
Preparation and properties of nano-CeO2/Zn composites
WANG Qian(王 乾)1, 2
1. Department of Die, Changzhou Institute of Light Industry Technology, Changzhou 213164, China;
2. Graduate School of Southeast University, Nanjing 210096, China
Received 15 July 2007; accepted 10 September 2007
Abstract: The nano-composite powders of CeO2/Zn were prepared with high energy ball milling and the nano-composite materials of CeO2/Zn were fabricated with vacuum sintering powder metallurgy. Meanwhile, the composite and structure were analyzed by the means of XRD and FESEM. From the comparison of different nano-CeO2 contents composites, the best corrosion resistance and hardness, and the optimum content of nano-CeO2 were achieved. The result shows that corrosion resistance, hardness and uniformity of metal structure can be improved significantly with nano-CeO2; at the same time, the optimal corrosion resistance, hardness and microstructure are obtained when the mass fraction of nano-CeO2 is 1%.
Key words: high energy ball milling; CeO2; nanocomposite; corrosion resistance; hardness
1 Introduction
The nanocomposite is the advanced subject of the research in high performance composite material. The performance of nanocomposite material is better than that of normal composite material[1-2]. Consequently, preparation of nanocomposite material is a valid method to obtain high performance material. Rare earth elements have a special function in improving material performance, especially corrosion resistance[3-5]. The improvement of corrosion resistance by rare earth elements added to pure Zn has been the purpose of studies by HINTON and WILSON firstly in 1980s[6]. It is suggested that the CeCl3 of 1.0 g/L can make the corrosion velocity of the pure zinc as low as 1/10 of original, and that of the electroplating zinc as low as 1/2 of original. After the corrosion experiments, there is a layer of yellow film on the surface of both the pure Zn and electroplating zinc. And then HINTON[7] studied the composition and structure of the film ulteriorly, and discovered that the main compositions in the film were CeO2 and Zn. In this work, the nano-CeO2 and Zn powder were used to prepare nano-composite materials of CeO2/Zn, and the performance was studied.
2 Experimental
2.1 Preparation of nano-CeO2/Zn composite powders with high energy ball milling
The homogenization of nano-CeO2/Zn composite materials prepared by vacuum sintering powder metallurgy were decided by the granularity difference of the two kinds of powders, nano-CeO2 and Zn[8]. The grain size of nano-CeO2 powder was so different from pure Zn powder that the homogenization becomes worse. And the nano-CeO2 particles are reunited in the compound based on Zn, so it can hardly carry out the homogenization of the material. To solve this problem, the key is to process nano-CeO2/Zn composite powders. Consequently, the composite powder was prepared by high energy ball milling[9-11], the nano-CeO2 particles were evenly distributed in the compound based on Zn, and the microstructure and organization were discussed.
The starting materials used in this study were Zn powders of 99.0% purity with a particle size range of 47-100 μm and CeO2 powder of 99.5% purity with a mean size of 30 nm. A water-cooled horizontal ball mill with the rotation speed of 15.18 and steel balls of 3, 6 and 10 mm in diameters with ratio of 3?6?10 were employed. Zn and CeO2 powders with mass ratio of 8?1 were added to the ball mill. The ball-to-powder mass ratio was selected to be 30?1. Pumping was repeated for three times to achieve a vacuum of 100 Pa, followed by argon gas charging. The powders were milled for 120 min[12-13].
2.2 Preparation of nano-CeO2/Zn composite blocks by vacuum sintering powder metallurgy
The cylindrical specimens were produced by hot-press sintering at 40 MPa and 370 ℃ for 2 h under vacuum[14-15]. The hot pressing parameters were as follows (values in parentheses are CeO2 mass fractions): Zn-CeO2 (0.1%): 9 t, 2 h, 370 ℃, 6 ℃/min; Zn-CeO2(1%): 9 t, 2 h, 370 ℃, 6 ℃/min; Zn-CeO2(5%): 9 t, 2 h, 370 ℃, 6 ℃/min.
2.3 Microstructure evaluation of nanocomposite materials
X-ray diffraction(XRD) patterns were obtained at a scanning rate of 2(?)/min by using a fully automated diffractometer (Model Bruker D8 ADVANCE, Germany) with Cu Kα (0.154 06 nm) radiation. A scanning electron microscopy (SEM) (Model LEO 1530 VP, Germany) coupled with energy dispersive X-ray spectrum (EDS) was used to investigate the morphology and the element distribution of the composites.
