盐酸溶液中双氧水浸出黄铜矿
来源期刊:中国有色金属学报(英文版)2018年第7期
论文作者:Sanja J. PETROVI? Grozdanka D. BOGDANOVI? Milan M. ANTONIJEVI?
文章页码:1444 - 1455
关键词:黄铜矿;浸出;双氧水;盐酸;硫
Key words:chalcopyrite; leaching; hydrogen peroxide; hydrochloric acid; sulphur
摘 要:本文旨在研究以双氧水为强氧化剂的黄铜矿精矿的盐酸浸出过程。研究搅拌速度、固液比、温度、HCl和H2O2浓度等浸出参数对金属浸出率的影响。室温下,用3.0 mol/L H2O2和0.5 mol/L HCl溶液与黄铜矿反应180 min后,获得33%的最大铜浸出率。结果表明,在反应的前60 min,铜的浸出率增大;此后,由于双氧水的快速催化分解,铜浸出率基本上保持不变。此外,固液比对铜的浸出率影响显著,而且在最稀的悬浮液中(即固液比1:100)铜的浸出率最高。溶出过程可用一级动力学方程描述,表观活化能为19.6 kJ/mol,表明溶出过程受扩散控制,对于HCl和H2O2的反应级数分别为0.30和0.53。浸出渣的XRD和SEM/EDS分析结果表明,矿物表面生成单质硫,抑制浸出率的提高。
Abstract: The aim of this work was to investigate the leaching of chalcopyrite concentrate in hydrochloric acid with hydrogen peroxide as a strong oxidizing agent. The effects of the leaching variables on metal extraction, such as stirring speed, solid-to-liquid ratio, temperature and HCl and H2O2 concentrations, were studied. The maximum final copper extraction of 33% was attained with 3.0 mol/L H2O2 in 0.5 mol/L HCl at room temperature after 180 min of the reaction. The results showed that the copper extraction was increased in the first 60 min of reaction, after which it essentially ceased due to the fast catalytic decomposition of hydrogen peroxide. Further, solid-to-liquid ratio affected the copper extraction significantly and the highest copper extraction was obtained in the most dilute suspension (i.e., S/L ratio of 1:100). The dissolution process was described by the first order kinetics equation. The apparent activation energy of 19.6 kJ/mol suggested that the dissolution process was under diffusion control. The reaction orders for HCl and H2O2 were established to be 0.30 and 0.53, respectively. The results of the XRD and SEM/EDS analysis of the leaching residue indicated the generation of the elemental sulphur on mineral surfaces which tended to inhibit the leaching rate.
Trans. Nonferrous Met. Soc. China 28(2018) 1444-1455
Sanja J. PETROVI 1, Grozdanka D. BOGDANOVI
1, Grozdanka D. BOGDANOVI 2, Milan M. ANTONIJEVI
2, Milan M. ANTONIJEVI 2
2
1. Mining and Metallurgy Institute Bor, Zeleni Bulevar 35, 19210 Bor, Serbia;
2. University of Belgrade, Technical Faculty in Bor, VJ 12, P. O. Box 50, 19210 Bor, Serbia
Received 27 July 2017; accepted 23 October 2017
Abstract: The aim of this work was to investigate the leaching of chalcopyrite concentrate in hydrochloric acid with hydrogen peroxide as a strong oxidizing agent. The effects of the leaching variables on metal extraction, such as stirring speed, solid-to-liquid ratio, temperature and HCl and H2O2 concentrations, were studied. The maximum final copper extraction of 33% was attained with 3.0 mol/L H2O2 in 0.5 mol/L HCl at room temperature after 180 min of the reaction. The results showed that the copper extraction was increased in the first 60 min of reaction, after which it essentially ceased due to the fast catalytic decomposition of hydrogen peroxide. Further, solid-to-liquid ratio affected the copper extraction significantly and the highest copper extraction was obtained in the most dilute suspension (i.e., S/L ratio of 1:100). The dissolution process was described by the first order kinetics equation. The apparent activation energy of 19.6 kJ/mol suggested that the dissolution process was under diffusion control. The reaction orders for HCl and H2O2 were established to be 0.30 and 0.53, respectively. The results of the XRD and SEM/EDS analysis of the leaching residue indicated the generation of the elemental sulphur on mineral surfaces which tended to inhibit the leaching rate.
Key words: chalcopyrite; leaching; hydrogen peroxide; hydrochloric acid; sulphur
1 Introduction
Demand for copper has increased dramatically in the world over the last few decades. For commercial exploitation, copper deposits generally need to be in excess of 0.5%, and preferably over 2% [1]. It is well known that most of the copper ore deposits belong to the sulphide deposits with chalcopyrite, covellite and chalcocite as the most important minerals. On the other hand, important part of the copper ore deposits are oxidized deposits, with malachite and azurite as copper minerals [2-4]. Among the mentioned minerals, chalcopyrite is the most important copper sulphide mineral since approximately 70% of world’s copper reserves are contained in the mineral chalcopyrite [5]. Extracting copper from its ores and concentrates is achieved by conventional pyrometallurgical and hydrometallurgical processes. In recent years, extensive research was performed on hydrometallurgical processes which could be used for low-grade ores and sulfide concentrates, without the emission of harmful sulphur dioxide. Given that the amount of rich copper ores is constantly decreasing, there is a growing need for the development of new technological processes to obtain copper from such mineral resources [6-8]. Since chalcopyrite is a highly refractory mineral for aqueous extraction processing and one of the most difficult-to- leach minerals in general, the focus of numerous laboratory investigations was directed towards the chloride solutions due to the advantage of this leaching system compared with the sulphate solutions [9-15]. These advantages were reflected in higher solubility of copper and iron, easier oxidation of ferrous ions and faster leaching kinetics of chalcopyrite compared to the sulphate systems [16]. In the chloride solution, Fe(III) ions in the form of Fe(III) chloride [9,17,18] and Cu(II) ions in the form of Cu(II) chloride [19-21] were most often used as the oxidizing agents for chalcopyrite leaching.
In addition to the mentioned oxidants, the investigations on chalcopyrite leaching on a laboratory scale were carried out using strong oxidizing agents, such as hydrogen peroxide [22-24], Cr(VI) ions [25-27] and ozone [28,29], in order to conduct the experiment at atmospheric pressure and temperatures below 100 °C.
Hydrogen peroxide is seen as one of the cleanest and most versatile chemicals which is widely used in many areas [30-32]. This fact, along with the high value of its oxidation-reduction potential (1.77 V, vs SHE), made this reagent extensively investigated in the oxidation processes of almost all sulphide minerals.
The oxidative action of hydrogen peroxide in acidic solutions is based on Eq. (1) [33,34]:
H2O2+2H++2e→2H2O, φ0=1.77 V (vs SHE) (1)
Dissolution of chalcopyrite by hydrogen peroxide in an acid medium is based on the following reactions [23]:
2CuFeS2+5H2O2+10H+→2Cu2++2Fe3++4S0+10H2O (2)
2CuFeS2+17H2O2+2H+→2Cu2++2Fe3++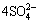 +18H2O (3)
+18H2O (3)
According to the present dissolution mechanism of chalcopyrite, sulphide sulphur can oxidize with hydrogen peroxide to the elemental sulphur (S0) (Reaction (2)) and/or 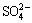 ions (Reaction (3)).
ions (Reaction (3)).
