配位沉淀体系中氯化锑水解分离的理论模拟及实验研究
来源期刊:中国有色金属学报(英文版)2016年第10期
论文作者:田庆华 辛云涛 杨丽 王学海 郭学益
文章页码:2746 - 2753
关键词:氯化锑;配位溶解-沉淀平衡;热力学平衡;验证
Key words:antimony chloride; complexation-precipitation; thermodynamics equilibrium; verification
摘 要:研究了Sb3+-OH--Cl-体系中配位溶解-沉淀平衡的理论模拟和实验验证,包括SbOCl、Sb4O5Cl2和Sb2O3的水解沉淀过程。对不同配体浓度、不同pH值条件下的锑离子平衡浓度进行理论计算及实验验证,同时分别从溶解平衡和物质转变吉布斯自由能的角度对沉淀产物进行理论分析,并开展验证实验。结果表明,实际锑离子平衡浓度大于理论计算的浓度,其中理论计算的锑离子最小平衡浓度在pH值4.6时为10-10.92 mol/L,而实验验证结果表明在pH值5.1时最小平衡浓度为10-3.8 mol/L。在一定pH值条件下可以得到不同沉淀产物,无论是在理论计算或是验证实验中均不存在SbOCl,验证实验中得到了产物Sb8O11Cl2?H2O。
Abstract: The theoretical simulation and verified experiments on metal separation in a Sb3+-OH--Cl- complexation-precipitation system involving hydrolysis-precipitation reactions of SbOCl, Sb4O5Cl2 and Sb2O3 were carried out. The equilibrium concentration of [Sb3+]T was obtained by calculation and verified by experiments. The precipitates SbOCl, Sb4O5Cl2 and Sb2O3 were analyzed through the equilibrium concentration of Sb3+ in the solution and the ΔrGΘm of transformation reactions of these materials. It is found that the concentration of [Sb3+]T in verified experiments was larger than the theoretical value, where the theoretical minimum concentration of [Sb3+]T was 10-10.92 mol/L at pH value of 4.6 and the minimum concentration obtained from the verified experiment was about 10-3.8 mol/L at pH value of 5.1. Different precipitates can be obtained at certain pH. The SbOCl cannot be obtained both in theoretic calculations and in verified experiments, while the Sb8O11Cl2?H2O was generated in the experiment.
Trans. Nonferrous Met. Soc. China 26(2016) 2746-2753
Qing-hua TIAN1,2, Yun-tao XIN1,2, Li YANG1, Xue-hai WANG1, Xue-yi GUO1,2
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Cleaner Metallurgical Engineering Research Center, Nonferrous Metal Industry of China, Central South University, Changsha 410083, China
Received 16 September 2015; accepted 19 May 2016
Abstract: The theoretical simulation and verified experiments on metal separation in a Sb3+-OH--Cl- complexation-precipitation system involving hydrolysis-precipitation reactions of SbOCl, Sb4O5Cl2 and Sb2O3 were carried out. The equilibrium concentration of [Sb3+]T was obtained by calculation and verified by experiments. The precipitates SbOCl, Sb4O5Cl2 and Sb2O3 were analyzed through the equilibrium concentration of Sb3+ in the solution and the ΔrGΘm of transformation reactions of these materials. It is found that the concentration of [Sb3+]T in verified experiments was larger than the theoretical value, where the theoretical minimum concentration of [Sb3+]T was 10-10.92 mol/L at pH value of 4.6 and the minimum concentration obtained from the verified experiment was about 10-3.8 mol/L at pH value of 5.1. Different precipitates can be obtained at certain pH. The SbOCl cannot be obtained both in theoretic calculations and in verified experiments, while the Sb8O11Cl2·H2O was generated in the experiment.
Key words: antimony chloride; complexation-precipitation; thermodynamics equilibrium; verification
1 Introduction
There exist a series of antimony oxides, such as Sb2O5, Sb6O13, Sb4O4, Sb2O3, Sb2O and gaseous SbO [1-5]. The most stable antimony oxide is Sb2O3, an important antimony product [6]. Most Sb2O3 products are produced by pyrometallurgy, while some are obtained by hydrometallurgy involving hydrolysis reactions of SbCl3 solution. Recently, hydrometallurgical processes of sulfide ores have greatly been promoted for lowering SO2 emission, and some advanced technologies, such as oxidation leaching and slurry electrolysis of sulfides [7], have already been developed and adopted. Generally, in an acid system of SbCl3, the Sb2O3 is obtained by hydrolysis of SbCl3, which involves the complexation of Sb3+ with Cl- and OH-. Because of the complexation, the stability of the SbCl3 system in hydrochloric acid is influenced by the concentrations of Cl- and OH-. When the system is changed or the equilibrium of complexation-precipitation is altered, either the antimony ion precipitates from the solution or the antimony precipitate dissolves in the solution. In the hydrolysis process of SbCl3 solution, SbOCl, Sb4O5Cl2, Sb2OCl4, Sb2O3, Sb4O3(OH)3Cl3 and Sb4O3(OH)5Cl. could be generated depending on certain conditions [8,9]. In the hydrometallurgy of antimony in a hydrochloric acid system and other hydrometallurgical processes involving SbCl3, the equilibrium of complexation- precipitation of Sb3+ with Cl- and OH- must be controlled to prevent unexpected reactions. Based on the known data on Sb compounds and other materials in complexation-precipitation system of Sb3+ with Cl- and OH-, theoretical simulation of the complexation- precipitation equilibrium could be achieved [10,11-13]. As to the system of SbCl3, researches on the hydrolysis equilibrium of the Sb3+-Cl--H2O system [14] are limited to achieve theoretical simulation without verification by experiments. And there exist some deficiencies in these theoretical analyses, such as limitation to acidic system, no consideration on the precipitates generated in the system neither in theoretical analyses nor in verification experiments, which will hinder the comprehensive understanding of the system [15].
