
Ligand selection for complex-leaching valuable metals in hydrometallurgy
YANG Tian-zu(杨天足), DOU Ai-chun(窦爱春), LEI Cun-mao(雷存茂), REN Jin(任 晋), LIU Zhen-zhen(刘珍珍)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 6 July 2009; accepted 19 November 2009
Abstract: A two-stage ligand selection method composed of a primary selection and a critical selection, for complex-leaching valuable metals was presented. At the primary selection stage, three conditions were discussed under a supposed ideal state by mathematical derivation. Generally, ligands selected under conditionⅠwere easier for complex-leaching valuable metals than that under condition Ⅱ, however, under condition Ⅲ, ligands selected were hard to complex-leaching the valuable metals. Ligands that were out of these three conditions could be disposed directly. In critical selection, ligands selected in primary selection can be finalized. Case applications were provided for verifying the method. The application indicated that iminodiacetate (Ida2-) can be used as a complex agent for complex-leaching smithsonite (ZnCO3); the leaching condition should be controlled with pH 8-11; the relative error of the minimum consumption of Ida2- between the predicted and the calculated results is 5.3%. The results indicate that the theoretical derivations in the ideal state are reliable, and the method for ligand selection is practical and operable.
Key words: ligand selection; complex-leaching; smithsonite; hydrometallurgy
1 Introduction
Nowadays, with the depletion of copper, zinc sulphuric ores are easily upgraded by floatation. However, the low grade oxide ores are difficult to be upgraded and smelt, which draws more and more attention and thus various hydrometallurgical methods have been developed to leach these low grade oxide ores. The processes include bioleaching, acid leaching, and alkaline leaching. In bioleaching[1-2] bacteria are used to leach ores. It is reported that the bacteria are difficult to be domesticated for the low metals recovery and slow reaction rate, which constrains the development of this process. Acid leaching[3-5] occurs in the acidic media. As we all know, impurities such as Ca, Mg, Fe and Si can be dissolved in these media, which contaminate the leaching liquor and consume leaching reagent. Si is always formed as silica gels in the system and affects the slurry filtration. This limits the process development. Recently, more and more studies focus on the alkaline leaching process in which valuable metals can be selectively separated with impurities by the formation of complexes. Alkaline leaching mainly contains ammonia leaching and caustic soda leaching by using different ligands. NH3 is used as a leaching ligand to coordinate with valuable metal ions (Cu2+, Zn2+) in ammonia leaching. In this process, ammonia evaporates, which causes the environment pollution. Current studies[6-8] still focus on utilizing ammonium to substitute part of ammonia to alleviate the environmental problem. Referring to caustic soda leaching[9-10], OH- is used as a leaching ligand to coordinate with metal ions. The ligand (OH-) and the metal ion (Zn2+) are combined to form the 4th complex Zn(OH)42- with high concentrated caustic soda in the solution, which requires large consumption of leaching reagent. While increasing the concentration of NaOH, SiO2 in the ore can be dissolved into the solution, which consumes more NaOH. The reasons mentioned above constrain the process development. Moreover, because the improper ligands used in the alkaline leaching process seriously affect the mineral resources utilization, it is urgent to study new ligands (or leaching reagents) to treat these low grade oxide ores, which is of great strategic significance for effective utilization and sustainable development of nonferrous metals.
The primary purpose of complex-leaching is to selectively separate valuable metals with impurities by generating complexes. And the main purpose is to dissolve the valuable metals Cu and Zn in leaching liquor and leave the impurities, such as Ca, Mg, Fe and Si in residue. Because the valuable metals in the oxide ore are mainly in form of carbonates, basic carbonates are insoluble compounds. The recovery of valuable metals by complex-leaching can be achieved by dissolving these insoluble compounds thermodynamically. The dissolving process for insoluble compounds is common in hydrometallurgical and chemical fields. Studies about ligand selection for dissolving the insoluble compound have not been reported in any of the studies published, only some studies[11-13] about leaching insoluble compounds with specific ligand were reported.
