Trans. Nonferrous Met. Soc. China 25(2015) 3286-3290
Green synthesis of silver nanoneedles using shallot and apricot tree gum
Mohammad Mahdi TAHERI1,2, Mohammed Rafiq ABDUL KADIR2, Noor Kamila AHMAD SHAFIAI2, Tolou SHOKUHFAR3,4, Mahtab ASSADIAN1, Mostafa Rezazadeh SHIRDAR1
1. Materials Engineering Department, Faculty of Mechanical Engineering, Universiti Teknologi Malaysia, 81310 Johor Bahru, Johor, Malaysia;
2. Medical Devices & Technology Group, Faculty of Bioscience and Medical Engineering, Universiti Teknologi Malaysia, 81310 Johor Bahru, Johor, Malaysia;
3. Department of Mechanical Engineering-Engineering Mechanics, Multiscale Technologies Institute, Michigan Technological University, Houghton, MI 49931, USA;
4. Department of Bioengineering, University of Illinois at Chicago, Chicago, IL 60607, USA
Received 5 November 2014; accepted 6 May 2015
Abstract: A green synthesis method to produce silver nanoneedles was described using shallot and apricot tree gum (ATG). A fast, simple, and low cost method was used to synthesize silver with nanoparticle and nanoneedle shape from the silver nitrate solution. Shallot as a reducing agent and apricot tree gum (ATG) as a stabilizer and a capping agent were utilized to reduce and form silver ions into silver atoms with needle and particle shape. Moreover, high crystalline structures of silver nanoparticles (AgNPs) with diameters of 8-20 nm and silver nanoneedles with average diameters of 50-60 nm and lengths of 5-10 μm were consequently synthesized by shallot and the mixture of shallot and ATG. A self-assembly mechanism was proposed to indicate the formation of needle-like structures of spherical AgNPs via carbon chains of ATG. The results indicate that the presence of ATG with shallot can transfer the reduced AgNPs into the silver nanoneedle. The findings were characterized using X-ray diffraction (XRD), ultra violet visible (UV-Vis) spectrometry, field emission scanning electron microscopy (FESEM), and transmission electron microscopy (TEM) techniques.
Key words: green synthesis; silver; nanoneedle; nanoparticle; shallot; apricot tree gum; self-assembly
1 Introduction
AgNPs have received a great deal of interest and have been used in many products such as optical sensors [1], semiconductors [2], and antibacterial agents [3]. Several methods to synthesize AgNPs such as laser ablation [4], electrochemical method [5], wet-chemical [6], mechanochemical [7], and green synthesis by plant extraction [8,9] have been described. Green synthesis is environmentally friendly, cost effective and therefore most likely to be massively produced [10]. Several studies [8-10] have reported the use of natural materials such as garlic and plant leaves extract as reduction agents to form spherical AgNPs [11]. Although, different surfactant and template agents such as nano-carbon [12], risodium citrate dehydrate and potassium tartrate [13] are utilized to prevent any agglomeration of synthesized AgNPs. However, it is more desirable to have green template or surfactant agents for AgNPs synthesis.
Recently, the nanoneedle and nanowire structures of silver with its great optical and electrical properties enable many applications such as chemical and biological sensors, airborne water-soluble molecules in military sensors, SERS micro-detection devices and restorative dental materials [14-17]. YANG et al [11] reported that silver nanoneedle structures can be synthesized by using CVD, PVD, electrical sputtering and other techniques. Because complex processes are needed to obtain needle structure of silver, the use of simple, eco-friendly, and environmentally friendly techniques are necessary to synthesize the needle structure of silver. The use of certain reducing and capping agents in suitable condition are desirable to eliminate the need for these complex processes. DARROUDI et al [18] reported that certain sugars such as furostanol saponins and flavonoids are potential good reducing agents, which can be found in shallots in high quantities [19]. However, the use of shallot as a reducing agent has not been previously described.
The present study was thus conducted to synthesize AgNPs from silver nitrate using shallot as a reducing agent and ATG as a stabilizer and a capping agent. Moreover, it was illustrated that ATG contains polysaccharides; therefore, it can help the formation of silver nanoneedles from AgNPs. Characterization of the nanoneedles and AgNPs were performed using XRD, UV-Vis, FESEM, and TEM.
2 Experimental
2.1 Materials
Silver nitrate (AgNO3, 99.9%) was purchased from Sigma Aldrich Company. ATG (Prunus Armenia) was obtained from the city of Taleghan, Iran, and shallots (Allium ascalonicum) were purchased from a local market.
