文章编号:1004-0609(2014)02-0506-05
铜镍硫化矿浮选中Cu(Ⅱ)和Ni(Ⅱ)离子对蛇纹石的活化机理
曹 钊1, 2,张亚辉1,孙传尧3,曹永丹2
(1. 东北大学 资源与土木工程学院,沈阳 110819;
2. 内蒙古科技大学 矿业工程学院,包头 014010;
3. 北京矿冶研究总院,北京 100044)
摘 要:通过纯矿物浮选、Zeta电位测试、红外光谱分析及溶液化学计算分析,研究Cu(Ⅱ)和Ni(Ⅱ)离子对蛇纹石浮选的活化机理。结果表明:溶液pH>6.5时,铜离子在水溶液中的优势组分为氢氧化铜沉淀;氢氧化铜沉淀覆盖在蛇纹石表面,与黄原酸根反应,从而活化蛇纹石浮选。溶液pH>8时,镍离子在水溶液中优势组分为氢氧化镍沉淀,能吸附于蛇纹石表面使其活化,但其活化作用弱于Cu2+。在酸性pH值范围内,铜、镍在水溶液中主要以Cu2+、Ni2+形式存在,对蛇纹石浮选没有活化作用。
关键词:铜镍硫化矿;浮选;活化;蛇纹石
中图分类号:TD951 文献标志码:A
Activation mechanism of serpentine by Cu(Ⅱ) and Ni(Ⅱ) ions in copper-nickel sulfide ore flotation
CAO Zhao1, 2, ZHANG Ya-hui1, SUN Chuan-yao3, CAO Yong-dan2
(1. College of Resources and Civil Engineering, Northeastern University, Shenyang 110819, China;
2. School of Mining Engineering, Inter Mongolia University of Science and Technology, Baotou 014010, China;
3. Beijing General Research Institute of Mining and Metallurgy, Beijing 100044, China)
Abstract: The activation mechanism of serpentine by Cu2+ and Ni2+ in copper-nickel sulfide ore floatation was investigated with pure mineral flotation tests, Zeta potential measurement, IR spectrum measurement and solution chemistry analysis. The results show that copper hydroxide Cu(OH)2 is the main component in cupric solution as pH>6.5, which will cover up serpentine and react with xanthate, activating the flotation of serpentine. And similarly, the nickel hydroxide Ni(OH)2 is the main component in nickelous solution at pH>8, which will be adsorbed onto serpentine and make it activated, and the activation of Ni2+ is weaker the that of Cu2+. While in acidic pH range, metal ions of Cu2+ and Ni2+ are the main species in solution, which can not activate the flotation of serpentine.
Key words: copper-nickel sulfide ore; flotation; activation; serpentine
铜镍硫化矿浮选中抑制MgO脉石矿物长期以来一直是选矿的技术难题,矿石中主要MgO脉石矿物有蛇纹石、滑石和绿泥石等[1]。研究表明,铜镍硫化矿浮选中MgO脉石矿物主要以如下3种方式进入浮选精矿:1) MgO脉石矿物与硫化铜镍矿以连生体方式进入精矿;2) 由于矿泥脉石罩盖在有用颗粒表面、粘附和疏水絮团夹杂方式进入精矿[2-4];3) 含镁层状硅酸盐矿物(如滑石)由于具有良好天然可浮性而进入浮选精矿[5-6]。