In this work, in order to well understand complexation-precipitation equilibrium of the Sb3+- OH--Cl- system, comprehensive theoretical simulations and verified experiments were carried out respectively. The thermodynamic equilibrium model was established and experiments were conducted for verifying the theoretical model.
2 Experimental
2.1 Thermodynamic simulation
The element of antimony, complexing agent of OH- and Cl-, precipitant of OH- and Cl- were selected for composing the complexation-precipitation system of Sb3+-OH--Cl-. The related reactions and constants are listed in Table 1, where the stability constants of complexes are cumulative. The equilibrium concentration of Sb was calculated based on the principle of mass balance and simultaneous equilibrium, where the Sb concentration comprises all soluble Sb materials such as Sb3+, SbO2-, SbO+ and complex of Sb3+ with Cl- and OH-. Some unknown parameters in the complex equations of complexation-precipitation system are obtained by Gibbs free energy changes of the related reactions or calculated with the data known in Table 2. The thermodynamic calculations are based on the assumptions: 1) system temperature is 25 °C and system is operated at atmospheric pressure; 2) thermal effect of reactions on the system is not considered; 3) activity coefficient is 1.0 and not affected by ionic strength and solution system; 4) the system is in equilibrium state; 5) no gas and other unexpected materials are generated. All the thermodynamic calculations were carried out by using excel 2010. The unstable antimony compounds and the compounds without thermodynamic data such as Sb2OCl4 and Sb4O3(OH)3Cl3 are not taken into account in the thermodynamic calculations. Only stable compounds SbOCl, Sb2O3 and Sb4O5Cl2 are considered in thermodynamic simulation.
Table 1 Complex constants of Sb3+ with Cl- and OH- [16]

Table 2 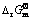 of related materials at 25 °C [12]
of related materials at 25 °C [12]

Equilibrium equations of Sb3+ and Cl- in this system:
Sb3++iCl-=SbCli3-i, i=1-6;
βi=[SbCli3-i]/([Sb3+][Cl-]i) (1)
Equilibrium equations of Sb3+ and OH-:
Sb3++iOH-=Sb(OH)i3-i, i=2-4
β′i=[Sb(OH)i3-i]/([Sb3+][OH-]i) (2)
Other equilibrium equations in this system:
Sb3++Cl-+H2O=SbOCl(s)+2H+,
K1=[H+]2/([Sb3+][Cl-]) (3)
Sb3++H2O=SbO++2H+,
K2=[H+]2[SbO+]/[Sb3+] (4)
Sb3++2H2O=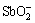 +4H+,
+4H+,
K3=[H+]4[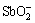 ]/[Sb3+] (5)
]/[Sb3+] (5)
4Sb3++2Cl-+5H2O=Sb4O5Cl2(s)+10H+,
K4=[H+]10/([Sb3+]4[Cl-]2) (6)
2Sb3++3H2O=Sb2O3(s)+6H+,
K5=[H+]6/[Sb3+]2 (7)
Sb(OH)3(s)=Sb3++3OH-,
K6=[Sb3+][OH-]3 (8)
The Ki listed above could be obtained by calculation with given data. Based on the principles of simultaneous equilibrium and mass conservation, the equilibrium equations of each ion in solution could be obtained.
