J. Cent. South Univ. Technol. (2007)06-0759-04
DOI: 10.1007/s11771-007-0144-7
Preparation and electrical properties of BaPbO3 thin film
LU Yu-dong(陆裕东)1,2, WANG Xin(王 歆)1, ZHUANG Zhi-qiang(庄志强)1
(1. College of Materials Science and Engineering, South China University of Technology, Guangzhou 510640, China;
2. National Key Laboratory for Reliability Physics and its Application Technology of Electrical Component, the 5th Electronics Research Institute of Ministry of Information Industry, Guangzhou 510610, China)
Abstract: BaPbO3 thin films were deposited on Al2O3 substrates by sol-gel spin-coating and rapid thermal annealing. The microstructure and phase of BaPbO3 thin films were determined by X-ray diffractometry, scanning electrons microscopy and energy dispersive X-ray spectrometry. The influence of annealing temperature and annealing time on sheet resistance of the thin films was investigated. The results show that heat treatment, including annealing temperature and time, causes notable change in molar ratio of Pb to Ba, resulting in the variations of sheet resistance. The variation of electrical properties demonstrates that the surface state of the film changes from two-dimensional behavior to three-dimensional behavior with the increase of film thickness. Crack-free BaPbO3 thin films with grain size of 90 nm can be obtained by a rapid thermal annealing at 700 ℃ for 10 min. And the BaPbO3 films with a thickness of 2.5 μm has a sheet resistance of 35 Ω?□-1.
Key words: BaPbO3; thin films; sol-gel; spin-coating; electrical resistivity; heat treatment
1 Introduction
The development of conductive oxide electrodes is extensively promoted as one of the solutions to the fatigue behavior of lead zirconate titanate (PZT) ferroelectrics[1-3]. BaPbO3 is currently considered one of the most promising perovskite materials for the bottom electrode of PZT films. BaPbO3 exhibits metallic conduction with the room-temperature resistivity of 5.3×10-4 Ω·cm for bulk ceramics[4]. BaPbO3 is stable in atmosphere and oxygen at high temperature[5-7]. Lead and barium do not negatively affect the leakage current of PZT thin films when they diffuse into the ferroelectric layer[8]. Thus, BaPbO3 is considered a promising electrode material for PZT ferroelectric thin films. Various physical and chemical methods including radio- frequency sputtering, sol-gel and chemical vapor deposition have currently been used to make BaPbO3 thin films[7-9]. Among these techniques, the sol-gel technique is regarded as a powerful method to synthesize BaPbO3 films with tailored properties. Sol-gel processing offers several advantages, including low processing temperature, non-vacuum process, low cost, uniform and large area deposition.
In this work, the influences of annealing temperature and annealing time on conductivity of BaPbO3 thin films were investigated. The crack-free BaPbO3 thin films with grain size of 90 nm on Al2O3 substrate were prepared by sol-gel spin-coating and rapid thermal annealing. The best electrical properties were obtained by a rapid thermal annealing (RTA) at 700 ℃ for 10 min.
2 Experimental
The BaPbO3 thin films were synthesized via a multistep sol-gel route, and the spin-coating was employed for formation of the multilayer structures. Starting materials for the sol solution were inorganic compounds Ba(OH)2?8H2O (98%, mass fraction, AR grade) and Pb(CH3COO)2?3H2O (99%, mass fraction, AR grade). Citric acid (CA 99.5%, mass fraction, AR grade) and ethylene diamine tetra-acetic acid EDTA, 99.5%, mass fraction, AR grade) were used as complex chelate agent, and distilled water as solvent. The concentration of BaPbO3 in the solution was controlled from 0.5 to 0.6 mol/L. Excessive 5%(mole fraction) lead was added to the composition in order to compensate for the likely loss of lead during the annealing process. The sol solution of BaPbO3 was spin-coated onto Al2O3 substrate at 3 000 r/min for duration of 20 s. The gel films were then dried at 415 ℃ for 10 min and annealed by RTA at different temperatures of 670, 700 and 720 ℃ for 10, 20 and 30 in, respectively. Such cycle was repeated until thedesired thickness of the thin films was achieved. The process flow chart is shown in Fig.1.
The sheet resistance of the BaPbO3 thin films was measured with the four-point direct current(DC) technique. The samples were characterized by X-ray diffraction (XRD, Rigaku D/max-IIIA) using Cu Kα radiation. The microstructural characterization and the measurements of film thickness were performed with a Hitachi S550 field-emission scanning electron microscope operating at an accelerating voltage of 15 kV. An EDS study was carried out to investigate the chemical compositions of the thin films.