In this work, the thermodynamic equilibrium of MaAm (insoluble compounds) dissolved by ligand in aqueous solution was analyzed. It is found that the independent complexation of the ligand with the metal ion M is a key factor to dissolve MaAm. Other factors could be simplified in a supposed ideal state. In this ideal state, the improper ligands could be directly rejected during primary selection, while the remained ligands could be selected carefully using the critical selection method in which all factors were considered. This ligand selection methodology provided fundamentals for studying new leaching reagents in hydrometallurgy.
For the sake of simplification, redox reaction of metal ions and the probable charge were not discussed in this work. The insoluble compounds were symbolised as MaAm, and the ligand was symbolized as L. For metal ion or ligand concentration in aqueous solution, there are two expressions, the total concentration and free concentration, which are unified as [M]T and [M], respectively.
2 Thermodynamic equilibrium and calcula- tion
2.1 Thermodynamic equilibrium
2.1.1 Solubility equilibrium of MaAm
The solubility equilibrium of MaAm is the basis of complex-leaching process. The corresponding reaction is as follows:
MaAm=aM+mA (1)
Its solubility product constant can be written as
Ksp=[M]a[A]m (2)
According to Eq.(1), there will be
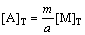 (3)
(3)
2.1.2 Equilibrium of M
The formation of polynuclear complex and mixed-ligands complex were not considered in the dissolution of MaAm, so reactions in aqueous solution will be simplified as follows:
1) Complex reaction of M with L
M+iL=MLi (i=1, 2, 3, …, x) (4)
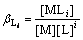
2) Complex reaction of M with OH-
M+iOH-=M(OH)i (i=1, 2, 3, …, y) (5)
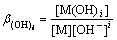
The complexation of A with M can be neglected without addition of A. Thereby, the total concentration of M can be expressed as
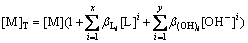 (6)
(6)
2.1.3 Equilibrium of A
The protonation of A occurs if A is a weak acid radical in the solution:
A+iH=HiA (i=1, 2, 3, …, z) (7)
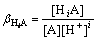
The total concentration of A can be expressed as
 (8)
(8)
2.1.4 Equilibrium of L
The protonation of L can be described as
L+iH+=HiL (i=1, 2, 3, …, q) (9)

According to Eqs.(4) and (9), the total concentration of L can be expressed as
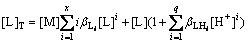 (10)
(10)
2.1.5 Dissociation equilibrium of H2O
In aqueous solution, there is always the following equilibrium:
Kw=[H+][OH-] (11)
Kw is a constant when the temperature and pressure are given.
2.2 Calculation
There are eight unknown numbers containing [M]T, [M], [L]T, [L], [A]T, [A], [OH-], [H+] in Eqs.(2), (3), (6), (8), (10) and (11). Constants in these equations can be obtained in handbooks of thermodynamic data or by calculation. While the leaching conditions, such as the pH value and [L]T value, are fixed, the other six unknown numbers can be calculated theoretically. This is a methodology for calculating solubility of MaAm. YANG and QIU[14] introduced the similar method. Obviously, the solubility calculated by this method is a comprehensive action of all the factors.
3 Ligand selection
It is known to all that there are multiple choices to select ligands which can complex with the metal ion M. It is necessary to select ligands (or leaching reagents) before conducting experiments to recover valuable metal M in oxide ores.
3.1 Primary selection
3.1.1 Independent complexation of ligand
It is said that the solubility of MaAm in L-H2O system which has been introduced in section 2.2 is a comprehensive action of all the factors. Actually, under a certain condition, some of the factors that have little influence on the solubility MaAm can be neglected for simplifying the calculation. Especially in selecting ligands, this simplification is necessary and can make primary selection efficient.
To determine whether a ligand is suitable for complex-leaching, it is necessary to exhibit its combining ability completely. Otherwise, the reason why the insoluble compound cannot be dissolved in the solution will be confused. Supposed an ideal state in the system, there is none but the independent complexation of L with M. The total concentration of L is enough to ensure that the metallic ions M are combined to form the most stable complex with L. In this state, the insoluble compound MaAm will be leached certainly by an arbitrary ligand which can complex with M. The consumption of the ligand (the total concentration of L) can be a criterion for ligand section.