1) Concentration of [SbCl]T formed by complexation of Sb3+ with Cl-
[SbCl]T=[SbCl2+]+[SbCl2+]+[SbCl3]+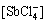 +
+ +
+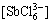 =
=
β1[Sb3+][Cl-]+β2[Sb3+][Cl-]2+β3[Sb3+][Cl-]3+
β4[Sb3+][Cl-]4+β5[Sb3+][Cl-]5+β6[Sb3+][Cl-]6=
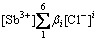 (9)
(9)
2) Concentration of [SbOH]T formed by complexation of Sb3+ with Cl-
[SbOH]T=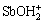 +[SbOH3]+
+[SbOH3]+ =
=
β′2[Sb3+][OH-]2+β′3[Sb3+][OH-]3+β′4[Sb3+][OH-]4=
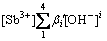 (10)
(10)
3) Total concentration of Sb in solution
[Sb3+]T=[Sb3+]+[SbCl]T+[SbOH]T+[SbO+]+ =
=

K2[Sb3+]/[H+]2+K3[Sb3+]/[H+]4=

K2/[H+]2+K3/[H+]4) (11)
4) Total concentration of Cl in solution
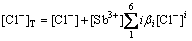 (12)
(12)
The αi (α0, α1, α2, α3, α4, α5, α6, α7, α8, α9, α10 and α11) are defined as the percentages of Sb3+, SbCl2+, 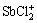 , SbCl3(l),
, SbCl3(l), 
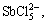
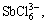
 Sb(OH)3(l),
Sb(OH)3(l), 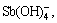 SbO+ and
SbO+ and  which are listed as follows:
which are listed as follows:
αi=[SbCli3-i]/[Sb3+]T, i=0-6 (13)
α7=[ ]/[Sb3+]T (14)
]/[Sb3+]T (14)
α8=[Sb(OH)3]/[Sb3+]T (15)
α9=[ ]/[Sb3+]T (16)
]/[Sb3+]T (16)
α10=[SbO+]/[Sb3+]T (17)
α11=[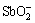 ]/[Sb3+]T (18)
]/[Sb3+]T (18)
As to each solid product, there exists one relationship expression on equilibrium constant. Together with the dissociation equilibrium of water, eight variables and six equilibrium equations could be used in every complexation-precipitation equilibrium system, where the degree of freedom is two. In order to study the hydrolysis process of complexation-precipitation equilibrium, the concentrations of antimony and chloridion are supposed to be constant, where the total concentration of antimony ([Sb3+]T0) is CSb0 which stands for initial antimony concentration in solution and chloridion ([Cl-]T0) is CCl0. Then, another equation of initial state is added, and there are eight variables and seven equilibrium equations in the complexation- precipitation system with the degree of freedom being one. After that, the other variables could be calculated by the given pH value.
i) The dissolution-precipitation equilibrium of SbOCl in complexation system
According to equations above, 1 mol Cl- and Sb3+ are consumed to generate 1.0 mol SbOCl, then the total concentration of chloridion is
[Cl-]T=CCl0-CSb0+[Sb3+]T (19)
The concentration of Sb3+ in solution is
[Sb3+]=[H+]2/(K1[Cl-]) (20)
Together with equations above, the total equation is obtained:
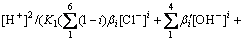 K2/[H+]2+K3/[H+]4+1)-[Cl-]2+(CCl0-CSb0)[Cl-]=0 (21)
K2/[H+]2+K3/[H+]4+1)-[Cl-]2+(CCl0-CSb0)[Cl-]=0 (21)
The concentration of Cl- could be calculated by dissolving equation under given CCl0, CSb0 and pH, and then [SbCl]T, [SbOH]T, [Sb3+]T and [Cl-]T could also be obtained.
ii) The dissolution-precipitation equilibrium of Sb4O5Cl2 in complexation system
Similarly, 2.0 mol Cl- and 4.0 mol Sb3+ are consumed to form 1.0 mol Sb4O5Cl2, and then the total concentration of chloridion is
[Cl-]T=CCl0-0.5CSb0+[Sb3+]T (22)
The concentration of Sb3+ in solution is
[Sb3+]=[H+]2.5/(K40.25[Cl-]0.5) (23)
Together with equations above, the total equation is obtained:


[Cl-]+(CCl0-0.5CSb0)=0 (24)
The concentration of Cl- could be calculated by dissolving equation under given CCl0, CSb0 and pH, and then [SbCl]T, [SbOH]T, [Sb3+]T and [Cl-]T could also be obtained.
iii) The dissolution-precipitation equilibrium of Sb2O3 in complexation system
Obviously, 2.0 mol Sb3+ is needed for 1.0 mol Sb2O3, and then the total concentration of chloridion is
[Cl-]T=CCl0 (25)
The concentration of Sb3+ in solution is
[Sb3+]=[H+]3/K50.5 (26)
Together with related equations, the total equation is obtained:
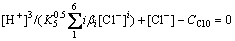 (27)
(27)
The concentration of Cl- could be calculated by dissolving equation under given CCl0 and pH, and then [SbCl]T, [SbOH]T, [Sb3+]T and [Cl-]T could also be obtained.
2.2 Precipitation experiments
The antimony chloride solution was prepared by leaching of antimony powder (AR, Sinopharm Chemical Reagent Co., Ltd.) by hydrochloric acid (AR) as leaching agent. The NaOH (AR, Sinopharm Chemical Reagent Co., Ltd.) was used for neutralization in the experiment.
The solution of antimony trichloride was put in water bath with magnetic stirring (DF-101, Yuhua Instrument, China) for controlling the experimental conditions. The pH value of the solution was adjusted by dropping NaOH slurry, and then liquid-solid separation was carried out by centrifuge (TDL-40B, Shanghai Anting Scientific Instrument Factory, China). After that, the separated solution was diluted and then detected, and precipitates were dried at 55 °C in dryer before further analysis. In the study, the influences of different pH values in the range of -0.5 to 14.0 at complexation- precipitation equilibrium of antimony compounds were investigated. The influence of chloridion concentration on the equilibrium process was also studied, in case that the concentration of chloridion was diluted by NaOH solution, NaOH was added in the form of NaOH slurry in stead of NaOH solution.