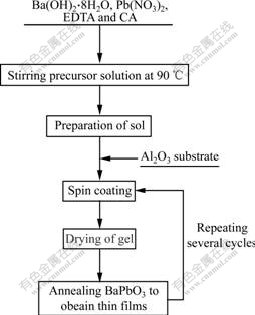
Fig.1 Flow chart of sol-gel route for preparation of BaPbO3 thin films
3 Results and discussion
Fig.2 shows the XRD pattern of the BaPbO3 thin films deposited on Al2O3 substrate and annealed at 700 ℃ for 10 min. The XRD pattern reveals that the films are polycrystalline perovskite BaPbO3 phases without preferred orientation. In addition, the films spectrum shows a small peak at 2θ=24.2?, which corresponds to the diffraction peak of BaCO3. This is due to the evaporation of lead oxide, leading to the surplus of barium during high temperature annealing treatment.
Fig.3 shows the EDS pattern of the BaPbO3 thin films annealed by RTA at 700 ℃ for 10 min. The peak energies corresponding to Ba, Pb, Al, O, and C are identified in the spectrum. No impurity element is detected in spectrum except for carbon, which is due to the surface contamination. The EDS analysis indicates that molar fractions of Ba and Pb are 18.032 % and 17.705% respectively, namely the molar ratio of Pb to Ba is 0.98?1.00. Although excessive lead of 5% was added to the composition, the molar fraction of Ba in the annealed films is still higher than that of Pb. This result is in agreement with that of the XRD analysis.
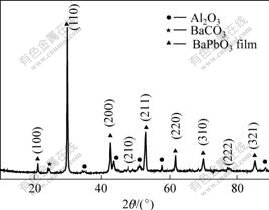
Fig.2 XRD spectrum for BaPbO3 films annealed by RTA at 700 ℃ for 10 min
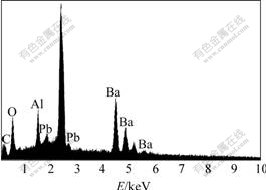
Fig.3 EDS pattern of BaPbO3 thin films annealed by RTA at 700 ℃ for 10 min
Fig.4 shows the variation of thickness as a function of spin-coating layers of the BaPbO3 thin films annealed at 670 ℃ for 10, 20 and 30 min, respectively. The film thickness was measured by the cross section SEM of films. The increase of thickness is roughly linear with the number of the spin-coating layer. For the same number of spin-coating layer, The BaPbO3 thin films with annealing time of 10 min show the largest thickness, followed by 20 and 30 min. The thickness increases with the reduction of annealing time. XRD and EDS analytical results show that the main constituent of volatile is Pb during the heat treatment. Therefore, the plot indicates that a part of films volatilize during the heat treatment and the loss of films, mainly Pb, increases with an increase in annealing time.
SEM image in Fig.5 shows the surface texture of BaPbO3 thin films by RTA at 700 ℃ for 10 min. Crack-free BaPbO3 thin films with an average grain size of 90 nm are obtained by the heat treatment mentioned above.
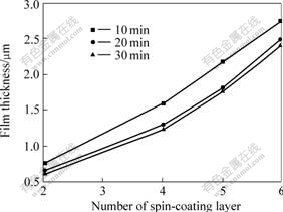
Fig.4 Dependence of thickness of BaPbO3 thin films annealed by RTA at 670 ℃ for different time on number of spin-coating layer
The sheet resistance(RS) of a thin film is given by where ρ is the resistivity of the film and l is the thickness of the film.
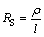 (1)
(1)
Figs.6(a)-(c) show the sheet resistance as a function of thickness of the BaPbO3 thin films annealed at 670, 700 and 720 ℃ for 10, 20 and 30 min, respectively. The sheet resistance is inversely related to the thickness of the BaPbO3 thin films in Figs.6(a) and (b). It is consistent with the relation of RS to l (Eqn.(1)). At the same time, the sheet resistance decreases with the reduction in annealing time. For the films annealed at 720℃(Fig.6(c)), the sheet resistance reduces abrupt up at first and beyond that the reduction is moderately. Fig.4 confirms that molar ratio of Pb to Ba is lower for the films annealed for longer time. The fact that resistivity increases with the reduction in molar ratio of Pb to Ba of BaPbO3 composition was reported in Ref.[5]. Therefore,the increase of sheet resistance with further annealing time is due to the evaporation of lead oxide, leading to the decrease of the molar ratio of Pb to Ba during high temperature annealing treatment. Fig.6(d) shows the sheet resistance as a function of film thickness annealed at 670, 700 and 720 ℃ for 10 min. BaPbO3 ceramics are black and stable in atmospheric condition, while the films annealed at 670 ℃ for 10 min are brown because the annealing temperature of 670 ℃ is lower to synthesize BaPbO3 composition. The sheet resistance of the BaPbO3 thin films annealed at 700 ℃ is lower than that annealed at 670 ℃. Sheet resistance is about 35 and 75 Ω·□-1 for the films with a thickness of 2.5 μm annealed at 700 and 670 ℃, respectively. For the films annealed at 670 and 700 ℃, the decrease of sheet resistance is roughly linear with annealing time. For the films annealed at 720 ℃, the sheet resistance reduces abrupt up to thickness of 1.3 μm and beyond that the reduction is moderately. This trend is consistent with all the films annealed at 720 ℃ for 10, 20 and 30 min (Fig.6(c)). This means that up to approximately 1.3 μm the films properties are highly dependent on the surface (two-dimensional behavior), while, for thicker films, resistivity is obviously dependent on the bulk properties of the materials (three-dimensional behavior). Therefore, the BaPbO3 thin films annealed at 700 ℃ for 10 min show the better sheet resistance of 35 Ω·□-1.