2.2 Shallot extraction
Shallots were peeled and washed several times using deionized water. 5 g of shallot was ground and immediately centrifuged at 6000 r/min for 2 min. 5 mL of the extracted solution was added to 10 mL of double deionized water. The concentration of the solution (35 μg/mL) was measured by evaporating 5.0 mL of the solution in a vacuum oven at 40 °C. The remaining solid particles were then weighed.
2.3 Synthesis of AgNPs
To prepare silver nitrate solution with concentration of 35 mmol/L, 0.6 g of silver nitrate was dissolved in 100 mL of double deionized water. The silver nitrate solution was then used to prepare 3 different samples: 2, 4, and 8 mL of shallot solution. The combined solutions were then stirred using a magnetic stirrer for 15 min. As time passes, the colour of solutions changed, indicating the formation of solid AgNPs. The solutions were centrifuged at 4000 r/min for 20 min to ensure no agglomerations or large particles present inside the solutions. 0.2 and 0.4 g ATG were added to the solutions using an ultrasonic device, respectively. Additionally, the sample without ATG was used as the control.
3 Results and discussion
It was reported that the shallot bulb (Allium ascalonicum) contains the flavonoids and volatile sulfur compounds while the extraction of shallot was composed of polar compounds like quercetine, isorhamnetin and glucose [19]. In addition, it was demonstrated that certain glucose, once oxidized into gluconic acid, reduced silver ions into silver atoms, which was the reason why shallot was chosen in the present study [18]. By adding extracted shallot solution as reducing agent to the silver nitride solution, the color of solution will be changed. It is directly related to the reduction of silver ions to silver atoms. As a result, the color changes of solutions were observed over time, possibly due to the change in the surface resonance of AgNPs, i.e., due to changes in the size and shape of AgNPs. Moreover, it was reported by ROSIK [20] that 80% of chemical composition of ATG contained polysaccharide which is composed of methyl-D-glucuronic acid, D-xylose, and D-galactose. The ATG which acts as a stabilizer and capping agent was added to prevent the agglomeration of AgNPs. It has been suggested that the increase in the bright yellow color indicates the decrease of obtained silver particles size [21-23].
Figure 1 demonstrates the UV-Vis result of AgNPs, which corresponds to the spectrum band of 300-570 nm. The addition of extracted shallot solution to AgNO3 solution changed the color of the solution from transparent to yellow due to the reduction of silver ions and the production of AgNPs. The existence of AgNPs also increased the excitation of surface plasmon vibration. The surface plasmon resonances peaked sharply at 320 nm, which indicated a particle size of less than 50 nm, which was also confirmed by TEM.
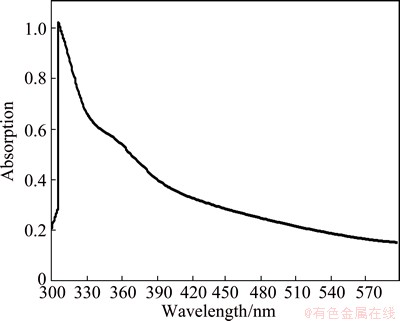
Fig. 1 UV-Vis spectrum of AgNPs
Figure 2 illustrates the X-ray diffraction pattern which demonstrates the crystallinity of AgNPs. The comparison between XRD standard patterns with the synthesized silver in this study confirmed the formation of nanocrystals, as proved by the peaks at 38°, 44°, 64° and 77° (2θ) corresponding to (111), (200), (220), and (311) planes, respectively [24]. Moreover, this pattern illustrates the face centered cubic (FCC) crystal structure of the AgNPs. In addition, sharp peaks of AgNPs reveal that AgNPs are highly crystallized, with diameters of 5-15 nm. The average size of AgNPs crystallite was calculated by using Debye-Scherrer equation [25,26]:
D1/4λ=βcos θ (1)
where D is the average crystallite size, λ is the wavelength of the X-ray (0.1541 nm for Cu Kα), β is the full width at half maximum of peak, K is the Scherrer constant (0.9-1), and θ is the Bragg angle.
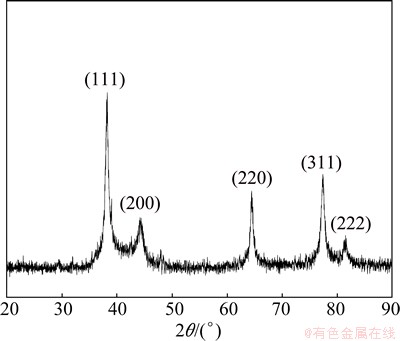
Fig. 2 XRD pattern of AgNPs
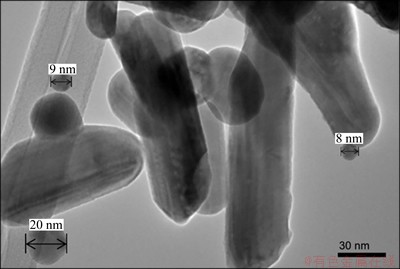
Fig. 3 TEM image of AgNPs

Fig. 4 FESEM images demonstrating nanoneedle structure of silver
Figure 3 illustrates the TEM image of the spherical silver particles of 8-20 nm in size. Figure 4 illustrates the FESEM images of the needle structure of accumulation of AgNPs. It can be seen that AgNPs have nanoneedle-like structure with diameters of 50-60 nm and lengths of 5-10 μm.