2.3 Analysis
The concentration of antimony ion in solutions was determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES, PS-6, Baird, USA). pH values of the solutions were measured with a pH/mV meter (PHS-3E, INESA Scientific Instrument, China). The precipitates obtained were detected by X-ray diffractometer (XRD, S0902240, Rigaku, Janpan).
3 Results and discussion
3.1 Thermodynamic simulation
The distribution of antimony compounds in solution could be derived from αi, which could be obtained with the given [Cl-], as shown in Fig. 1.
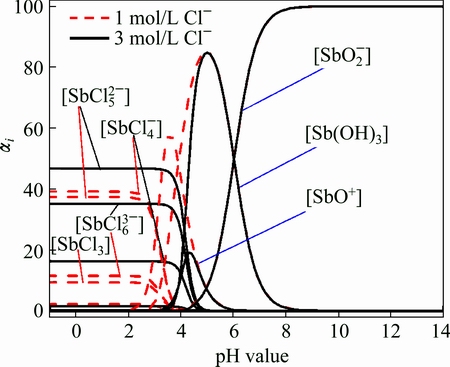
Fig. 1 Curves of αi vs pH
From Fig. 1, one can see that the antimony is mainly in the form of chloridion complex (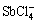 ,
, 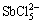 ,
,  ) when the pH is below 4.1. The α8 firstly increases and then decreases in the pH range of 3.4-8.0, and the α10 has the same trend between pH 3.4 and 6.0. With the increase of pH value, the antimony complex with chloridion decreases and the α11 increases. It is speculated that the hydrolysis reaction mainly involves the formation of
) when the pH is below 4.1. The α8 firstly increases and then decreases in the pH range of 3.4-8.0, and the α10 has the same trend between pH 3.4 and 6.0. With the increase of pH value, the antimony complex with chloridion decreases and the α11 increases. It is speculated that the hydrolysis reaction mainly involves the formation of 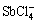 ,
, 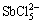 and
and  when the pH is below 3.5, and the hydrolysis products would be antimony oxychlorides. The hydrolysis reaction mainly involves the formation of SbO+, Sb(OH)3 and
when the pH is below 3.5, and the hydrolysis products would be antimony oxychlorides. The hydrolysis reaction mainly involves the formation of SbO+, Sb(OH)3 and 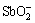 when the pH is higher than 4.0 (where [Cl-] is 3.0 mol/L), and the products of the hydrolysis would be antimony oxides.
when the pH is higher than 4.0 (where [Cl-] is 3.0 mol/L), and the products of the hydrolysis would be antimony oxides.
The thermodynamic simulation of the complexation-precipitation equilibrium of the compounds SbOCl, Sb4O5Cl2 and Sb2O3 is shown in Fig. 2, where the influence of initial total Cl- concentration and pH value on the equilibrium concentration of [Sb3+]T is easily seen, where the [Sb3+]T stands for all the soluble Sb in the system.
As can be seen in Fig. 2(a), the hydrolysis equilibrium concentration of Sb3+ of SbOCl decreases with the increase of pH value at first, and then increases when the pH is higher than 4.3. The concentration of [Sb3+]T is the largest when the initial concentration of Cl- is 1.0 mol/L, and reaches its minimum when the initial concentration of Cl- is 2.0 mol/L. Compared with 1.0 mol/L Cl-, the increasing Cl- concentration could enhance the hydrolysis of Sb3+ with Cl- and H2O, leading to less [Sb3+]T left in the solution when the Cl- is 2.0 mol/L. When the initial concentration of Cl- is more than 2.0 mol/L, the concentration of [Sb3+]T slightly increases with the increase of Cl- because of the complex reactions. In the acid system the Sb3+ is in the form of chloride complex (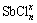 ), while in the alkaline system it is in the form of antimonite (
), while in the alkaline system it is in the form of antimonite (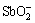 ), so the Cl- has influence on the equilibrium concentration of [Sb3+]T in acid system but has little influence in alkaline system.
), so the Cl- has influence on the equilibrium concentration of [Sb3+]T in acid system but has little influence in alkaline system.
The hydrolysis equilibrium concentration of [Sb3+]T of Sb4O5Cl2 versus pH value at various initial concentrations of Cl- is demonstrated in Fig. 2(b). In acid system the Sb3+ slightly increases along with the initial concentration of Cl- because of the complex reaction, and the Cl- has no influence on the concentration of [Sb3+]T in alkaline system because there are rarely 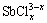 but mainly SbO+,
but mainly SbO+,  and Sb(OH)3 in the alkaline system. As can be seen in Fig. 2(c), the influence of pH value and initial concentration of Cl- on the hydrolysis of Sb2O3 is similar to that of Sb4O5Cl2.
and Sb(OH)3 in the alkaline system. As can be seen in Fig. 2(c), the influence of pH value and initial concentration of Cl- on the hydrolysis of Sb2O3 is similar to that of Sb4O5Cl2.