FORNASIERO等[7]和MALYSIAK等[8]研究表明MgO脉石矿物易受矿浆中Cu2+、Ni2+活化而能被黄药捕收上浮,并提出采用乙二胺等络合剂表面清洗方法去除蛇纹石、辉石、长石等脉石矿物表面金属离子的活化作用[9-13]。
本文作者通过纯矿物浮选、Zeta电位测试、红外光谱测试和溶液化学计算等方法,研究了硫化矿浮选中Cu2+、Ni2+金属离子对蛇纹石脉石矿物的活化作用,为进一步研究铜镍硫化矿浮选中MgO脉石矿物抑制剂提供理论及技术基础。
1 实验
1.1 矿样和试剂
蛇纹石纯矿物取自山东临朐,块矿经锤碎手选后用瓷球磨磨细,得到37~74 μm粒级的样品用于纯矿物浮选试验。
试验用盐酸、氢氧化钠、CuSO4·5H2O、NiSO4·6H2O均为分析纯,丁基钠黄药为工业纯,试验用水为一次蒸馏水。
1.2 研究方法
1.2.1 纯矿物浮选试验
纯矿物浮选试验采用40 mL XFG 型挂槽式浮选机。每次称取蛇纹石纯矿物2 g,置于浮选槽内,加入一定浓度浮选药剂(以调整剂、捕收剂、起泡剂顺序添加)并每次加药后搅拌5 min,测pH值后,浮选手工刮泡5 min,将槽内产品和泡沫产品烘干、称量后计算回收率。
1.2.2 Zeta电位测试
将蛇纹石纯矿物细磨至粒径<2 μm,取一定量配制成浓度为0.6 g/L的溶液,按试验条件调浆,采用Malvern Zetasizer Nano ZS90测量调浆后蛇纹石Zeta电位,每点测3次后取平均值。
1.2.3 红外光谱分析
称取粒径<2 μm的蛇纹石纯矿物2 g,按照试验条件调整pH值,加入10-4 mol/L的Cu2+和100 mg/L的丁黄药,充分搅拌后,静置待矿物沉降后,用吸管吸出上层清液,然后用蒸馏水充分洗涤矿物,固液分离后自然晾干,采用Nicolet FTIR-670 型傅立叶变换红外光谱仪进行红外光谱测试。
1.2.4 溶液化学计算分析方法
使用IUPAC SC-database数据库及SCDbase Species.exe溶液化学计算软件,计算并绘制出Cu2+、Ni2+溶液的优势组分,用OriginPro 8.0软件绘图。
2 结果和讨论
2.1 纯矿物浮选
在丁基黄药用量为1.2×10-4 mol/L、松醇油用量为15 mg/L时,考察不同Cu2+浓度下pH值对蛇纹石浮选的影响,结果如图1所示。由图1可以看出,溶液中无Cu2+且pH值在5~11范围内时,蛇纹石回收率均低于10%。溶液中存在不同浓度Cu2+时,蛇纹石回收率先随pH值升高而增大,在pH=9左右达到最大值,之后随pH值增大而减小。
相同pH值下不同Cu2+浓度对蛇纹石浮选影响表明,Cu2+浓度为5×10-4 mol/L时,蛇纹石浮选回收率最高;Cu2+浓度增加至1×10-3 mol/L蛇纹石回收率反而下降。这表明Cu2+浓度为5×10-4 mol/L时蛇纹石表面已被羟基铜络合物饱和吸附,溶液中过量Cu2+和羟基铜络合离子会额外消耗黄药而造成回收率下降。在适宜Cu2+浓度及弱碱性pH值条件下,Cu2+对蛇纹石浮选有最大活化作用。
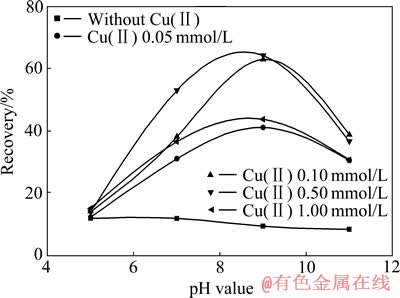
图1 pH值及Cu2+浓度对蛇纹石回收率的影响
Fig. 1 Effects of pH value and Cu2+ concentration on recovery of serpentine
图2所示为不同Ni2+浓度下pH值对蛇纹石浮选影响(丁基黄药用量1.2×10-4 mol/L、松醇油15 mg/L)。由图2可见,溶液中Ni2+浓度为5×10-4 mol/L时,蛇纹石回收率在pH=9左右达到最大值,表明Ni2+在一定浓度及弱碱性pH值条件下对蛇纹石有一定的活化作用,但其活化能力比Cu2+的弱。
2.2 Zeta电位测试分析

图2 pH值及Ni2+浓度对蛇纹石回收率影响
Fig. 2 Effects of pH value and Ni2+ concentration on recovery of serpentine
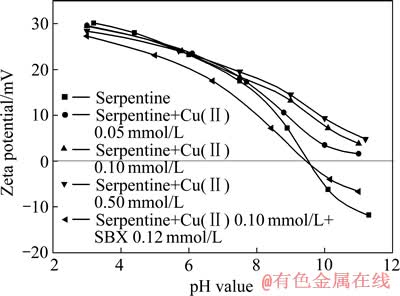
图3 pH值和Cu2+浓度对蛇纹石Zeta电位的影响
Fig. 3 Effects of pH value and Cu2+ concentration on Zeta potential of serpentine