LEIGHTON et al [27] have suggested that the presence of flavonols in onion varieties and gums may be the reason for the formation needle-structures when they are used to prepare AgNPs, although the exact mechanism remains unknown. It is possible that the self-assembly formation of the silver nanoneedles observed in the present study may be due to the assembly of many spherical-shaped particles with carbon chains as a connector into the needle-shaped structure. The obtained nanoneedles also may be the final product of a conversion of spherically-shaped particles to a columnar-shape structure which was then collectively connected by carbon chains to form a needle-shaped structure. In both cases, we can therefore suggest that the hydrogen bonding formation between carbon chains may be a key factor in the formation of needle-like structures (Fig. 6), which has also been suggested in previous studies [28].
As shown in Fig. 5, the absence of ATG as capping agent in the control sample causes the AgNPs to agglomerate. The agglomeration of AgNPs results in large silver spheres. It is illustrated that the presence of ATG may prevent the AgNPs from agglomerating and it can provide a suitable condition to form nanoneedle structure of silver. The mechanism of ATG existence as stabilizing agent while the shallot was used as reducing agent is demonstrated in Fig. 6. It can be seen that by adding shallot as reducing agent to the silver nitride solution, the silver ions transferred to the solid silver atoms. It is interpreted that the first nucleation of solid silver atoms tends to connect to the other transferred silver atoms which create agglomerated particles of silver. This may be due to the proper position of silver nucleation on the solid silver atoms. However, in the presence of ATG, it is mechanistically illustrated that ATG initially prevents agglomeration of silver atoms and then plays a capping agent rule which is described in Fig. 7. It is supposed that the ATG surrounds the particles of solid silver atoms and prevents any further agglomeration.

Fig. 5 FESEM image of AgNPs reduced by shallot without adding ATG (control sample)
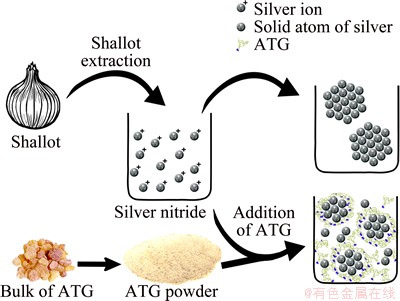
Fig. 6 Suggested mechanism of silver nitride reduction by shallot reduction with ATG as stabilizing and capping agent
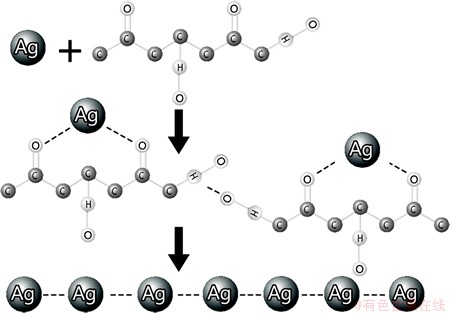
Fig. 7 Suggested mechanism of AgNPs self-assembly for formation of silver nanoneedles
As can be seen in Fig. 7, the organic carbon chain plays a major role in the formation of nanoneedles. The formation may be described by the intermolecular force bonding between silver and oxygen atoms. This electrostatic bonding leads to a connection between particles for the formation of nanoneedles.
4 Conclusions
Nanoneedle structure of AgNPs can be obtained via a green synthesis method using shallot as a reducing agent and ATG as capping agent without the need of complex processes. The technique produced not only AgNPs of highly crystalline spherical shaped particles of 8-20 nm, but also nanoneedle structures with diameters of 50-60 nm and lengths of 5-10 μm. This was achieved by applying 8 mL of shallot solution and 0.2 g of ATG as capping agent at 35 μg/mL concentration with 35 mmoL of silver nitrate.
Acknowledgement
This study was supported by the international doctoral fellowship (IDF) scheme from Universiti Teknologi Malaysia. The authors would like to thank Mr. Abolfazl MIRJALILI from the Material and Energy Research Centre of Iran for providing the TEM analysis.