3.1.1 Complexation-precipitation equilibrium of [Sb3+]T at various pH values
Three concentrations of [Sb3+]T with 3.0 mol/L Cl- and 5.0 mol/L Cl- are put together as shown in Fig. 3.
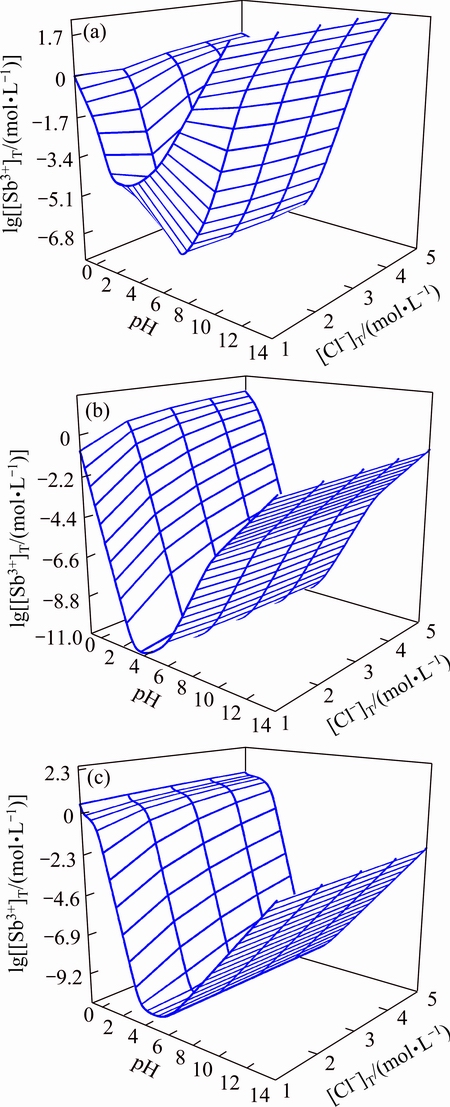
Fig. 2 Thermodynamic simulation curves of total antimony at complexation-precipitation equilibrium of SbOCl (a), Sb4O5Cl2 (b) and Sb2O3 (c)
It is easily seen that the concentration of [Sb3+]T in the complexation-precipitation curve of hydrolysis reaction of SbOCl is minimum when the pH is less than -0.2, which means that the hydrolysis reaction of SbOCl happens more easily than that of Sb4O5Cl2 and Sb2O3 in this range of pH, and the Sb is precipitated in the form of SbOCl. In the same way, the Sb4O5Cl2 rather than others is generated when the pH is between -0.2 and 6.6, and the hydrolysis reaction generates Sb2O3 when the pH is higher than 6.6. The predicted stable interval of these materials without any transformation is demonstrated in Fig. 3(b).

Fig. 3 lg[Sb3+]-pH curves of antimony (a) and stability interval of these materials generated (b)
The influence of Cl- on the concentration of [Sb3+]T could also be analyzed from Fig. 3(a). The Sb3+ is in the form of complexes because of the complexation of Cl- in an acidic system. The [Sb3+]T with 5.0 mol/L Cl- is larger than that with 3.0 mol/L Cl-. When the system is alkali the Sb3+ is in the form of antimonate hydrate, so there is no difference between the concentration of [Sb3+]T with different concentrations of Cl- where the hydrolysis product is Sb2O3. While the products are SbOCl and Sb4O5Cl2 (the Cl- takes part in the hydrolysis reaction), the Cl- will promote the hydrolysis and the Sb concentration with 5.0 mol/L Cl- is smaller than that with 3.0 mol/L Cl-.
3.1.2 Theoretical precipitates at various pH values
A preliminary deduction of the precipitates could be obtained from involving three complexation- precipitation equilibriums of Sb4O5Cl2, SbOCl and Sb2O3, then the three precipitates, Sb4O5Cl2, SbOCl and Sb2O3, will be theoretically analyzed from the perspective of thermodynamic conversion. Some related published literatures already illustrate the hydrolysis behavior of SbCl3, which are in the order of SbCl3→SbOCl→Sb4O5Cl2→Sb2O3 [5,17,18], and there exist rarely researches about the reverse reactions. To further clarify the relationship between pH and the products, the transformation Gibbs free energy of the three precipitates is calculated and is shown below, where the activities of related components are their concentrations and the initial concentrations of Sb3+ and Cl- are 1.0 mol/L and 3.0 mol/L, respectively.