Based on the literature data, the studies on chalcopyrite leaching in the presence of hydrogen peroxide were mainly conducted in the sulphuric acid solutions [23,24,35,36]. The leaching of the sulphide minerals in H2SO4-H2O2 solution was proven to be very efficient. For instance, ADEBAYO et al [35] found, by examining the kinetics of the chalcopyrite oxidation, that copper extraction significantly increased as the concentrations of both hydrogen peroxide and sulphuric acid were increased. The reaction orders were 1.45 and 0.77 with respect to concentration of H2O2 and H2SO4, respectively. The dissolution kinetics was found to follow a shrinking-core model with surface chemical reaction as the rate-determining step. Similar conclusions were made by other researchers [23,36]. On the other hand, hydrogen peroxide is a highly unstable compound which decomposes in mineral dissolution processes. Its decomposition can be accelerated by the presence of catalytic metal, mineral particles, as well as the impurities. In order to avoid rapid decomposition of hydrogen peroxide, some stabilizers, such as phosphoric acid, acetic acid and polar organic solvents, are recently used in the leaching processes [37-39].
However, while the numerous researches were performed in the sulphuric acid solution, hydrogen peroxide was not often investigated for the oxidation of chalcopyrite in hydrochloric acid solution. Leaching system of HCl-H2O2 was used for leaching of pyrite concentrate [33], zinc-lead bulk sulphide concentrate at atmospheric pressure [40], galena ore [41] and scheelite concentrate [42]. Hydrogen peroxide was also used in combination with perchloric acid in leaching of the pyrite concentrate [43].
Considering the fact which indicated there were no detailed studies on chalcopyrite leaching with hydrogen peroxide in hydrochloric acid, the objective of this work was to investigate the leaching of chalcopyrite concentrate using hydrogen peroxide as a strong oxidant in hydrochloric acid solution in order to define the effects of various parameters on the dissolution of chalcopyrite as well as to define the dissolution kinetics.
2 Experimental
2.1 Material
The experiments were carried out with chalcopyrite sample obtained by refloating the chalcopyrite concentrate from the Veliki Krivelj Copper Mine (Serbia). The <75 μm size fraction was used for all the experiments.
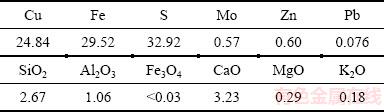
Quantitative determination of the elements in the starting chalcopyrite sample was performed by different analytical methods (gravimetric, volumetric, atomic emission spectroscopy with inductively coupled plasma, and atomic absorption spectrometry). Table 1 shows the chemical analysis result of the sample.
Table 1 Chemical composition of chalcopyrite concentrate (mass fraction, %)
The phase composition of the initial chalcopyrite sample was determined by the X-ray analysis (PHILIPS, model PW-1710) with a Cu Kα radiation (0.154 nm). The operating conditions were 40 kV and 30 mA. Figure 1 shows an XRD pattern for a chalcopyrite sample. The XRD peaks indicate the presence of chalcopyrite as a predominant mineral, as well as pyrite, sphalerite, molybdenite and gangue minerals (calcite and quartz).
Quantitative mineralogical composition analysis of the concentrate showed that the sample contained:
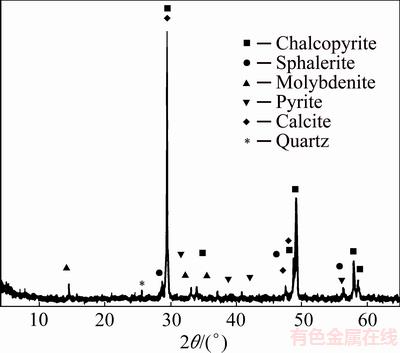
Fig. 1 X-ray diffraction pattern of initial chalcopyrite concentrate
chalcopyrite (64.79%), pyrite (18.78%), covelite (0.86%), chalcocite (0.37%), sphalerite (0.91%), molybdenite (0.88%), bornite (0.08%), galena (0.09%), rutile (0.02%), cassiterite (0.03%), magnetite (0.07%) and gangue minerals (13.12%). Gangue minerals dominantly consisted of carbonate, mainly calcite and quartz and significantly less silicate.
Qualitative microscopic investigation was performed in the reflected light on Carl Zeiss-Jena, JENAPOL-U microscope. Microscopic photograph of the initial concentrate sample (Fig. 2) showed chalcopyrite as the main mineral phase, then pyrite and gangue minerals.

Fig. 2 Microscopic photograph of chalcopyrite sample (chalcopyrite (cp), pyrite (py) and gangue minerals (gn))
The morphology of the chalcopyrite sample and leaching residues samples was examined by a JEOL JSM 5800 scanning electron microscope, operated at 20 keV coupled with energy dispersive spectroscopy (EDS) type Oxford Inca 3.2 and by Tescan VEGA 3 LM with Oxford EDS X-act 10 mm2 SDD (an accelerating voltage of 30 kV). SEM photograph of initial chalcopyrite sample (Fig. 3) showed that the crystals were mainly anhedral, rarely subhedral.
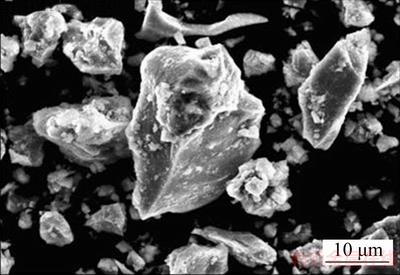
Fig. 3 SEM photograph of chalcopyrite sample
2.2 Reagents and experimental procedure
Leaching solutions were prepared using the analytical grade chemicals: hydrochloric acid (37% HCl, ρ=1.18 g/cm3), hydrogen peroxide (30% H2O2, ρ=1.11 g/cm3) and distilled water.
Leaching experiments were carried out in a 600 mL five-necked glass tempering beaker equipped with magnetic stirrer, thermometer and a glass condenser to prevent evaporation (Fig. 4). The reactor was filled with 200 mL of hydrochloric acid-hydrogen peroxide leaching solution of a certain concentration and heated to the required temperature. When the required temperature was achieved, a 2.0 g of chalcopyrite concentrate sample was added to the leaching solution and then stirred at the constant temperature.

Fig. 4 Experiment set-up for performing leaching tests
The leaching liquor samples (1 mL of the leaching liquor) were taken at certain time intervals, diluted to 50 mL in a volumetric flask and analyzed on dissolved copper/iron by the atomic absorption spectrophotometry (Atomic absorption spectrophotometer, Perkin Elmer model 403). The leaching residues obtained after oxidation were filtered, washed with distilled water, dried and characterized using the X-ray diffraction analysis (XRD).
3 Results and discussion
3.1 Effect of stirring speed
The effect of the stirring speed (0, 200, 400 and 600 r/min) on the dissolution of chalcopyrite was investigated in solutions containing 2.0 mol/L H2O2 and 0.5 mol/L HCl at 40 °C. The results are given in Fig. 5.