考察不同Cu2+浓度对蛇纹石Zeta电位的影响,结果如图3所示。由图3可见,溶液中没有Cu2+存在 时,蛇纹石Zeta电位随pH值增大而减小,零电点pH值为9.4;溶液中加入Cu2+时,pH<6.5,蛇纹石Zeta电位没有变化,表明Cu2+在蛇纹石表面吸附较少;pH>6.5,蛇纹石Zeta电位正移,且Cu2+浓度越大,蛇纹石Zeta电位正移幅度越大,表明Cu2+或铜的羟基络合物能吸附于矿物表面,增大蛇纹石表面Zeta电位。加入丁基黄药后,黄原酸根与蛇纹石表面吸附的铜离子反应生成黄原酸铜,从而屏蔽Cu2+的正电荷,降低蛇纹石表面Zeta电位。
图4所示为不同Ni2+浓度对蛇纹石Zeta电位的影响。由图4可见,溶液中存在Ni2+时,pH<8,蛇纹石Zeta电位没有变化,表明Ni2+在蛇纹石表面吸附较少;pH>8,蛇纹石Zeta电位正移,且Ni2+浓度越大,蛇纹石Zeta电位正移幅度越大,表明Ni2+ 或镍的羟基络合物能吸附于矿物表面,增大蛇纹石表面Zeta电位。加入丁基黄药后,黄原酸根与蛇纹石表面吸附的镍离子反应生成黄原酸镍,从而降低蛇纹石表面Zeta电位。
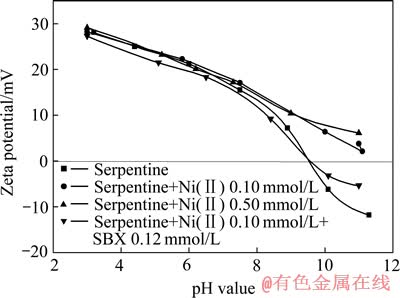
图4 pH值和Ni2+浓度对蛇纹石Zeta电位的影响
Fig. 4 Effect of pH value and Ni2+ concentration on Zeta potential of serpentine
2.3 红外光谱分析

图5 蛇纹石与丁黄药作用前后的红外光谱
Fig. 5 Contrast infrared spectra of adsorbed onto serpentine
为了研究蛇纹石被Cu2+活化前后与丁黄药的作用情况,进行了红外光谱分析,结果如图5所示。由图5可见,谱线a为丁基钠黄药的红外光谱,1 178.5和1 155.4 cm-1处为C—O—C键不对称伸缩振动峰,1 104.8 cm-1处为C—O—C键对称伸缩振动峰,1 071.5 cm-1处为C=S键伸缩振动峰[14-16];谱线b为黄原酸铜的红外光谱,1 145.7 cm-1处为C—O—C键不对称伸缩振动峰,1 110.3 cm-1处为C—O—C键对称伸缩振动峰,1 020.8 cm-1处为C=S键伸缩振动峰;谱线c为蛇纹石与Cu2+作用后,与丁黄药反应的红外光谱,可见丁黄药与蛇纹石表面铜反应后,出现了黄药中C—O—C键伸缩振动峰(移至1 125.2和1 085.3 cm-1处)及C=S键伸缩振动峰(移至1 042.6 cm-1处),表明蛇纹石表面吸附的铜离子能与黄原酸根反应生成黄原酸铜;谱线d为蛇纹石的红外光谱;谱线e为蛇纹石与丁黄药反应后的红外光谱,与谱线a、b、c及d对比,谱线e只有蛇纹石对应吸收峰,没有出现丁黄药及黄原酸铜所含基团的吸收峰,表明丁黄药没有在蛇纹石表面吸附,不能与蛇纹石表面直接反应。
2.4 Cu(Ⅱ)和Ni(Ⅱ)水溶液的优势组分计算分析
溶液中Cu2+浓度为10-4 mol/L,计算得Cu2+水溶液中优势组分分布如图6所示。由图6可见,溶液pH<6.5时,溶液中主要组分为Cu2+。纯矿物浮选和Zeta电位测试表明:溶液pH<6.5时,Cu2+不能在蛇纹石表面吸附,所以对蛇纹石浮选无活化作用,也不会改变蛇纹石表面Zeta电位。溶液pH>6.5时,溶液中优势组分为氢氧化铜沉淀(Cu(OH)2)。氢氧化铜沉淀覆盖在蛇纹石表面,与溶液中黄原酸根反应,从而活化蛇纹石浮选。
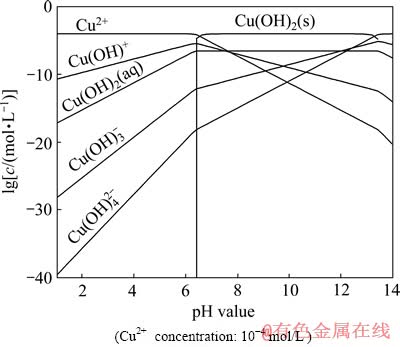
图6 Cu2+溶液体系lg c—pH图
Fig. 6 lg c—pH diagram of Cu2+ solution system
溶液中Ni2+浓度为10-4 mol/L,计算得Ni2+水溶液中优势组分分布如图7所示。由图7可见,溶液pH<8时,溶液中主要组分为Ni2+。纯矿物浮选和Zeta电位测试表明:pH<8时,Ni2+不能在蛇纹石表面吸附,所以对蛇纹石浮选没有活化作用,也不会改变蛇纹石表面Zeta电位。溶液pH>8时,溶液中优势组分为氢氧化镍沉淀(Ni(OH)2)。纯矿物浮选和Zeta电位测试表明:pH>8时,氢氧化镍沉淀覆盖在蛇纹石表面,从而对蛇纹石浮选起到活化作用。
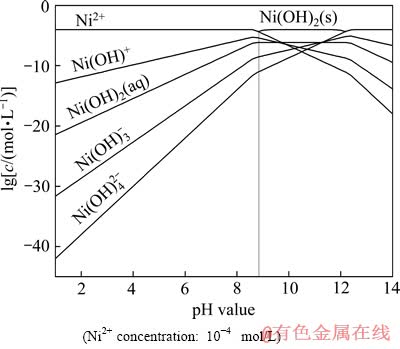
图7 Ni2+溶液体系lg c — pH图
Fig. 7 lg c — pH diagram of Ni2+ (Ni2+ concentration: 10-4 mol/L)
3 结论
1) 纯矿物浮选表明:黄药作捕收剂时,Cu2+、Ni2+分别在溶液pH>6.5和pH>8时对蛇纹石浮选具有活化作用,且Cu2+对蛇纹石的活化能力大于Ni2+对蛇纹石的活化能力。