References
[1] MCFARLAND A D, van DUYNE R P. Single silver nanoparticles as real-time optical sensors with zeptomole sensitivity [J]. Nano Letters, 2003, 3: 1052-1057.
[2] COZZOLI P D, COMPARELLI R, FANIZZA E, CURRI M L, AGOSTIANO ALAUB D. Photocatalytic synthesis of silver nanoparticles stabilized by TiO2 nanorods: A semiconductor/metal nanocomposite in homogeneous nonpolar solution [J]. Journal of the American Chemical Society, 2004, 126: 3868-3879.
[3] SONDI ISALOPEK-SONDI B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for gram-negative bacteria [J]. Journal of Colloid and Interface Science, 2004, 275: 177-182.
[4] PYATENKO A, SHIMOKAWA K, YAMAGUCHI M, NISHIMURA OSUZUKI M. Synthesis of silver nanoparticles by laser ablation in pure water [J]. Applied Physics A, 2004, 79: 803-806.
[5] YIN B, MA H, WANG SCHEN S. Electrochemical synthesis of silver nanoparticles under protection of poly (N-vinylpyrrolidone) [J]. The Journal of Physical Chemistry B, 2003, 107: 8898-8904.
[6] ZHAI Ai-xia, CAI Xiong-hui, JIANG Xiao-ye, FAN Guo-zhi. A novel and facile wet-chemical method for synthesis of silver microwires [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(4): 943-948.
[7] KHAYATI G RJANGHORBAN K. Preparation of nanostructure silver powders by mechanical decomposing and mechanochemical reduction of silver oxide [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(5): 1520-1524.
[8] KARUPPIAH MRAJMOHAN R. Green synthesis of silver nanoparticles using ixora coccinea leaves extract [J]. Materials Letters, 2013, 97: 141-143.
[9] KOUVARIS P, DELIMITIS A, ZASPALIS V, PAPADOPOULOS D, TSIPAS S AMICHAILIDIS N. Green synthesis and characterization of silver nanoparticles produced using arbutus unedo leaf extract [J]. Materials Letters, 2012, 76: 18-20.
[10] SHARMA V K, YNGARD R, ALIN Y. Silver nanoparticles: Green synthesis and their antimicrobial activities [J]. Advances in Colloid and Interface Science, 2009, 145: 83-96.
[11] YANG Y, LI Z Y, YAMAGUCHI K, TANEMURA M, HUANG Z, JIANG D, CHEN Y, ZHOU FNOGAMI M. Controlled fabrication of silver nanoneedles array for sers and their application in rapid detection of narcotics [J]. Nanoscale, 2012, 4: 2663-2669.
[12] ZHENG Min, WANG Zuo-shan, ZHU Ya-wei. Preparation of silver nanoparticle via active template under ultrasonic [J]. Transactions of Nonferrous Metals Society of China, 2006, 16(6): 1348-1352.
[13] YANG Yan, CHEN Ke-bin, LI Hao-ran, WEI Hong, HU Xiao-bin, ZHOU Xu. Capping effect of reducing agents and surfactants in synthesizing silver nanoplates [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(11): 3732-3738.
[14] RAJENDRAN A, VINOTH G, SHANTHI V, BARIK RPATTANAYAK D. Silver nano particle incorporated Ti metal prepared by chemical treatment for antibacterial and corrosion resistance study [J]. Materials Technology: Advanced Biomaterials B, 2014, 29: 26-34.
[15] VERNE E, FERRARIS S, MIOLA M, FUCALE G, MAINA G, ROBOTTI P, BIANCHI G, MARTINASSO G, CANUTO RVITALE-BROVARONE C. Synthesis and characterisation of bioactive and antibacterial glass-ceramic. Part 2: Plasma spray coatings on metallic substrates [J]. Advances in Applied Ceramics, 2008, 107: 245-253.
[16]  W, SCHWEIGER M, RHEINBERGER VKAPPERT H. Bioceramics and their application for dental restoration [J]. Advances in Applied Ceramics, 2009, 108: 373-380.
W, SCHWEIGER M, RHEINBERGER VKAPPERT H. Bioceramics and their application for dental restoration [J]. Advances in Applied Ceramics, 2009, 108: 373-380.
[17] JASTRZEBSKA A, KUNICKI A, OLSZYNA AKARWOWSKA E. Al2O3-Ag nanopowders: New method of synthesis, characterisation and biocidal activity [J]. Advances in Applied Ceramics, 2011, 110: 108-113.
[18] DARROUDI M, AHMAD M B, ABDULLAH A, HIBRAHIM N A. Green synthesis and characterization of gelatin-based and sugar-reduced silver nanoparticles [J]. International Journal of Nanomedicine, 2011, 6: 569-574.