4SbOCl+H2O=Sb4O5Cl2+2H++2Cl-,
ΔrGm=ΔrGΘm-2×2.303×TR×pH+2×2.303×TR×lg[Cl-] (28)
2SbOCl+H2O=Sb2O3+3H++3Cl-,
ΔrGm=ΔrGΘm-3×2.303×TR×pH+3×2.303×TR×lg[Cl-] (29)
Sb4O5Cl2+H2O=2Sb2O3+2H++2Cl-,
ΔrGm=ΔrGΘm-2×2.303×TR×pH+2×2.303×TR×lg[Cl-] (30)
The ΔrGm of transformation reactions (16)-(18) are shown in Fig. 4. In the given conditions, the SbOCl cannot exist in the system because it will turn into Sb4O5Cl2 spontaneously and then Sb2O3 is generated when pH is larger than 1.9. The Sb4O5Cl2 could only exist stably when the pH is less than 6.3. So, when the pH is less than 6.3 the Sb4O5Cl2 could be obtained directly or by transformation, while Sb2O3 could be obtained directly or by transformation when the pH is higher than 6.3.
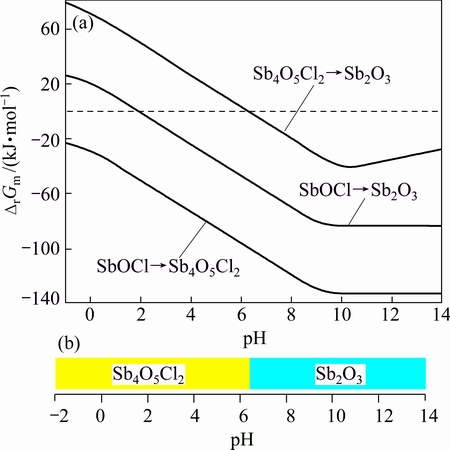
Fig. 4 ΔrGm of transformation reactions of three materials (a) and stable interval of these materials generated (b)
3.2 Precipitation
In the verified experiments, the precipitates came into being when the alkali slurry was added into the solution and the equilibrium of dissolution (complexation)-precipitation was altered. After keeping certain pH for 2.0 d, the solution and precipitates were separated and detected. Then, the results in verified experiments were compared with the theoretical ones, which were calculated from the equilibrium concentrations of SbOCl, Sb4O5Cl2 and Sb2O3.
3.2.1 Equilibrium concentration of [Sb3+]T in verified experiments on complexation-precipitation equilibrium of [Sb3+]T with 3.0 and 5.0 mol/L Cl- at various pH values
From the equilibrium of [Sb3+]T in the solution, the final concentration of [Sb3+]T should be the minimum value in any of the three concentrations as shown in Fig. 5(a). The results of verified experiments are shown in Figs. 5(a) and (b).
Fig. 5 lg[Sb3+]-pH curves of thermodynamic simulation (a) and verified experiments (b)
From the result, it could easily be seen that the concentration of Sb3+ in verified experiments has the same tendency with those obtained from the thermodynamic simulation: with the increase of pH value, the concentration firstly decreases and then increases. The concentration of [Sb3+]T with 5.0 mol/L Cl- is slightly larger than that with 3.0 mol/L Cl- because of the complexation of Cl-. Because there are some unknowns in the system such as multi-core complexes (SbaClb(3a-b)+ or Sba(OH)b(3a-b)+, a>1, b>0), mixed-type complexes (SbClb(OH)c(3-b-c)+ or SbaClb(OH)c(3-b-c)+, a>1, b>0, c>0), and the reactions corresponding to these complexes, the concentration of verified experiments is larger than the theoretical one, where the theoretical minimum concentration of Sb is 7.94×10-10 mol/L at pH value of 4.6 and the minimum verified concentration is about 6.31×10-3 mol/L at pH value of 5.1, which is drawn from the curve in Fig. 5(b).
3.2.2 XRD pattern of precipitates
In verified experiment of 1.0 mol/L Sb3+ with 3.0 mol/L Cl-, the precipitates obtained were detected by XRD. Certain pH values (pH=0, 2.0, 3.0, 4.0, 5.0, 6.0, 9.0, 12.0 and 14.0) were selected for this study, the results of XRD pattern are shown in Fig. 6.