The results shown in Fig. 5 reveal that the copper extraction values were at the lowest when the mechanical stirring of the solution was absent. The copper extraction increased slowly as the stirring speed increased up to 400 r/min (copper extraction was 26%). A possible explanation for this could be found in better contact between chalcopyrite particles and leaching solution when the stirring was present in the system. Therefore, the optimum speed of the 400 r/min was selected for all subsequent experiments. The results from previous studies on oxidation of the sulphide minerals with hydrogen peroxide suggested that efficient dissolution was achieved without the mechanical stirring of the solution [35,44,45]. This could be related to the influence of stirring on an accelerated degradation of hydrogen peroxide in the system. For instance, ADEBAYO et al [35] showed that the decomposition of hydrogen peroxide was accelerated with the increase of the stirring speed. During the leaching of pyrite with hydrogen peroxide in sulphuric acid, the agitation had a negative influence on the pyrite oxidation and this influence was not caused by decomposition of hydrogen peroxide at higher stirring speed but probably by the better contact between pyrite particles and hydrogen peroxide without stirring of the solution [44].
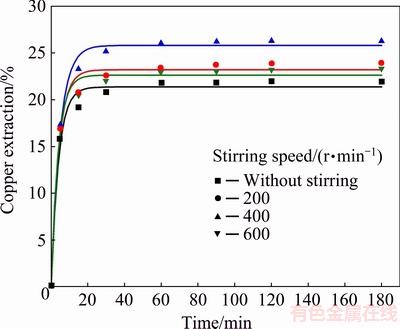
Fig. 5 Effect of stirring speed on copper extraction from chalcopyrite concentrate (Conditions: 0.5 mol/L HCl, 2.0 mol/L H2O2, 40 °C)
3.2 Effect of solid/liquid ratio
The experiments were carried out in the solution of 0.5 mol/L HCl and 2.0 mol/L H2O2, at temperature of 40 °C in various solid/liquid ratio (1:100, 1:50, 1:25 and 1:10).
As seen in Fig. 6, the solid/liquid ratio affects the copper extraction significantly. The dissolution curves show that the highest final copper extraction (26% Cu) was obtained in the most dilute suspension (i.e., at solid/liquid ratio of 1:100). With the increase of solid/liquid ratio from 1:50 to 1:10, copper extraction was reduced, from 14.2% to 6% Cu, respectively. Therefore, the solid/liquid ratio of 1:100 remained as constant for further experimental work. Lower values of copper extraction obtained in more dense suspensions (S/L ratio from 1:50 to 1:10) were probably the result of insufficient amounts of necessary reagents in the leaching solution. Very rapid decomposition of the hydrogen peroxide in more dense suspensions caused low copper extraction (6% Cu in the densest suspension, S/L ratio of 1:10). The increase of the solid phase content led to the increase of viscosity of the suspension, which affected the efficiency of the leaching process and caused the lowering of the dissolution rate of the mineral.
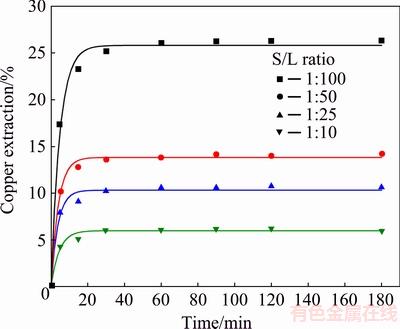
Fig. 6 Influence of solid/liquid ratio on copper extraction from chalcopyrite concentrate (Conditions: 0.5 mol/L HCl, 2.0 mol/L H2O2, 40 °C, 400 r/min)
3.3 Effect of initial hydrogen peroxide concentration
The effect of the initial hydrogen peroxide concentration on chalcopyrite dissolution was studied by varying the initial hydrogen peroxide concentration (0.5-3.0 mol/L) in 0.5 mol/L hydrochloric acid at room temperature (without thermostating). The results are given in Fig. 7.
Figure 7 shows that, when the concentration of the hydrogen peroxide was increased, it had a positive effect on chalcopyrite dissolution in hydrochloric acid solution. However, the final copper extractions were relatively low (9%-33%). Also, the copper extraction essentially ceased after 60 min. With regard to the iron extractions, they were slightly lower than the copper extractions, and varied from 8.3% to 20.2% for the lowest and highest hydrogen peroxide concentration, respectively. The decomposition rate of H2O2 was proportional to its concentration, which meant that decomposition of hydrogen peroxide was faster at higher concentrations of H2O2 [44].
In the HCl-H2O2 mixed solution, there was catalytic and self-decomposition reactions of hydrogen peroxide (Reactions (4) and (5)) [46] which led to a significant reduction of its concentration:
2H++2Cl-+H2O2→Cl2+2H2O (4)
Cl2+H2O2→O2+2Cl-+2H+ (5)
The overall reaction can be presented by
H2O2→H2O+1/2O2 (6)
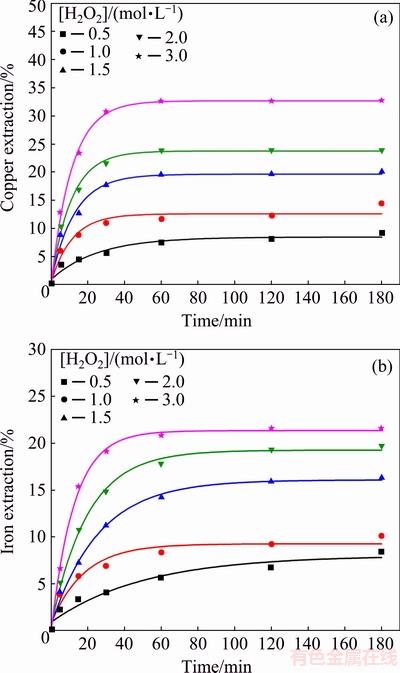
Fig. 7 Copper (a) and iron (b) extraction from chalcopyrite concentrate as function of time at different hydrogen peroxide concentrations (Conditions: room temperature, without thermostating, 0.5 mol/L HCl, 400 r/min)
The Reaction (6) was very favorable; it had a ΔH0 of -98.2 kJ/mol and a ΔG0 of -119.2 kJ/mol. The rate of decomposition of hydrogen peroxide depended on the concentration of the peroxide and temperature, as well as on pH and the presence of impurities [47]. It can be said that the higher the concentration of H2O2 was, the greater the extraction of copper was achieved and the catalytic decomposition of peroxide to oxygen and water was also more intense (Reaction (6)).
Due to the oxidation of chalcopyrite (Reactions (2) and (3)) and the oxidation of pyrite present in the examined sample of chalcopyrite concentrate, ferric ions were liberated into solution according to Reactions (7) and (8) [48,49]:
2FeS2+15H2O2→2Fe3++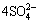 +2H++14H2O (7)
+2H++14H2O (7)
2FeS2+7.5H2O2+H+→Fe3++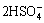 +7H2O (8)
+7H2O (8)
The resulting Fe(III) ions led to catalytic decomposition of H2O2 [50] which caused significant decrease of its concentration and release of heat and gaseous oxygen [51].
In the current work, visual observation of the experiments showed that the leaching reaction was very turbulent in the first 60 min (accompanied by hissing and boiling) due to the intensive hydrogen peroxide decomposition and oxygen effervescence, especially at higher hydrogen peroxide concentrations (i.e., equal to or in excess of 1.5 mol/L). As a result of the catalytic decomposition of peroxide, which was an exothermic process followed by turbulent generation of oxygen, there was an increase in temperature (as shown in Table 2). This increase led to faster peroxide decomposition and significant loss of the active oxygen in the form of bubbles. Due to rapid exothermic decomposition of hydrogen peroxide in solution, isothermal leaching conditions could disappear and the active oxygen might not be sufficiently used for oxidation of chalcopyrite [37], which had a negative effect on the dissolution rate.