2.4 Corrosion resistance test
The effect of CeO2 nanoparticles on the corrosion resistance was measured. And two kinds of corrosive media were used.
1) The specimens were soaked in the spray tank full of 3.5% NaCl. After corroded for 36 h, they were rinsed, dried and weighted.
2) The specimens were soaked in 0.5 mol/L MgSO4 solution (pH=1) at room temperature. After corroded for 0.5 h, they were rinsed, dried and weighted. The process was repeated six times, and the total corrosion time was 3 h.
The corrosion resistance was evaluated by the mass loss per area, ?m/S (?m is the mass loss and S is the surface area).
2.5 Hardness test
The hardness of specimens was measured by a Rockwell hardness tester.
3 Results and discussion
3.1 Corrosion resistance
The corrosive results of the specimens on salt-spray condition are shown in Fig.1. It can be seen that, when the mass fraction of nano-CeO2 is 1% the corrosion resistance is the best. With further increase of nano-CeO2 to 5%, the corrosion resistance reduces.
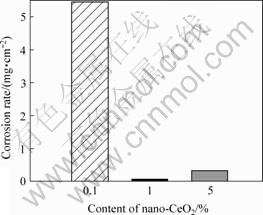
Fig.1 Corrosion rates of samples in salt-spray corrosion
The corrosive results of the specimens in MgSO4 solution are shown in Fig.2. The similar results are found to those in salt-spray corrosion.
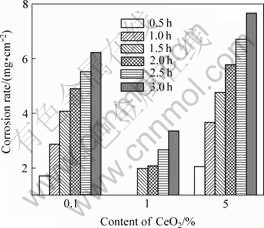
Fig.2 Corrosion rates of samples in 0.5 mol/L MgSO4 with pH=1.0
3.2 Hardness performance
The Rockwell hardness of samples is shown in Fig.3. The hardness of the specimen with nano-CeO2 content of 0.1% is lower. When nano-CeO2 is increased to 1%, the hardness is the highest. With further increasing the content of nano-CeO2 to 5%, the hardness reduces.
The addition of nano-CeO2 can increase the hardness of the material based on Zn, due to the fact that the nano-CeO2 particles are very fine and they are distributed homogeneously on the Zn matrix. It engenders the dispersion strengthening of the matrix metal obviously. But an excessive amount of nano-CeO2 is reunited and unevenly distributed, resulting in the reduction of hardness.
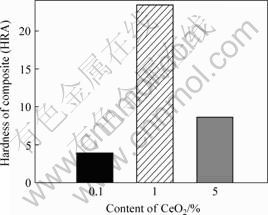
Fig.3 Hardness (HRA) of samples
3.2 Analysis of organization structure
The XRD patterns of nano-CeO2/Zn composite are shown in Fig.4. It can be seen that except a small amount of zinc oxidized because of operational reasons, there is no new alloy phase, which indicates the nano-composite materials of CeO2/Zn are gained.
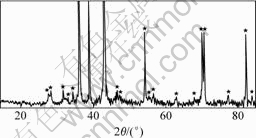
Fig.4 XRD pattern of nano-CeO2/Zn composite
The SEM micrograph for the powders milled for 120 min is shown in Fig.5. The nano-CeO2 particles distribute either on the surface of Zn micro-flake or around the peripheral layer of Zn particle. The Zn particles become small obviously, with the CeO2 particles in a nano size.
The SEM micrographs of sintered nano-CeO2/Zn composite is shown in Fig.6. Fig.6(a) shows that the grains of the bulk are fine and the structure is dense. Fig.6(b) shows that the pores distribute homogeneously. This indicates that the sintered material has an uniform structure and steady performance.
After sintering, a few of CeO2 particles dissolve into the Zn grains, which makes the structure more even and denser, the cell lattice increased, the grains refined and the preferred orientation promoted to single-crystal plane in the crystallization process. This is a reason of the corrosion resistance increasing of nano-composite of CeO2/Zn.
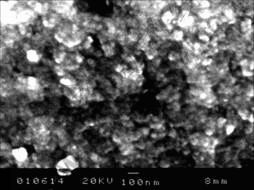
Fig.5 SEM micrograph of milled CeO2/Zn powder
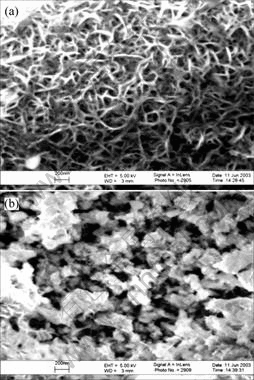
Fig.6 SEM micrographs of sintered nano-CeO2/Zn composite
4 Conclusions
1) High energy ball milling and vacuum sintering powder metallurgy are effective techniques for preparing nano-composite of CeO2/Zn. The XRD pattern and FESEM micrograph show that there is no new alloy phase, and the products have uniform structure and steady performance.