3.1.2 Derivation in ideal state
In the ideal state, the cumulative stable constant of the complex can be written as 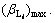 Setting the solubility of MaAm as S, there will be equilibrium equations as follows:
Setting the solubility of MaAm as S, there will be equilibrium equations as follows:
 (12)
(12)
[A]T=[A]=mS (13)
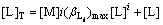 (14)
(14)
According to Eqs.(12)-(13), there will be
 (15)
(15)
According to solubility product rule of MaAm, Eq.(15) can be written as
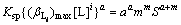 (16)
(16)
From Eq.(16), following expression can be obtained:
 (17)
(17)
Inserting Eq.(12) into Eq.(14), an equation can be deduced as follows:
[L]T-iaS=[L] (18)
Inserting Eq.(18) into Eq.(17), (βLi)max can be described as
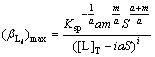 (19)
(19)
In Eq.(19),  can be constrained in a range with the constraint of [L]T. Deducing the range of
can be constrained in a range with the constraint of [L]T. Deducing the range of  by discussing the range of [L]T using Eq.(19), so, three conditions can be deduced as a result.
by discussing the range of [L]T using Eq.(19), so, three conditions can be deduced as a result.
Ⅰ) 0<[L]T-iaS≤1, (βLi)max must satisfy
 ≥
≥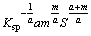
Ⅱ) 1≤[L]T-iaS≤10,  must satisfy
must satisfy
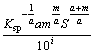 ≤
≤ ≤
≤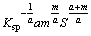
Ⅲ) 10≤[L]T-iaS≤100,  must satisfy
must satisfy
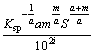 ≤(βLi)max≤
≤(βLi)max≤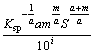
From the view of mathematics, the range of [L]T must contain [L]T-ias≤0, [L]T-ias≥100. In practice, the range of [L]T-ias≤0 is not existent, and the range of [L]T-ias≥100 has no meaning to dissolve MaAm in so high concentration of ligands. While doing primary selection, ligands whose  satisfies condition Ⅰ should be selected priorly, then condition Ⅱ, and the last one is condition Ⅲ. Ligand whose
satisfies condition Ⅰ should be selected priorly, then condition Ⅱ, and the last one is condition Ⅲ. Ligand whose  does not satisfy any one of the three conditions can be deserted directly. In addition, the total concentration of L can be calculated approximately as
does not satisfy any one of the three conditions can be deserted directly. In addition, the total concentration of L can be calculated approximately as
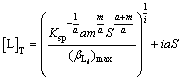 (20)
(20)
3.2 Critical selection
The remained ligands that had been selected by primary selection should be selected carefully in this section. According to the calculation method in section 2.2 with fixed value of pH and [L]T, the other unknown numbers can be calculated from the six equilibrium equations. Drawing a 3D graph using pH, [L]T, and [M]T as coordinates, the change tendency of [M]T can be represented apparently with the change of pH and [L]T. The ligand should be finally determined in thermodynamics from the analysis of the 3D graph. In addition, the distribution of M and L can help us to analyze the affections of all the factors in dissolving process.
Actually, factors that affect ligand selection are variable. Factors such as the combining ability of the ligand with impurities, the price of the ligand, and the environmental factors of the ligand which are commonly used in hydrometallurgy need to be considered during selection.
4 Case application
A sample of a low-grade zinc oxide ore, needs to be leached for recovery of valuable metal Zn. The main phase of Zn is smithsonite (ZnCO3) in the sample, as such we take ZnCO3 as the insoluble compound for ligand selection.
4.1 Primary selection process
At 298 K, the solubility product constant of ZnCO3 is Ksp=1×10-10. Given that S=0.469 mol/L ([Zn2+]T=30 g/L), the complex levels i are 1-4. The requirements of  are given in Table 1. Part of the common inorganic ligands and amino acidic ligands was investigated, and the results are given in Table 2.
are given in Table 1. Part of the common inorganic ligands and amino acidic ligands was investigated, and the results are given in Table 2.