[19] FATTORUSSO E, IORIZZI M, LANZOTTI VTAGLIALATELA- SCAFATI O. Chemical composition of shallot (Allium Ascalonicum Hort.) [J]. Journal of Agricultural and Food Chemistry, 2002, 50: 5686-5690.
[20] ROSIK J. Structural features of the polysaccharide of apricot gum in dependence on the infection with fungi, application of a synthetic material and vegetative period [C]//Proceedings of the IV International Symposium on Apricots and Apricot Culture 1968. Cacak, Yugoslavia: ISHS Acta Horticulturae, 1968, 11: 523-528.
[21] RAVEENDRAN P, GOYAL A, BLATCHFORD M AWALLEN S L. Stabilization and growth of silver nanocrystals in dendritic polyol dispersions [J]. Materials Letters, 2006, 60: 897-900.
[22] ZHAO S, ZHANG K, AN J, SUN YSUN C. Synthesis and layer-by-layer self-assembly of silver nanoparticles capped by mercaptosulfonic acid [J]. Materials Letters, 2006, 60: 1215-1218.
[23] MITRA ABHAUMIK A. Nanoscale silver cluster embedded in artificial heterogeneous matrix consisting of protein and sodium polyacrylate [J]. Materials Letters, 2007, 61: 659-662.
[24] KHALIL M M, ISMAIL E H, EL-BAGHDADY K, ZMOHAMED D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity [J]. Arabian Journal of Chemistry, 2014, 7: 1131-1139.
[25] AHMAD N, SHARMA S, ALAM M K, SINGH V, SHAMSI S, MEHTA BFATMA A. Rapid synthesis of silver nanoparticles using dried medicinal plant of basil [J]. Colloids and Surfaces B: Biointerfaces, 2010, 81: 81-86.
[26] VIDHU V, AROMAL S APHILIP D. Green synthesis of silver nanoparticles using macrotyloma uniflorum [J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2011, 83: 392-397.
[27] LEIGHTON T, GINTHER C, FLUSS L, HARTER W K, CANSADO JNOTARIO V. Molecular characterization of quercetin and quercetin glycosides in allium vegetables: their effects on malignant cell transformation [C]//Proceedings of ACS Symposium Series, 1992. CA, USA: ACS, 1992: 220-238.
[28] JIANG X, YU A. One-step approach for the synthesis and self-assembly of silver nanoparticles [J]. Journal of Nanoscience and Nanotechnology, 2010, 10: 7643-7647.
利用葱和杏树胶绿色合成银纳米针
Mohammad Mahdi TAHERI1,2, Mohammed Rafiq ABDUL KADIR2, Noor Kamila AHMAD SHAFIAI2, Tolou SHOKUHFAR3,4, Mahtab ASSADIAN1, Mostafa Rezazadeh SHIRDAR1
1. Materials Engineering Department, Faculty of Mechanical Engineering, Universiti Teknologi Malaysia, 81310 Johor Bahru, Johor, Malaysia;
2. Medical Devices & Technology Group, Faculty of Bioscience and Medical Engineering, Universiti Teknologi Malaysia, 81310 Johor Bahru, Johor, Malaysia;
3. Department of Mechanical Engineering-Engineering Mechanics, MultiScale Technologies Institute, Michigan Technological University, Houghton, MI 49931, USA;
4. Department of Physics and Department of Mechanical and Industrial Engineering,
University of Illinois at Chicago, Chicago, IL 60607, USA
摘 要:使用葱和杏树胶为原料,采用一种快速、简单和低成本的绿色方法在硝酸银溶液中合成银纳米颗粒和纳米针。葱作为还原剂,杏树胶为稳定剂和覆盖剂,银离子被还原成颗粒状和针状的银原子。利用葱或葱与杏树胶混合物合成的银纳米颗粒的直径为8~20 nm,银纳米针的直径为50~60 nm,长度为5~10 μm。提出一个自组装机理来阐明球形银纳米颗粒通过杏树胶的碳链形成针状结构,通过这种方式,葱和杏树胶可将还原的银纳米颗粒转变成银纳米针。采用XRD、紫外-可见光谱、场发射电子扫描显微镜和透射电镜对所得样品进行表征。
关键词:绿色合成;银;纳米针;纳米颗粒;葱;杏树胶;自组装
(Edited by Yun-bin HE)
Corresponding author: M. R. ABDUL KADIR; Tel: +60-7-55-58514/+60-7-55-57998; Fax: +60-7-55-58515; E-mail: rafiq@biomedical.utm.my
DOI: 10.1016/S1003-6326(15)63965-6