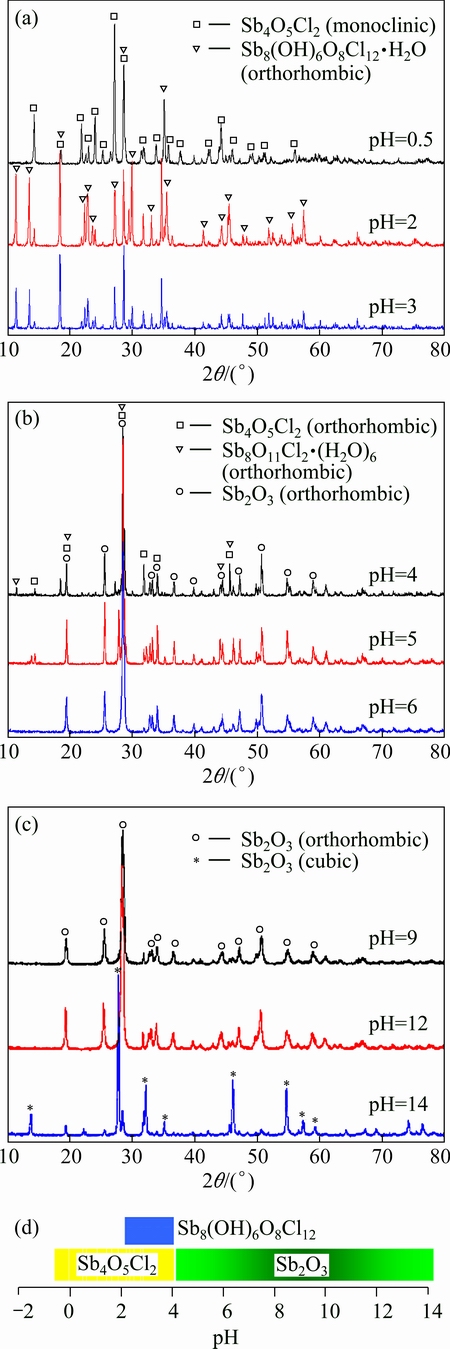
Fig. 6 XRD patterns of precipitates at different pH values (a, b and c) and stable interval of precipitates obtained in verified experiment (d)
Figure 6 shows that no SbOCl is obtained in the conditions of this study. When the pH value is less than 4.0, there exists monoclinic Sb4O5Cl2 (JCPDS No. 30- 0091) in precipitates. The orthorhombic transition material Sb8O11Cl2·6H2O (JCPDS No. 77-1584) could also be detected when the pH is between 2.0 and 4.0, and the Sb8O11Cl2 will transform to Sb2O3 when reacting with alkali liquor, which involves the Eqs. (19) and (20) [19]. Available data of Sb8O11Cl2 are hard to be found so that Sb8O11Cl2 is not discussed in this work. Orthorhombic Sb2O3 (JCPDS No. 11-0689) could be found in precipitates when the pH value is equal to or higher than 4.0, and when the pH is 14.0, cubic Sb2O3 (JCPDS No. 43-1071) is obtained. It is known that orthorhombic Sb2O3 is stable crystal at low temperature while the cubic one is stable at high temperature. The steric configuration of orthorhombic Sb2O3 is Pccn, and there are four Sb2O3 molecules in a unit cell. The steric configuration of cubic Sb2O3 is 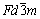 , there are eight Sb4O6 molecules (or sixteen Sb2O3 molecules) in a unit cell.
, there are eight Sb4O6 molecules (or sixteen Sb2O3 molecules) in a unit cell.
8SbCl3+11H2O=Sb8O11Cl2+22HCl (31)
Sb8O11Cl2+H2O=4Sb2O3+2HCl (32)
According to the Ostwald law and the granularity effects [20], the atoms (molecules) of a unit cell at metastable phase are less than those at stable phase, thus the number of molecules and the nucleation energy needed for the formation of critical nucleus to generate a unit cell at metastable phase are less than those to generate a unit cell at stable phase. Therefore, the precipitate at metastable phase is generated prior to the final product at stable phase. Besides that, the precursor of Sb2O3 is monoclinic Sb4O5Cl2, which is easily to transform into orthorhombic ones.
4 Conclusions
1) The effect of Cl- on the separation (hydrolysis) process was discussed according to the calculation, and at the same time the precipitates obtained at different pHs were speculated from the perspective of equilibrium concentration of [Sb3+]T in the solution and from the ΔrGm of transformation reactions of the material.
2) Most results from the verified experiments and from the theoretical simulation match up each other, but there exist some differences. For example, the precipitates obtained do not totally agree with those drawn from the theoretical calculation.
3) There still exists room for further investigations on the complexation-precipitation system of Sb3+-OH-- Cl-.
References
[1] ZHAO Rui-rong, SHI Xi-chang. Physical chemistry of antimony metallurgy [M]. Changsha: Central South University Press, 2006. (in Chinese)
[2] LIU Ke-song, ZHAI Jin, JIANG Lei. Fabrication and characterization of superhydrophobic Sb2O3 films [J]. Nanotechnology, 2008, 19(16): 165604-1-6.
[3] LIU Kuo, XU Rui-dong, HE Shi-wei, CHEN Han-sen, ZHU Yun, HUA Hong-quan, SHU Bo. Arsenic and antimony removal from bismuth-rich lead anode slime by alkaline pressure oxidation leaching [J]. The Chinese Journal of Nonferrous Metals, 2015, 25(5): 1394-1400. ( in Chinese)
[4] XIAO Fa-xin, CAO Dao, MAO Jian-wei, SHEN Xiao-ni, REN Feng-zhang. Role of Sb(V) in removal of As, Sb and Bi impurities from copper electrolyte [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(1): 271-278.
[5] TIAN Qing-hua, WANG Heng-li, XIN Yun-tao, LI Dong, GUO Xue-yi. Ozonation leaching of a complex sulfidic antimony ore in hydrochloric acid solution [J]. Hydrometallurgy, 2016, 159(1): 126-131.