Table 1 Requirements of 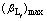 composing certain level complex
composing certain level complex

Table 2 Results of primary selection
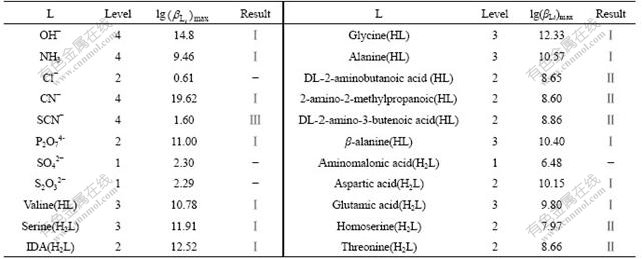
Table 2 shows that  of ligands Cl-, SCN-, SO42-, S2O32- and aminomalonic acid does not satisfy any of the three conditions, so they can be deserted directly.
of ligands Cl-, SCN-, SO42-, S2O32- and aminomalonic acid does not satisfy any of the three conditions, so they can be deserted directly.  of inorganic ligands OH-, NH3, CN- and P2O74- satisfy conditionⅠ. It seems that they all can be remained for the critical selection. NH3 and CN- which are toxic to human beings and environment will be deserted. OH- and Zn2+ are combined together to form its 4th complex which needs the total concentration of OH- to be above 1.876 mol/L. Meanwhile, SiO2 in the ore can be dissolved into the solution at this concentration of OH-, which causes more consumption of OH-. So, the remained ligand is P2O74-.
of inorganic ligands OH-, NH3, CN- and P2O74- satisfy conditionⅠ. It seems that they all can be remained for the critical selection. NH3 and CN- which are toxic to human beings and environment will be deserted. OH- and Zn2+ are combined together to form its 4th complex which needs the total concentration of OH- to be above 1.876 mol/L. Meanwhile, SiO2 in the ore can be dissolved into the solution at this concentration of OH-, which causes more consumption of OH-. So, the remained ligand is P2O74-.
All the amino acidic ligands seem to be remained except for aminomalonic acid ligand. It is known that the price of organic agents is generally expensive. The economic factors should be adequately considered while carrying on the primary selection of these amino acidic ligands. While the specific price of these ligands is unknown, according to Eq.(20), ligand whose  satisfies the conditionⅠand the complex level is lower can be selected priorly. So, the remained ligands are IDA and Aspartic acid.
satisfies the conditionⅠand the complex level is lower can be selected priorly. So, the remained ligands are IDA and Aspartic acid.
4.2 Critical selection process
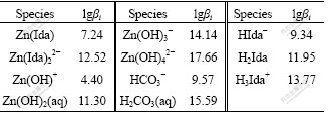
Taking IDA (C4H7O4N iminodiacetic acid) as an example for critical selection, the ligand of IDA can be written as Ida2-. The thermodynamic data[15-17] are given in Table 3.
Table 3 Thermodynamic data of related species at 298 K
Unknown numbers were calculated and a 3D graph (Fig.1) was given by MATLAB software. When the total concentration of the ligand is 1.0 mol/L ([Ida2-]T=1.0 mol/L), the distributions of Zn2+, Ida2- and CO32- are represented in Figs.2-4, respectively. The distribution is described as its concentration that occupied in the total concentration. This helps us to understand Fig.1 better.
