(Ⅵ)[J]. 过程工程学报, 2009, 9(5): 871-876.
SHEN Ni, LIN Xiao, CAO Hong-bin, GUO Fen, ZHANG Yi. Removal of vanadium (Ⅴ) and chromium (Ⅵ) from high concentration sodium phosphate solution with ion exchange resins[J]. The Chinese Journal of Process Engineering, 2009, 9(5): 871-876.
[6] LI Ping, ZHENG Shi-li, QING Peng-hui, CHEN Yong-an, LEI Tian, ZHENG Xiao-dan, ZHANG Yi. The vanadate adsorption on a mesoporous boehmite and its cleaner production application of chromate[J]. Green Chemistry, 2014, 16(9): 4214-4222.
[7] 梁书婷, 张红玲, 雒敏婷, 刘红霞, 白玉兰, 徐红彬, 张 懿. 热分解CrOOH制备具有高近红外反射率的Cr2O3颜料[J]. 中国有色金属学报, 2015, 25(8): 2646-2652.
LIANG Shu-ting, ZHANG Hong-ling, LUO Min-ting, LIU Hong-xia, BAI Yu-lan, XU Hong-bin, ZHANG Yi. Preparation of Cr2O3-based pigments with high NIR reflectance via thermal decomposition of CrOOH[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(8); 2646-2652.
[8] 雷细平. 镁合金沉积耐蚀金属镀层机理及工艺研究[D]. 长沙: 湖南大学, 2012.
LEI Xi-ping. Study on mechanism and technology of deposited metal plating on magnesium alloy deposition[D]. Changsha: Hunan University, 2012.
[9] CHRISTENSEN A N, HANSEN P, LEHMANN M S. Isotope effects in the bonds of β-CrOOH and β-CrOOD[J]. Journal of Solid State Chemistry, 1976, 19(3): 299-304.
[10] FUJIHARA T, ICHIKAWA M, GUSTAFSSON T, OLOVSSON I, TSUCHIDA T. Powder neutron diffraction studies on CrOOH- type zero-dimensional H-bonded crystals[J]. Ferroelectrics, 2001, 259(1):133-138.1.
[11] KNAAK J F. γ-CrOOH fluorination catalysts: US Patent 3992325[P]. 1976-11-16.
[12] CHRISTENSEN A N, LARSEN E, MURTO J. Hydrothermal preparation and magnetic properties of alpha-CrOOH, beta- CrOOH, and gamma-CrOOH[J]. Acta Chemica Scandinavica, 1976, A30(2): 133-136.
[13] ABECASSIS-WOLFOVICH M, ROTTER H, LANDAU M V, KORIN E, ERENBURG A I, MOGILYANSKY D, GARTSTIN E. Texture and nanostructure of chromia aerogels prepared by urea-assisted homogeneous precipitation and low-temperature supercritical drying[J]. Journal of Non-crystalline Solids, 2003, 318(1): 95-111.
[14] LIANG Shu-ting, ZHANG Hong-ling, LUO Min-ting, LUO Ke-jun, LI Ping, XU Hong-bin, ZHANG Yi. Colour performance investigation of a Cr2O3 green pigment prepared via the thermal decomposition of CrOOH[J]. Ceramics International, 2014, 40(3): 4367-4373.
[15] LIU Qing, GAO Jia-jian, GU Fang-na, LU Xiao-peng, LIU You-jun, LI Hui-fang, ZHONG Zi-yi, LIU Bin, XU Guang-wen, SU Fa-bing. One-pot synthesis of ordered mesoporous Ni-V-Al catalysts for CO methanation[J]. Journal of Catalysis, 2015, 326: 127-138.
[16] WEI Guang-ye, QU Jing-kui, YU Zhi-hui, LI Yong-li, GUO Qiang, QI Tao. Mineralizer effects on the synthesis of amorphous chromium hydroxide and chromium oxide green pigment using hydrothermal reduction method[J]. Dyes & Pigments, 2015, 113: 487-495.
[17] 李小燕, 刘义保, 花明, 李金轩, 高 柏. 改性玉米芯吸附溶液中U(Ⅵ)的热力学特征[J]. 中国有色金属学报, 2013, 23(4): 1168-1172.
LI Xiao-yan, LIU Yi-bao, HUA Ming, LI Jin-xuan, GAO Bai. Adsorption thermodynamic characteristics of U(Ⅵ) on modified corncob in aqueous solution[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(4): 1168-1172.
[18] MANOHAR D M, NOELINE B F, ANIRUDHAN T S. Removal of vanadium(Ⅳ) from aqueous solutions by adsorption process with aluminum-pillared bentonite[J]. Industrial & Engineering Chemistry Research, 2005, 44(17): 6676-6684.
[19] SHPACHENKO A K, SOROKHTINA N V, CHUKANOV N V. Genesis and compositional characteristics of natural γ-CrOOH[J]. Geochemistry International, 2006, 44(7): 681-689.
[20] KAVAKLI PA, SEKO N, TAMADA M. Adsorption efficiency of a new adsorbent towards uranium and vanadium ions at low concentrations[J]. Separation Science and Technology, 2005, 39(7): 1631-1643.
[21] GROSS T, TREU D, KEMNITZ E. Characterization of Cr(Ⅲ) compounds of O, OH, F and Cl by XPS[J]. Surface Science Spectra, 2008, 15(1):77-123.
Synthesis of hydroxyl chromium oxide and its application in removal of vanadium from chromate solution
CHEN Xin1, 2, ZHENG Shi-li2, ZHANG Hai-lin2, CUI Wen-wen2, WANG Shu-lei2, LI Ping2, ZHANG Yi1, 2
(1. National Engineering Research Center of Distillation Technology, School of Chemical Engineering, Tianjin University, Tianjin 300350, China;
2. National Engineering Laboratory for Hydrometallurgical Cleaner Production Technology, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China)
Abstract: A guyanaite (β-CrOOH) with nanometer flower morphology synthesised by hydrothermal precipitation method and vanadate (V5+) ion adsorption on the β-CrOOH were investigated. In the alkaline system, the synthetic specific surface area of the β-CrOOH is 174.882 m2/g, the pore volum is 0.602 cm3/g. The β-CrOOH possesses the maximum V5+ ion adsorption capacity of 32.66 mg/g with pH=4 at 65 ℃. The adsorption mechanism results show that the β-CrOOH liberated surface hydroxyls to form coordinatively unsaturated Cr6+ centres, which further adsorbs V5+ ions through connecting mono-oxo, V=O terminal double bonds with oxygen of the Cr6+ centres. In the cleaner production application of chromate, the β-CrOOH is placed in the Na2CrO4-NaVO3-H2O solutions to selectively adsorb the V5+ ions, and 91.57% vanadium could be effectivly removed and Cr6+ is rarely adsorbed, realizing effective separation of vanadium and chromium.
Key words: β-CrOOH; separation; chromate solution; vanadium; hydrothermal method; selective adsorption
Foundation item: Projects(51574212, U1403195) supported by the National Natural Science Foundation of China
Received date: 2017-05-03; Accepted date: 2017-07-15
Corresponding author: LI Ping; Tel: +86-13718635790; E-mail: lipinggnipil@ipe.ac.cn
(编辑 龙怀中)
基金项目:国家自然科学基金资助项目(51574212, U1403195)
收稿日期:2017-05-03;修订日期:2017-07-15
通信作者:李 平,副研究员,博士;电话:13718635790;E-mail: lipinggnipil@ipe.ac.cn