Table 2 Change of temperature (°C) of reaction mixture with time as function of hydrogen peroxide concentration (Conditions: 0.5 mol/L HCl, 400 r/min)
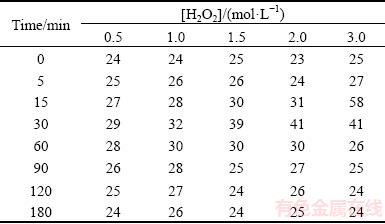
Table 2 shows that maximum temperatures were registered mainly within 30-60 min of reaction. The shape of the dissolution curves (Fig. 7) shows a tendency to increase mainly up to 60 min, which means that the oxidation of chalcopyrite practically occurred up to that time. Based on the results, it can be concluded that the catalytic decomposition of hydrogen peroxide was accelerated with the increase of temperature, which led to a significant lowering of its concentration in the leaching system (reduction of the oxidizing power of hydrogen peroxide).
A significant slowing of the leaching rate (Fig. 7) indicates that a product layer on the mineral surface was created which also reduced the reaction rate. The previous research results have shown that iron was released from the chalcopyrite matrix with formation of product layer of the following composition Cu1-xFe1-yS2-z. This layer was an intermediate product, which, mixed with sulfur, formed a passive electron conductive layer on the mineral surface [52]. HABASHI and TOOR [53] emphasized that at the constant temperature, time, O2 pressure, and molar ratio of CuFeS2/HCl above 0.25, an inverse relation between Cu and Fe in solution was noted, which suggested that iron(III) ions had influence on chalcopyrite leaching in HCl solution (Reaction (9)):
CuFeS2+4Fe3+→Cu2++5Fe2++2S (9)
The formation of iron compounds as reaction products can lead to the inhibition of reaction rate (Reaction (10)):
2Fe2++1/2O2+3H2O→2FeOOH+4H+ (10)
It was observed that the greater part of unreacted chalcopyrite was attached to the elemental sulphur pellets [53].
The XRD analysis of solid residue produced after oxidation in 0.5 mol/L HCl and 2.0 mol/L H2O2 (Fig. 8) revealed the presence of elemental sulphur traces on the mineral surface. This indicated that sulphide sulphur from chalcopyrite was oxidized according to Eq. (2) and that it could act as a diffusion barrier which limited the overall leaching rate.
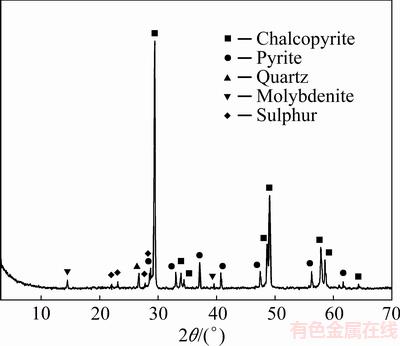
Fig. 8 X-ray diffraction pattern of leaching residue (Conditions: 0.5 mol/L HCl, 2.0 mol/L H2O2, room temperature, without thermostating, time 180 min, 400 r/min)
3.4 Effect of temperature
The effect of temperature was investigated in a temperature range from room temperature (without thermostating) to 60 °C in solution containing 0.5 mol/L HCl and 2.0 mol/L H2O2 (Fig. 9).
From Fig. 9, it is evident that increase in temperature led to an increase in copper extraction at the initial leaching stage. The final copper extractions were relatively low and varied from 24% to 29% in the studied temperature range. The obtained results show that the temperature did not have a significant influence on the rate of chalcopyrite oxidation. A significant slowing of the leaching rate in the final leaching stage was noted. The form of the dissolution curves showed a formation of plateau after 60 min of oxidation at temperatures up to 30 °C. The same occurrence was noticed at 40-60 °C after 30 min of oxidation, which could be attributed to a more rapid reaction on higher temperatures and formation of the reaction products. Therefore, a temperature of 40 °C was selected. The intensive decomposition of hydrogen peroxide with the decrease of reaction rate of chalcopyrite and pyrite was also observed at temperatures above 40 °C [23,43]. AGACAYAK et al [22] suggested that Cu extraction rate increased with increasing temperatures in the hydrogen peroxide solution during the early period of the leaching process. However, a significant slowing of the leaching rate in the final stage of leaching was noted due to the decomposition of peroxide at higher temperatures (above 60 °C).
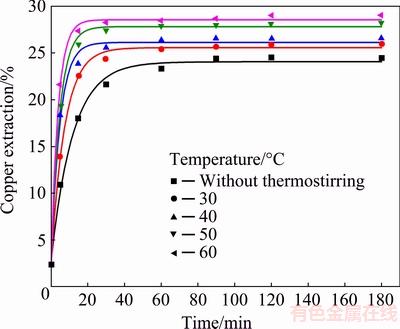
Fig. 9 Copper extraction from chalcopyrite concentrate as function of time at different temperatures (Conditions: 0.5 mol/L HCl, 2.0 mol/L H2O2, 400 r/min)
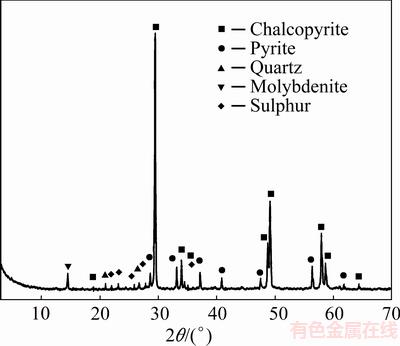
Fig. 10 X-ray diffraction pattern of leaching residue (Conditions: 0.5 mol/L HCl, 2.0 mol/L H2O2, 40 °C, time 90 min)
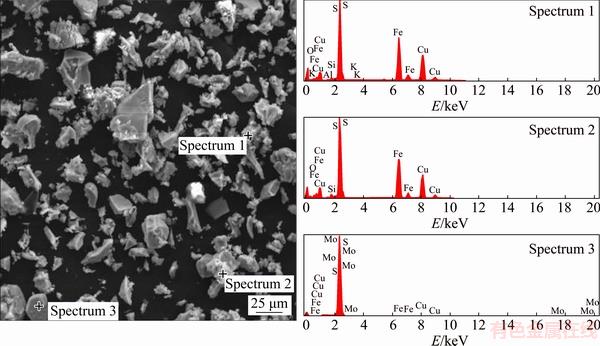
Fig. 11 SEM photograph with EDS spectra of leach residue (leached in 0.5 mol/L HCl, 2.0 mol/L H2O2, 40 °C, time 90 min, 400 r/min)
Table 3 EDS results of leaching residue
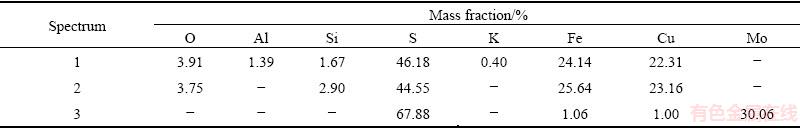
The XRD analysis of the leached residue (Fig. 10) shows that, along with the presence of detected mineral phases, the elemental sulphur was present as well. The formed elemental sulfur created a passive layer on the chalcopyrite surface, and thereby hindered the transfer of both lixiviate to the mineral’s surface and the solubilized species from the mineral’s surface to the leaching solution. SEM/EDS analysis (Fig. 11 and Table 3) of the leaching residue (leached in 0.5 mol/L HCl, 2.0 mol/L H2O2, 40 °C, time 90 min, 400 r/min) supported the results of the XRD analysis.