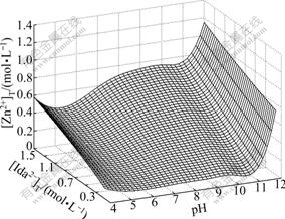
Fig.1 Tendency of [Zn2+]T with pH and [Ida2-]T
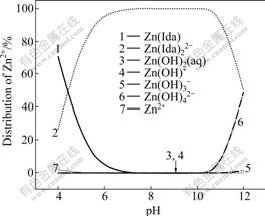
Fig.2 Distribution of Zn2+ vs pH
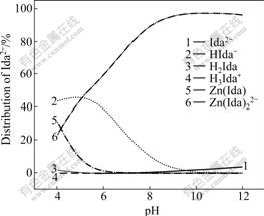
Fig.3 Distribution of Ida2- with pH
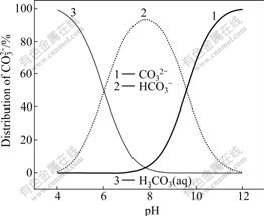
Fig.4 Distribution of CO32- with pH
Fig.1 shows that the [Zn2+]T decreases with the increase of pH value between 4 and 5. While in Fig.2 with pH of 4-5, the distribution of free Zn2+ decreases, meaning that acid-leaching action of ZnCO3 decreases with the decrease of acidity. While in Fig.3 with pH of 4-5, the distribution of HIda- increases, which causes the complexation of Ida2- with Zn2+ decreasing. Because of these two decreasing actions, the [Zn2+]T decreases.
Fig.1 shows that the [Zn2+]T increases with the increase of pH value between 5 and 9.6, and then decreases slightly till pH nearly reaches 11. [Zn2+]T approaches the highest value when pH reaches 9.6. Fig.2 shows that Zn(Ida) decreases and Zn(Ida)22- increases till pH=7. In the pH range of 7-11, above 99% of Zn are Zn(Ida)22-. In Fig.3, HIda- decreases and Zn(Ida)22- increases strongly from pH 5 to 9; from pH 9 to 11, above 98% of Ida2- are combined with Zn2+ as Zn(Ida)22-.
From Figs.2 and 3, it seems that the [Zn2+]T will not change much from pH 9 to 11. In fact, after the pH value approaches nearby 9.6 in Fig.1, [Zn2+]T decreases slightly. The change of this decrease can be explained in Fig.4. In Fig.4, the protonation of CO32- is dominant when pH<9.6, which is promotional to dissolve ZnCO3; after pH approaches 9.6, the free CO32- becomes dominant. [CO32-] increases dramatically when pH>9.6, which causes the decline of [Zn2+] because of solubility product rules. The decrease of [Zn2+] causes a slight decrease of [Zn2+]T.
Fig.1 shows that [Zn2+]T increases sharply with the raise of pH value between 11 and 12. In Fig.2, with the pH of 11-12, Zn(Ida)22- decreases strongly, while Zn(OH)42- increases strongly. Both Ida2- and OH- complex with Zn2+ causing the increase of [Zn2+]T. When pH>11 in Fig.3, Zn(Ida)22- decreases slightly, and free Ida2- increases slightly. From Figs.2 and 3, it can be predicted that the complexation of OH- would replace the complexation of Ida2- gradually after pH approaches 11, and the consumption of total concentration of OH- would be more higher because of its 4th complex with Zn2+.
In addition, when we use the Eq.(20) to predict the minimum consumption of [Ida2-]T, the calculation result is 0.938 mol/L less than 0.99 mol/L (pH=9.6, [Zn]T= 0.469 mol/L) in Fig.1. The relative error is only 5.3%. When pH ranges from 9 to 11, the complexation of OH- with Zn2+, the pronation of CO32- and the protonation of Ida2- can be neglected. The complexation of Ida2- with Zn2+ can be regarded as the only action in this condition. This proves that the independent complexation of L with M is existent in practice.
Therefore, Ida2- can be used as a complex agent to leach the sample, and the pH value must be controlled within the range of 8-11.
5 Conclusions
1) The key factor for ligand selection is independent complexation of the ligand.
2) The method of ligand section contains primary selection and critical selection. In primary selection, ligands selected under conditionⅠare more easily for complex-leaching valuable metals than that under condition Ⅱ, and ligands selected under condition Ⅲ are hard to complex-leach the valuable metals. Ligands that are out of these three conditions can be deserted directly. Ligands selected in primary selection will be finally determined in critical selection.
3) Case application indicates that Ida2- can be used as a complex agent complex-leaching smithsonite, and pH should be controlled to be 8-11. The relative error of the minimum consumption of Ida2- between predicted and calculated results is 5.3%.
4) It has been proved that independent complexation of the ligand is existent in practice, and the theoretical derivations in the ideal state are reliable.