[6] CORBY G A. The metallurgy of antimony [J]. Chemie der Erde - Geochemistry, 2012, 72(S4): s3-s8.
[7] WANG Cheng-yan, QIU Ding-fan, JIANG Pei-mei. Status and development of antimony metallurgy technology in China [J]. Nonferrous Metals (Extractive Metallurgy), 2002(5): 6-10. ( in Chinese)
[8] LIU Lei, HU Zhao-lin, CUI Yu-ming, LI Bo, ZHOU Xing-fu. A facile route to the fabrication of morphology-controlled Sb2O3 nanostructures [J]. Solid State Sciences, 2010, 12(5): 882-886.
[9] JIANG Han-ying. Physical chemistry of hydrometallurgy [M]. Beijing: Metallurgical Industry Press, 1984. ( in Chinese)
[10] ZHANG Chuan-fu, YAO Yong-lin, ZHAN Jing. Thermodynamics of precipitation-coordination equilibrium in Fe2+-Ni2+-NH3- NH4+-C2O42--H2O system [J]. The Chinese Journal of Nonferrous Metals, 2012, 22(10): 2938-2943. ( in Chinese)
[11] ZHANG Xiang-lin, KANG Heng. Coordination chemistry [M]. Changsha: Central South University of Technology Press, 1986. ( in Chinese)
[12] CHEN Jin-zhong, CAO Hua-zhen, LI Bo, YUAN Hai-jun, ZHENG Guo-qu, YANG Tian-zu. Thermodynamic analysis of separating lead and antimony in chloride system [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(3): 730-734.
[13] MA Li-wen, NIE Zuo-ren, XI Xiao-li, LI Xiao-kang. Theoretical simulation and experimental study on nickel, cobalt, manganese separation in complexation-precipitation system [J]. Separation and Purification Technology, 2013, 108: 124-132.
[14] DU Xin-ling. Research on the hydrolysis equilibrium of antimony trichloride in the Sb3+-Cl--H2O system [J]. China Nonferrous Metallurgy, 2012, 41(5): 75-79. ( in Chinese)
[15] YANG Jian-guang, TANG Mo-tang, YANG Sheng-hai, TANG Chao-bo, CHEN Yong-ming. Thermodynamic analysis of Sn(IV)-Sb(III)-NH3-NH4Cl-H2O system and its application [J]. J Cent South Univ: Science and Technology, 2005, 36(4): 582-586. ( in Chinese)
[16] DEAN J A. Handbook of chemistry [M]. Beijing: Science Press, 1990. ( in Chinese)
[17] DU Xin-ling, TANG Chang-qing. Production technology of high- purity antimony white [J]. Liaoning Chemical Industry, 2007, 36(12): 853-856. (in Chinese)
[18] LEI Xin-you, LIU Xin-wen, ZUO Guo-fang, DU Shu-rong. The technology control of reducing solution hydrolysis in the production of antimony white with wet method [J]. Applied Chemical Industry, 2001, 30(1): 42-43. ( in Chinese)
[19] TANG Jun-Jun, WANG Yong, JIAO Zheng, WU Ming-hong. Self-assembly nanostructures of one-dimensional antimony oxide and oxychloride [J]. Materials Letters, 2009, 63(17): 1481-1484.
[20] ZHANG Duo-mo, XIAO Song-wen, LIU Zhi-hong, LI Qi-hou. Ostwald rule and the polymorphous control of antimony white prepared by wet method [J]. Journal of Central South University of Technology: Natural Science, 2000, 31(2): 121-123. ( in Chinese).
田庆华1,2,辛云涛1,2,杨 丽1,王学海1,郭学益1,2
1. 中南大学 冶金与环境学院,长沙 410083;
2. 中南大学 有色金属工业协会清洁冶金工程研究中心,长沙 410083
摘 要:研究了Sb3+-OH--Cl-体系中配位溶解-沉淀平衡的理论模拟和实验验证,包括SbOCl、Sb4O5Cl2和Sb2O3的水解沉淀过程。对不同配体浓度、不同pH值条件下的锑离子平衡浓度进行理论计算及实验验证,同时分别从溶解平衡和物质转变吉布斯自由能的角度对沉淀产物进行理论分析,并开展验证实验。结果表明,实际锑离子平衡浓度大于理论计算的浓度,其中理论计算的锑离子最小平衡浓度在pH值4.6时为10-10.92 mol/L,而实验验证结果表明在pH值5.1时最小平衡浓度为10-3.8 mol/L。在一定pH值条件下可以得到不同沉淀产物,无论是在理论计算或是验证实验中均不存在SbOCl,验证实验中得到了产物Sb8O11Cl2·H2O。
关键词:氯化锑;配位溶解-沉淀平衡;热力学平衡;验证
(Edited by Xiang-qun LI)
Foundation item: Project (51474257) supported by the National Natural Science Foundation of China
Corresponding author: Xue-yi GUO; Tel: +86-731-88876255; Fax: +86-731-88836207; E-mail: xyguo@csu.edu.cn
DOI: 10.1016/S1003-6326(16)64370-4