It is well known that the mineralogical origin and genesis have a great influence on the oxidation of chalcopyrite. The difference in the obtained results in numerous studies of chalcopyrite concentrate leaching can be attributed to different chemical and mineralogical compositions of chalcopyrite samples (the presence of other sulfide minerals, the presence of different gangue minerals, impurities, etc).
3.5 Effect of initial hydrochloric acid concentration
The experiments on the effect of the initial hydrochloric acid concentration (0.1-3.0 mol/L) on chalcopyrite dissolution were performed using 2.0 mol/L H2O2 at 40 °C.
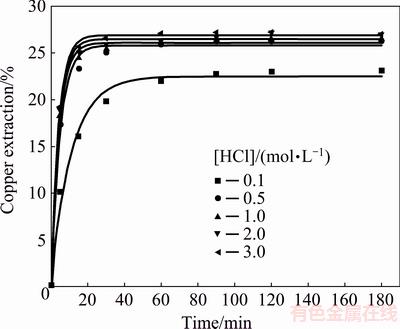
Fig. 12 Copper extraction from chalcopyrite concentrate as function of time at different HCl concentrations (Conditions: 2.0 mol/L H2O2, 40 °C, 400 r/min)
The results (Fig. 12) showed that copper extraction increased slightly with increasing hydrochloric acid concentration. This was specifically indicated at HCl concentration from 0.1 to 0.5 mol/L that higher acid concentration (0.5-3.0 mol/L) did not have a significant effect on the reaction rate and final copper extraction. The final copper extractions attained were relatively low (23%-27% Cu) in the studied concentration range (0.1-3.0 mol/L). The presented results led to a conclusion that the oxidation effect of the hydrogen peroxide was stronger in the acidic environment (0.5-3.0 mol/L HCl). It could be further noticed that the copper extraction slightly depended on the concentration of H+ ions in the given range of the acid concentrations. Under these conditions, the chalcopyrite oxidation reaction was very fast in the first 10 min, and after 30 min a plateau was formed on the curves, showing that the oxidation reaction was practically over and that the degree of the copper extraction was relatively low (up to 27%). It was previously discussed that the catalytic and self-decomposition reactions of hydrogen peroxide (Reactions (4) and (5)) occurred in the system of HCl-H2O2, following the occurrence of a certain amount of the molecular oxygen. Considering the fact that after 30 min there was no significant increase in the copper extraction, the molecular oxygen did not act as an oxidant arose. In addition to this fact, a layer of reaction products, which passivized the surface, was formed due to the rapid chalcopyrite oxidation in the presence of hydrogen peroxide. This hindered further dissolution of chalcopyrite. The existence of a layer of elemental sulphur was confirmed with the XRD and SEM/EDS analysis of the examined system.
By comparing the results obtained in the present study with those found during leaching of pyrite in HCl-H2O2 solutions [33], it can be seen that the concentration of the acid had different influence on the dissolution kinetics of these two minerals. The oxidation rate of pyrite decreased with the increase of the HCl concentration, probably due to the adsorption of chloride ions on the surface of the pyrite particles. Chloride ions had a high adsorption capacity and their adsorption on the mineral surface diminished the contact surface between peroxide and mineral particles, which led to a reduction in dissolution rate.
4 Kinetics analysis
Dissolution process of chalcopyrite may be described by a number of reaction models for the heterogeneous solid-liquid reactions already proposed in the literatures [54-59]. Generally, the dissolution mechanism of chalcopyrite is based on three main kinetics models: surface reaction model, diffusion model through the fluid film or through the product layer and mixed kinetic model containing diffusion and surface reaction components, which simultaneously take place. In chemical controlled processes, the rise in temperature usually has a pronounced positive effect on the reaction kinetics. On the other hand, if reaction kinetics is slightly affected by temperature together with a mild effect of agitation, it can be concluded that the kinetics of leaching is controlled by diffusion. A value of activation energy less than 40 kJ/mol suggests that the leaching process is controlled by diffusion (parabolic leaching), while activation energy over 40 kJ/mol implies chemical reaction (linear leaching) [59].
The obtained experimental results of the chalcopyrite oxidation in the present work were used in the process of choosing the appropriate kinetics model. The basic criterion for applying each kinetic equation in the determining of the kinetic process parameters is its linearization in an appropriate coordinate system using the experimental results X=f(τ). According to obtained shape of dissolution curves in this work, it can be seen that curves have an exponential dependence. Function inverse to exponential function is a logarithmic function that gives the best linearization of obtained results, so a logarithmic equation was needed to describe the leaching kinetics. Therefore, the kinetic analysis in this work was evaluated via Kazeev-Erofeev equation [60].
According to Eq. (11),
1-X=exp(-kt) (11)
where X is the degree of copper reacted, k is the apparent rate constant, and t is time.
And introducing the parameter Xm, which represents the maximum extraction of copper in the system determined from the dissolution curves (for t→∞), allowed the use of a classical first order kinetics equation:
X=Xm[1-exp(-kt)] (12)
The logarithm of Eq. (12) gave following equation:
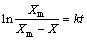 (13)
(13)
The selected kinetics model (Eq. (13)) was the most appropriate for fitting experimental results in a wide temperature range and low extraction values.
4.1 Effect of temperature
Based on the experimental results in the present work, the leaching kinetics was mildly affected by temperature. The kinetic curves (Fig. 9) were linearized by means of Eq. (13), as shown in Fig. 13. The results from Fig. 13 indicated that the good linearization existed and that this model could be used to describe the kinetics of the chalcopyrite oxidation for given experimental conditions with satisfying correlation coefficients values, R2. Straight lines started from the origin of the coordinate system. Apparent rate constants, ks, for each temperature were obtained from the slopes of the lines in Fig. 13. The results obtained under conditions without thermostating of the solution were not taken into consideration when the activation energy was determined due to the change in the reaction temperature of the system. Therefore, the activation energy was determined for the temperature range of 30-60 °C.
The activation energy in the range of temperatures studied was calculated to be 19.6 kJ/mol from the diagram of ln ks versus 1/T, as shown in Fig. 14. This value of apparent activation energy (<40 kJ/mol) supported diffusion control mechanism [58,59] for the dissolution process of chalcopyrite with H2O2 in HCl solution.
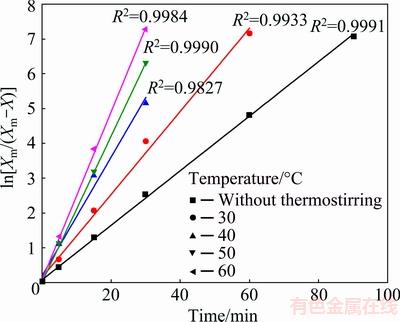
Fig. 13 Variation in ln[Xm/(Xm-X)] with time at different temperatures
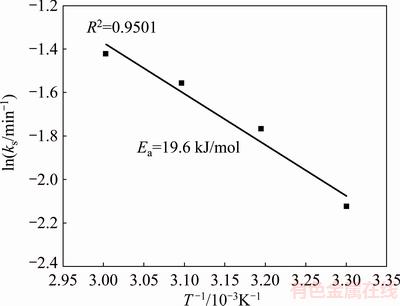
Fig. 14 Arhenius plot for chalcopyrite dissolution
Similar value of activation energy of 24 kJ/mol was found for the chalcopyrite dissolution in acidic potassium dichromate solution [26]. It was found that the kinetics of dissolution of chalcopyrite in acidic potassium dichromate in temperature range of 50-97 °C could be fitted by the shrinking core model with diffusion through a porous sulphur layer. The activation energies of 60 kJ/mol [23] and 39 kJ/mol [35] were found in the investigations of chalcopyrite oxidation by hydrogen peroxide in sulphuric acid.