References
[1] CASTRO I M, FIETTO J L R, VIEIRA R X. Bioleaching of zinc and nickel from silicates using Aspergillus niger cultures [J]. Hydrometallurgy, 2000, 57(1): 39-49.
[2] MULLIGAN C N, KAMALI M, GIBBS B F. Bioleaching of heavy metals from a low-grade mining ore using Aspergillus niger [J]. Journal of Hazardous Materials, 2004, 110: 77-84.
[3] HUA Y, LIN Z, YAN Z. Application of microwave irradiation to quick leach of zinc silicate ore [J]. Minerals Engineering, 2002, 15(6): 451-456.
[4] QIN Wen-qing, LI Wei-zhong, LAN Zhuo-yue. Simulated small-scale pilot plant heap leaching of low-grade oxide zinc ore with integrated selective extraction of zinc [J]. Minerals Engineering, 2007, 20(7): 694-700.
[5] SHAYESTEHFAR M R, NASAB S K, MOHAMMADALIZADEH H. Mineralogy, petrology, and chemistry studies to evaluate oxide copper ores for heap leaching in Sarcheshmeh copper mine, Kerman, Iran [J]. Journal of Hazardous Materials, 2008, 154(1/3): 602-612.
[6] JU Shao-hua, TANG Mo-tang, YANG Sheng-hai. Dissolution kinetics of smithsonite ore in ammonium chloride solution [J]. Hydrometallurgy, 2005, 80(1/2): 67-74.
[7] BING?L D, CANBAZO?LU M, AYDO?AN S. Dissolution kinetics of malachite in ammonia/ammonium carbonate leaching [J]. Hydrometallurgy, 2005, 76(1/2): 55-62.
[8] FENG Lin-yong, YANG Xian-wan, SHEN Qing-feng. Pelletizing an alkaline leaching powdery low grade zinc oxide ores [J]. Hydrometallurgy, 2007, 89(3/4): 305-310.
[9] FRENAY J. Leaching of oxidized zinc ores in various media [J]. Hydrometallurgy, 1985, 15: 243-253.
[10] ZHAO You-cai, STANFORTH R. Production Zn powder by alkaline treatment of smithsonite Zn-Pb ores [J]. Hydrometallurgy, 2000, 56(2): 237-249.
[11] YANG Sheng-hai, TANG Mo-tang. Thermodynamics of Zn(Ⅱ)- NH3-NH4Cl-H2O system [J]. Transactions of Nonferrous Metals Society of China, 2000, 10(6): 830-833.
[12] JU Shao-hua, TANG Mo-tang, YANG Sheng-hai. Thermodynamics and technology of extracting gold from low-grade gold ore in system of NH4Cl-NH3-H2O [J]. Transactions of Nonferrous Metals Society of China, 2006, 16(1): 203-208.
[13] WANG Rui-xiang, TANG Mo-tang, YANG Sheng-hai. Thermodynamics of Ni(Ⅱ) complex equilibrium in system of Ni(Ⅱ)-NH3-Cl—H2O [J]. Journal of Central South University: Science and Technology, 2008, 39(1): 891-895. (in Chinese)
[14] YANG Xian-wan, QIU Ding-fan. Hydrometallurgy [M]. Beijing: Metallurgical Industry Press, 2001: 111-113. (in Chinese)
[15] MARTELL E, SMITH R M. Critical stability constants (volume 1: Amino acids) [M]. New York and London: Plenum Press, 1974: 116-118.
[16] MARTELL E, SMITH R M. Critical stability constants (volume 4: Inorganic Ligands) [M]. New York and London: Plenum Press, 1974: 37-38.
[17] DEAN J A. Lange’s handbook of chemistry (15th edition) [M]. Simplified Chinese Translation Edition (2003). Beijing: Science Press McGraw-Hill Education (Asia) Co, 1998. (in Chinese)
Foundation item: Project(2007CB613604) supported by the National Basic Research Program of China
Corresponding author: YANG Tian-zu; Tel: +86-731-88836791; E-mail: tianzuyang@163.com
DOI: 10.1016/S1003-6326(09)60270-3
(Edited by LI Xiang-qun)