2) The corrosion resistance, hardness and uniformity of metal structure can be improved significantly with nano-CeO2, the optimal corrosion resistance, hardness and microstructure are obtained when the mass fraction of nano-CeO2 is 1%.
References
[1] WANG Xiao-yan, ZHAO Yu-tao, DAI Qi-xun, LI Gui-rong, ZHANG Hong-jie. Research development on preparation, property and application of block mtal matrix nanocomposites[J]. Materials Review, 2005, 19(F11): 186-190.
[2] HUANG Xin-ming, WU Yu-cheng, ZHENG Yu-chun. Effect of nanometer particles on properties of electroless composite coatings[J]. Ordnance Material Science and Engineering, 1999, 22(6): 11-14.
[3] LUO Xin-yi, HE Jian-ping, ZHU Zheng-hou. Effect of ceria nanoparticles on corrosion resistance of electrodeposited zinc coating[J]. Materials Protection, 2003, 36(1): 1-4.
[4] LI Shi-jia, HE Jian-ping, SUN Li. The effect of rare earth elements on corrosion resistance of electrodeposited zinc coating[J]. Materials Protection, 1991, 24(3): 4-10.
[5] YU Sheng-xue, XIA Yuan, YAO Mu. Effect of rare earth element cerium on properties and microstructure of hot dip aluminum coating on Q235 steel[J]. Materials For Mechanical Engineering, 2006, 30(6): 77-79.
[6] HINTON B R W, WILSON L. The corrosion inhibition of zinc with cerous chloride[J]. Corrosion Science, 1989, 29(8): 967-975.
[7] HINTON B R W. Corrosion inhibition with rare earth metal salts[J]. Journal of Alloys and Compounds, 1992, 180(1/2): 15-25.
[8] DENG Shu-hao, GONG Zhu-qing, CHENG Wen-gu. Research status and development of nano-material electrodeposition[J]. Electroplating and Finishing, 2001, 20(4): 35-39.
[9] MANNA I, CHATTOPADHYAY P P, BANHART F, FECHT H J. Development of amorphous and nanocrystalline Al65-Cu35-xZrx alloys by mechanical alloying[J]. Materials Science and Engineering A, 2004, 379(1/2): 360-365.
[10] DEGMOVA J, ROTH S, ECKERT J, GRAHL H, SCHULTZ L. Magnetic properties of bulk amorphous FeAlGaPCBSi samples prepared by ball milling and subsequent hot pressing[J]. Materials Science and Engineering A, 2004, 375/377: 265-269.
[11] SHERIF EL-ESKANDARANY M, BAHGAT A A, GOMAA N S, EISSA N A. Kinetics and formation mechanism of amorphous Fe52Nb48 alloy powder fabricated by mechanical alloying[J]. Journal of Alloys and Compounds, 1999, 290(1/2): 181-190.
[12] LU Xiang, ZHU Zheng-hou, LUO Xin-yi, LI Shun-lin. Synthesization and characterization of NiO/Zn nanocomposite powders[J]. Materials for Mechanical Engineering, 2003, 27(12): 38-41. (in Chinese)
[13] LUO Xin-yi, ZUO Dun-wen, WANG Min, LI Shun-lin, YANG Wen-tao, CHANG Hua. Preparation and characterizations of CeO2/Zn nanocomposite powder by high-energy ball milling[J]. Journal of Nanjing University of Aeronautics and Astronautics, 2006, 38(6): 383-387. (in Chinese)
[14] LUO Xin-yi, ZUO Dun-wen, WANG Min, LI Shun-lin, CHANG Hua. Preparation and hot-dip galvanizing application of CeO2/Zn nanocomposite[J]. Trans Nonferrous Met Soc China, 2005, 15(s3): 203-207.
[15] CHANG Hua, LUO Xin-yi, LI Shun-lin, YANG Wen-tao. Hot-press sintering of CeO2/Zn nanocomposite powders prepared by high-energy ball milling[J]. Journal of Materials Engineering, 2006, 7: 35-38.
(Edited by YANG Bing)
Foundation item: Project (BG2001026) supported by Advanced Technology Item of Jiangsu Province, China
Corresponding author: WANG Qian; Tel: +86-519-6335160; E-mail: trumpwq@sohu.com