4.2 Effect of hydrochloric acid and hydrogen peroxide concentrations
The results of the effect of hydrochloric acid and hydrogen peroxide concentrations were applied to Eq. (13). The kinetic curves (Fig. 7(a) and Fig. 12) were linearized by means of Eq. (13), as shown in Fig. 15.
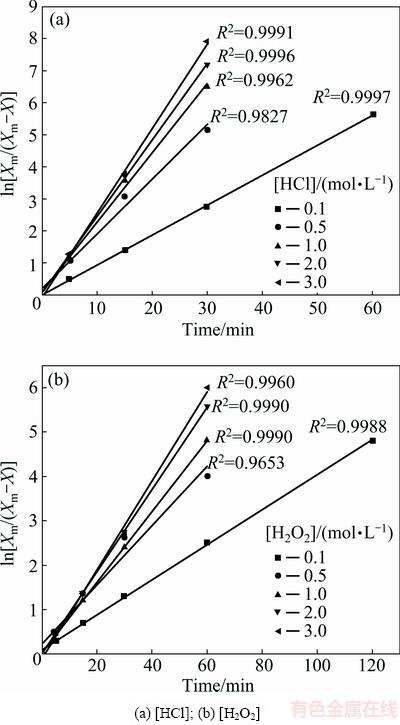
Fig. 15 Variation in ln[Xm/(Xm-X)] with time at different concentrations
The results from Fig. 15 indicated that the good linearization existed. The rate constants were calculated from the slopes of the straight lines from Fig. 15. Logarithms of these values were drawn as a function of the logarithm of concentrations of HCl and H2O2, respectively (as shown in Fig. 16). The reaction orders for HCl and H2O2 were determined to be 0.30 and 0.53, respectively.
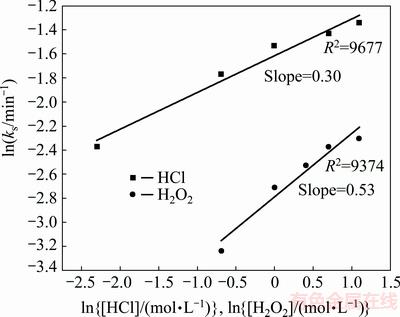
Fig. 16 Plot of ln ks vs ln[HCl] and ln[H2O2]
5 Conclusions
1) Leaching of chalcopyrite concentrate with mixed lixiviant containing hydrochloric acid and hydrogen peroxide was investigated. The leaching results showed that even the application of strong oxidant, such as hydrogen peroxide, did not result in complete metal dissolution from chalcopyrite concentrate in hydrochloric acid solution. In most of the experiments, copper was dissolved in the first 60 min of reaction. The maximum final copper extraction of 33% was attained with 3.0 mol/L H2O2 in 0.5 mol/L HCl at room temperature.
2) The dissolution process was described by first order kinetics equation, X=Xm[1-exp(-kt)]. The apparent activation energy of 19.6 kJ/mol indicated that the leaching process is controlled by diffusion through the product layer. The reaction orders for HCl and H2O2 were evaluated to be 0.30 and 0.53, respectively.
3) The behavior of chalcopyrite observed could be explained from both hydrogen peroxide decomposition and sulfur formation viewpoints. In the hydrochloric acid/hydrogen peroxide leaching system, the decomposition of hydrogen peroxide was catalyzed by Fe(III) ions, chlorine and solid mineral particles, which led to reduction of the oxidizing power of hydrogen peroxide and decrease of the dissolution rate.
4) The XRD and SEM/EDS analysis of leaching residue showed the formation of elemental sulfur that probably passivized the chalcopyrite surface and thus reduced the initial reactivity of the mineral.
Acknowledgments
The authors acknowledge the financial support from the Ministry of Education, Science and Technological Development of the Republic of Serbia (Projects No. 34025 and No. 172031).
References
[1] DAVENPORT W G, KING M, SCHLESINGER M, BISWAS A K. Extractive metallurgy of copper [M]. 4th ed. UK: Pergamon, 2002.
[2] ZHAO Hong-bo, HU Ming-hao, LI Yi-ni, ZHU Shan, QIN Wen-qing, QIU Guan-zhou, WANG Jun. Comparison of electrochemical dissolution of chalcopyrite and bornite in acid culture medium [J]. Transactions of Nonferrous Metals Society of China, 2015, 25: 303-313.
[3] DENG J, WEN S, YIN Q, WU D, SUN Q. Leaching of malachite using 5-sulfosalicylic acid [J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 71: 20-27.
[4] BOGDANOVI G D, STANKOVI
G D, STANKOVI V D, TRUMI
V D, TRUMI M S, ANTI
M S, ANTI D V, TRUMI
D V, TRUMI M
M  . Leaching of low-grade copper ores: A case study for “Kraku Bugaresku-Cementacija” Deposits (Eastern Serbia) [J]. Journal of Mining and Metallurgy A, 2016, 52: 45-56.
. Leaching of low-grade copper ores: A case study for “Kraku Bugaresku-Cementacija” Deposits (Eastern Serbia) [J]. Journal of Mining and Metallurgy A, 2016, 52: 45-56.
[5] WANG S. Copper leaching from chalcopyrite concentrates [J]. JOM, 2005, 57: 48-51.
[6] DREISINGER D. Copper leaching from primary sulfides: Options for biological and chemical extraction of copper [J]. Hydrometallurgy, 2006, 83: 10-20.
[7] QIU Ting-sheng, NIE Guang-hua, WANG Jun-feng, CUI Li-feng. Kinetic process of oxidative leaching of chalcopyrite under low oxygen pressure and low temperature [J]. Transactions of Nonferrous Metals Society of China, 2007, 17: 418-422.
[8] PAN Hao-dan, YANG Hong-ying, TONG Lin-lin, ZHONG Cong-bin, ZHAO Yu-shan. Control method of chalcopyrite passivation in bioleaching [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 2255-2260.
[9] DUTRIZAC J E. Elemental sulphur formation during the ferric chloride leaching of chalcopyrite [J]. Hydrometallurgy, 1990, 23: 153-176.
[10] HARMER S, THOMAS J E, FORNASIERO D, GERSON A R. The evolution of surface layers formed during chalcopyrite leaching [J]. Geochimica et Cosmochimica Acta, 2006, 70: 4392-4402.
[11] LI J, KAWASHIMA N, KAPLUN K, ABSOLON V J, GERSON A R. Chalcopyrite leaching: The rate controlling factors [J]. Geochimica et Cosmochimica Acta, 2010, 74: 2881-2893.
[12] LU J, DREISINGER D. Copper leaching from chalcopyrite concentrate in Cu(II)/Fe(III) chloride system [J]. Minerals Engineering, 2013, 45: 185-190.
[13] LUNDSTROM M, LIIPO J, TASKINEN P, AROMAA J. Copper precipitation during leaching of various copper sulfide concentrates with cupric chloride in acidic solutions [J]. Hydrometallurgy, 2016, 166: 136-142.