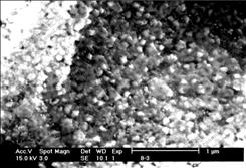
Fig.5 SEM image of BaPbO3 thin films annealed by RTA at 700 ℃ for 10 min
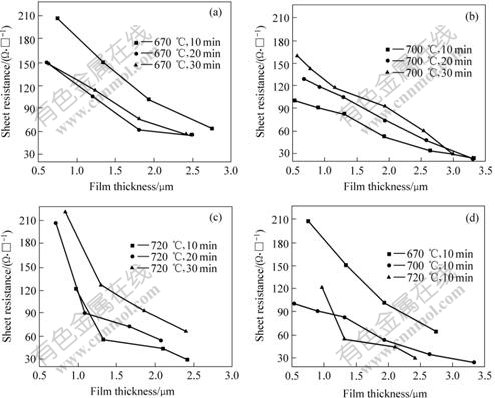
Fig.6 RS-l curves for BaPbO3 thin films annealed at different temperatures for different times
(a) 670 ℃; (b) 700 ℃; (c) 720 ℃; (d) Comparison of sheet resistance of BaPbO3 thin films annealed at 670, 700 and 720 ℃ for 10 min
4 Conclusions
1) Crack-free BaPbO3 thin films with homogeneous surfaces are prepared by a modified sol-gel route by using citric acid and EDTA as complex chelate agent and distilled water as solvent.
2) Heat treatment, including annealing temperature and time, affects the molar ratio of Pb to Ba, resulting in the variation of sheet resistance. The increase of sheet resistance with increase of annealing time is due to the evaporation of lead oxide, leading to the decrease of molar ratio of Pb to Ba during high temperature annealing treatment. And the films annealed by RTA at 700 ℃ for 10 min show the lowest sheet resistance of 35 Ω·□-1when the film thickness is 2.5 μm.
References
[1] SHANNON R D, BIERSTEDT P E. Single-crystal growth and electrical properties of BaPbO3[J]. J Am Ceram Soc, 1970, 11(53): 635-636.
[2] MOUSSA S M, KENNEDY B J, VOGT T. Structure variants in ABO3 type perovskite oxides—On the structure of BaPbO3[J]. Solid State Comm, 2001, 119: 549-552.
[3] LUO Y R, WU J M. RF-magnetron sputtered conductive perovskite BaPbO3 films[J]. Jpn J Appl Phys, 2003, 42(1): 242-246.
[4] LU Yu-dong, WANG Xin, ZHUANG Zhi-qiang. Electrical properties of sol-gel-derived Ba1-xKxPbO3 conductive ceramics[J]. Chinese Journal of Rare Metals, 2005, 29(5): 643-646. (in Chinese)
[5] WANG F, UUSIMAKI A, LEPPAVUORI S, et al. Preparation of conductive barium metaplumbate thin film using solution method[J]. Mater Res Bull, 1996, 31(1): 37-46.
[6] LU Yu-dong, WANG Xin, ZHUANG Zhi-qiang. Preparation, microstructure, and conductive properties of BaPbO3 thin films[J]. Journal of Inorganic Materials, 2007, 22(1): 138-142. (in Chinese)
[7] WANG Xin, LU Yu-dong, ZHUANG Zhi-qiang. Electrical properties of sol-gel-derived BaPbO3 conductive ceramic films[J]. Journal of the Chinese Ceramic Society, 2006, 34(9): 1140-1142. (in Chinese)
[8] LIANG C S, WU J M, CHANG M C. Ferroelectric BaPbO3/ PbZr0.53Ti0.47/BaPbO3 heterostructures[J]. Appl Phys Lett, 2002, 81(4): 3624-3626.
[9] LUO Y R, WU J M. BaPbO3 perovskite electrode for lead zirconate titanate ferroelectric thin films[J]. Appl Phys Lett, 2001, 79(22): 3669-3671.
(Edited by CHEN Wei-ping)
Foundation item: Project(033177) supported by the Natural Science Foundation of Guangdong Province, China; Project(040140) supported by the Natural Science Foundation of South China University of Technology
Received date: 2007-04-20; Accepted date: 2007-05-23
Corresponding author: WANG Xin, PhD; Tel: +86-20-87114627; E-mail: g96217@scut.edu.cn