[14] MIKI H, NICOL M. The dissolution of chalcopyrite in chloride solutions: IV. The kinetics of the auto-oxidation of copper(I) [J]. Hydrometallurgy, 2011, 105: 246-250.
[15]  L, NICOL M, MIKI H. The dissolution of chalcopyrite in chloride solutions: Part 1. The effect of solution potential [J]. Hydrometallurgy, 2010, 103:108-113.
L, NICOL M, MIKI H. The dissolution of chalcopyrite in chloride solutions: Part 1. The effect of solution potential [J]. Hydrometallurgy, 2010, 103:108-113.
[16] WATLING H R. Chalcopyrite hydrometallurgy at atmospheric pressure: 2. Review of acidic chloride process options [J]. Hydrometallurgy, 2014, 146: 96-110.
[17] HAVLIK T, SKROBIAN M, BALAZ P, KAMMEL R. Leaching of chalcopyrite concentrate with ferric chloride [J]. International Journal of Mineral Processing, 1995, 43: 61-72.
[18] TURKMEN Y, KAYA E. Acidified ferric chloride leaching of a chalcopyrite concentrate [J]. The Journal of Ore Dressing, 2009, 11: 16-24.
[19] NICOL M, MIKI H,  L. The dissolution of chalcopyrite in chloride solutions: Part 3. Mechanisms [J]. Hydrometallurgy, 2010, 103: 86-95.
L. The dissolution of chalcopyrite in chloride solutions: Part 3. Mechanisms [J]. Hydrometallurgy, 2010, 103: 86-95.
[20] LUNDSTROM M, AROMAA J, FORSEN O, HYVARINEN O, BARKER M H. Leaching of chalcopyrite in cupric chloride solution [J]. Hydrometallurgy, 2005, 77: 89-95.
[21] TURKMEN Y, KAYA E, YAVUZHAN O. Leaching of chalcopyrite flotation concentrate with CuCl2-NaCl-HCl [C]//Proceedings of the XIII International Mineral Processing Symposium. Bodrum, 2012: 361.
[22] AGACAYAK T, ARAS A, AYDOGAN S, ERDEMOGLU M. Leaching of chalcopyrite concentrate in hydrogen peroxide solution [J]. Physicochemical Problems of Mineral Processing, 2014, 50: 657-666.
[23] ANTONIJEVI M M, JANKOVI
M M, JANKOVI Z D, DIMITRIJEVI
Z D, DIMITRIJEVI M D. Kinetics of chalcopyrite dissolution by hydrogen peroxide in sulphuric acid [J]. Hydrometallurgy, 2004, 71: 329-334.
M D. Kinetics of chalcopyrite dissolution by hydrogen peroxide in sulphuric acid [J]. Hydrometallurgy, 2004, 71: 329-334.
[24] TURAN M D, ALTUNDOGAN H S. Leaching of chalcopyrite concentrate with hydrogen peroxide and sulphuric acid in an autoclave system [J]. Metallurgical and Materials Transactions B, 2013, 44: 809-819.
[25] ANTONIJEVI M M, JANKOVI
M M, JANKOVI Z D, DIMITRIJEVI
Z D, DIMITRIJEVI M D. Investigation of the kinetics of chalcopyrite oxidation by potassium dichromate [J]. Hydrometallurgy 1994, 35: 187-201.
M D. Investigation of the kinetics of chalcopyrite oxidation by potassium dichromate [J]. Hydrometallurgy 1994, 35: 187-201.
[26] AYDOGAN S, UCAR G, CANBAZOGLU M. Dissolution kinetics of chalcopyrite in acidic potassium dichromate solution [J]. Hydrometallurgy, 2006, 81: 45-51.
[27] UCAR G, BOYRAZLI M, AYDOGAN S. Dissolution of fine particle chalcopyrite concentrate in acidic potassium dichromate solution [C]//Proceedings of 7th International Scientific Conference of Modern Management of Mine Producing, Geology and Environmental Protection. Albena: SGEM, 2007: 44-51.
[28] HAVLIK T, SKROBIAN M. Acid leaching of chalcopyrite in the presence of ozone [J]. Canadian Metallurgical Quarterly, 1990, 29: 133-139.
[29] PEDROZA F R C, AGUILAR M J S, TREVINO T P, LUEVANOS A M, CASTILLO M S. Treatment of sulfide minerals by oxidative leaching with ozone [J]. Mineral Processing and Extractive Metallurgy Review, 2012, 33: 269-279.
[30] KERTALLI E, van RIJNSOEVER L S, PAUNOVIC V, NEIRA D'ANGELO M F, SCHOUTEN J C, NIJHUIS T A. Propylene epoxidation with hydrogen peroxide in acidic conditions [J]. Chemical Engineering Science, 2016, 156: 36-43.
[31] HE Mei-feng, WANG Hao, JIANG Hong, ZHAO Su, PAN Deng. Effect of hydrogen peroxide concentration on surface properties of Ni-Cr alloys [J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 1353-1358.
[32] LI Qian, RAO Xue-fei, XU Bin, YANG Yong-bin, LIU Ting, JIANG Tao, HU Long. Extraction of manganese and zinc from their compound ore by reductive acid leaching [J]. Transactions of Nonferrous Metals Society of China, 2017, 27: 1172-1179.
[33] DIMITRIJEVI M, ANTONIJEVI
M, ANTONIJEVI M M, DIMITRIJEVI
M M, DIMITRIJEVI V. Investigation of the kinetics of pyrite oxidation by hydrogen peroxide in hydrochloric acid solutions [J]. Minerals Engineering, 1999, 12: 165-174.
V. Investigation of the kinetics of pyrite oxidation by hydrogen peroxide in hydrochloric acid solutions [J]. Minerals Engineering, 1999, 12: 165-174.
[34] GREENWOOD N N, EARNSHAW A. Chemistry of the elements [M]. New York: Pergamon Press, 1984.
[35] ADEBAYO A O, IPINMOROTI K O, AJAYI O O. Dissolution kinetics of chalcopyrite with hydrogen peroxide in sulphuric acid medium [J]. Chemical and Biochemical Engineering Quarterly, 2003, 17: 213-218.
[36] MAHAJAN V, MISRA M, ZHONG K, FUERSTENAU M C. Enhanced leaching of copper from chalcopyrite in hydrogen peroxide–glycol system [J]. Minerals Engineering, 2007, 20: 670-674.
[37] TURAN D, SARI Z A, MILLER J D. Leaching of blended copper slag in microwave oven [J]. Transactions of Nonferrous Metals Society of China, 2017, 27: 1404-1410.
[38]  O J, LAPIDUS G T. Improvement of chalcopyrite dissolution in acid media using polar organic solvents [J]. Hydrometallurgy, 2013, 131-132: 120-126.
O J, LAPIDUS G T. Improvement of chalcopyrite dissolution in acid media using polar organic solvents [J]. Hydrometallurgy, 2013, 131-132: 120-126.
[39]  A, LAPIDUS G T. Study of chalcopyrite leaching from a copper concentrate with hydrogen peroxide in aqueous ethylene glycol media [J]. Hydrometallurgy, 2017, 169: 192-200.
A, LAPIDUS G T. Study of chalcopyrite leaching from a copper concentrate with hydrogen peroxide in aqueous ethylene glycol media [J]. Hydrometallurgy, 2017, 169: 192-200.
[40] VAZARLIS H G. Hydrochloric acid-hydrogen peroxide leaching and metal recovery from a Greek zinc-lead bulk sulphide concentrate [J]. Hydrometallurgy, 1987, 19: 243-251.
[41] BABA A A, ADEKOLA F A. Comparative analysis of the dissolution kinetics of galena in binary solutions of HCl/FeCl3 and HCl/H2O2 [J]. International Journal of Minerals, Metallurgy and Materials, 2011, 18: 9-17.
[42] HE Gui-xiang, ZHAO Zhong-wei, WANG Xiao-bo, LI Jiang-tao, CHEN Xing-yu, HE Li-hua, LIU Xu-heng. Leaching kinetics of scheelite in hydrochloric acid solution containing hydrogen peroxide as complexing agent [J]. Hydrometallurgy, 2014, 144-145: 140-147.
[43] DIMITRIJEVI M, ANTONIJEVI
M, ANTONIJEVI M M, JANKOVI
M M, JANKOVI Z. Kinetics of pyrite dissolution by hydrogen peroxide in perchloric acid [J]. Hydrometallurgy, 1996, 42: 377-386.
Z. Kinetics of pyrite dissolution by hydrogen peroxide in perchloric acid [J]. Hydrometallurgy, 1996, 42: 377-386.
[44] ANTONIJEVI M, DIMITRIJEVI
M, DIMITRIJEVI D, JANKOVI
D, JANKOVI Z. Leaching of pyrite with hydrogen peroxide in sulphuric acid [J]. Hydrometallurgy, 1997, 46: 71-83.
Z. Leaching of pyrite with hydrogen peroxide in sulphuric acid [J]. Hydrometallurgy, 1997, 46: 71-83.
[45] AYDOGAN S. Dissolution kinetics of sphalerite with hydrogen peroxide in sulphuric acid medium [J]. Chemical Engineering Journal, 2006, 123: 65-70.
[46] SHU Chi-Min, YANG Yuh-Joang. Using VSP2 to separate catalytic and self-decomposition reactions for hydrogen peroxide in the presence of hydrochloric acid [J]. Thermochimica Acta, 2002, 392-393: 259-269.
[47] VOLL F A P, PALU P, SANTOS J B O. Influence of catalyst treatments on the decomposition of hydrogen peroxide on supported palladium catalysts [J]. Latin American Applied Research, 2011, 41: 305-310.
[48] EARY L E. Catalytic decomposition of hydrogen peroxide by ferric ion in dilute sulfuric acid solutions [J]. Metallurgical Transactions B, 1985, 16: 181-186.
[49] CARRILLO-PEDROZA F R, DAVALOS-SANCHEZ A, SORIA- AGUILAR M, PECINA-TREVINO E T. Coal desulfurization in oxidative acid media using hydrogen peroxide and ozone: A kinetic and statistical approach [J]. Energy & Fuel, 2009, 23: 3703-3710.
[50] CHIRITA P. Hydrogen peroxide decomposition by pyrite in the presence of Fe(III)-ligands [J]. Chemical and Biochemical Engineering Quarterly, 2009, 23: 259-265.
[51] ETO I, AKIYOSHI M, MIYAKE A, OGAWE T, MATSUNAGA T. Hazard evaluation of runaway reaction of hydrogen peroxide– Influence of contamination of various ions [J]. Journal of Loss Prevention in the Process Industries, 2009, 22: 15-20.
[52] LU Z Y, JEFFREY M I, LAWSON F. An electrochemical study of the effect of chloride ions on the dissolution of chalcopyrite in acidic solutions [J]. Hydrometallurgy, 2000, 56: 145-155.
[53] HABASHI F, TOOR T. Aqueous oxidation of chalcopyrite in hydrochloric acid [J]. Metallurgical Transactions B, 1979, 10: 49-56.
[54] LI Y, KAWASHIMA N, LI J, CHANDRA A, GERSON A. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite [J]. Advances in Colloid and Interface Science, 2013, 197: 1-32.
[55] LI Xin, XING Pengfei, DU Xinghong, GAO Shuaibo, CHEN Chen. Influencing factors and kinetics analysis on the leaching of iron from boron carbide waste-scrap with ultrasound-assisted method [J]. Ultrasonics Sonochemistry, 2017, 38: 84-91.
[56] VELOSO T C, PEIXOTO J J M, PEREIRA M S, LEAO V A. Kinetics of chalcopyrite leaching in either ferric sulphate or cupric sulphate media in the presence of NaCl [J]. International Journal of Mineral Processing, 2016, 48: 147-154.
[57] LIU Zhi-xiong, XIANG Yan-hong, YIN Zhou-lan, WU Xian-wen, JIANG Jian-bo, CHEN Yi-guang, XIONG Li-zhi. Oxidative leaching behavior of metalliferous black shale in acidic solution using persulfate as oxidant [J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 565-574.
[58] MA Jia-yu, DU Xue-lan, QIN Yuan-hang, WU Zai-kun, CHI Ru-an, WANG Cun-wen. Kinetics on leaching of potassium from phosphorus-potassium associated ore in HCl-H3PO4 media [J]. Transactions of Nonferrous Metals Society of China, 2017, 27: 1870-1877.
[59] DREISINGER D, ABED N. A fundamental study of the reductive leaching of chalcopyrite using metallic iron: Part I. Kinetic analysis [J]. Hydrometallurgy, 2002, 66: 37-57.
[60] FATEMI N, WHITEHEAD R, PRICE D, DOLLIMORE D. Some comments on the use of Avrami-Erofeev expressions and solid state decomposition rate constants [J]. Thermochimica Acta, 1986, 104: 93-100.
Sanja J. PETROVI 1, Grozdanka D. BOGDANOVI
1, Grozdanka D. BOGDANOVI 2, Milan M. ANTONIJEVI
2, Milan M. ANTONIJEVI 2
2
1. Mining and Metallurgy Institute Bor, Zeleni Bulevar 35, 19210 Bor, Serbia;
2. University of Belgrade, Technical Faculty in Bor, VJ 12, P. O. Box 50, 19210 Bor, Serbia
摘 要:本文旨在研究以双氧水为强氧化剂的黄铜矿精矿的盐酸浸出过程。研究搅拌速度、固液比、温度、HCl和H2O2浓度等浸出参数对金属浸出率的影响。室温下,用3.0 mol/L H2O2和0.5 mol/L HCl溶液与黄铜矿反应 180 min后,获得33%的最大铜浸出率。结果表明,在反应的前60 min,铜的浸出率增大;此后,由于双氧水的快速催化分解,铜浸出率基本上保持不变。此外,固液比对铜的浸出率影响显著,而且在最稀的悬浮液中(即固液比1:100)铜的浸出率最高。溶出过程可用一级动力学方程描述,表观活化能为19.6 kJ/mol,表明溶出过程受扩散控制,对于HCl和H2O2的反应级数分别为0.30和0.53。浸出渣的XRD和SEM/EDS分析结果表明,矿物表面生成单质硫,抑制浸出率的提高。
关键词:黄铜矿;浸出;双氧水;盐酸;硫
(Edited by Bing YANG)
Corresponding author: Grozdanka D. BOGDANOVI ; E-mail: gbogdanovic@tfbor.bg.ac.rs
; E-mail: gbogdanovic@tfbor.bg.ac.rs
DOI: 10.1016/S1003-6326(18)64788-0