2) Zeta电位测试表明:Cu2+在溶液pH<6.5时对蛇纹石Zeta电位无影响,在溶液pH>6.5时能增大蛇纹石表面Zeta电位。在溶液pH>6.5时,Cu2+在溶液主要组分为氢氧化铜沉淀,覆盖在蛇纹石表面使其活化。Ni2+在溶液pH<8时对蛇纹石Zeta电位无影响,在溶液pH>8时能使蛇纹石表面Zeta电位正移。在溶液pH>8时,Ni2+在溶液主要组分为氢氧化镍沉淀,覆盖在蛇纹石表面,从而活化蛇纹石浮选。在酸性pH值范围内,铜、镍在水溶液中主要以Cu2+、Ni2+形式存在,对蛇纹石浮选没有活化作用。
3) 红外光谱分析表明:在溶液pH=9时蛇纹石与Cu2+作用后(优势组分为氢氧化铜沉淀),再与丁黄药反应的红外光谱中出现了黄药中C—O—C键伸缩振动峰(相对丁黄药移至1 125.2和1 085.3 cm-1处)及C=S键伸缩振动峰(移至1 042.6 cm-1处),表明覆盖在蛇纹石表面的铜离子能与黄原酸根反应生成黄原酸铜,从而导致蛇纹石表面的活化。
REFERENCES
[1] 张亚辉, 孟凡东, 孙传尧. 铜镍硫化矿浮选过程中MgO脉石矿物抑制途径探析[J]. 矿冶, 2012, 21(2): 1-5.
ZHANG Ya-hui, MENG Fan-dong, SUN Chuan-yao. Analysis of effective approaches for depressing MgO-containing gangue minerals in copper-nickel sulfide ore floatation[J]. Mining & Metallurgy, 2012, 21(2): 1-5.
[2] 龙 涛, 冯其明, 卢毅屏. 六偏磷酸钠在硫化铜镍矿浮选中的分散机理[J]. 中国有色金属学报, 2012, 22(6): 1763-1769.
LONG Tao, FENG Qi-ming, LU Yi-ping. Dispersive mechanism of sodium hexametaphosphate on flotation of copper-nickel sulphide[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(6): 1763-1769.
[3] EDWARDS C R, KIPKIE W B, AGAR G E. The effect of slime coatings of the serpentine minerals, chrysotile and lizardite, on pentlandite flotation[J]. International Journal of Mineral Processing, 1980, 7(1): 47-50.
[4] 唐 明, 张文彬. 在微细粒铜镍硫化矿浮选中蛇纹石类脉石矿物浮选行为研究[J]. 中国矿业, 2008, 17(2): 47-50.
TANG Min, ZHANG Wen-bin. A study on floatability of serpentine in ultra-fine copper-nickel sulfide[J]. China Mining Magazine, 2008, 17(2): 47-50.
[5] BEATTIE D A, HUYNH L, KAGGWA G B N, RALSTON J. The effect of polysaccharides and polyacrylamides on the depression of talc and the flotation of sulphide minerals[J]. Minerals Engineering, 2006, 19(6/8): 596-608.
[6] 卢烁十. 滑石的晶体化学研究及其在有色金属硫化矿选矿中的浮选现状和实践[J]. 矿冶, 2010, 19(3): 8-11.
LU Shuo-shi. Research on crystal chemistry of talc and review and application of talc flotation in minerals processing of nonferrous sulphide ores[J]. Mining & Metallurgy, 2010, 19(3): 8-11.
[7] FORNASIERO D, RALSTON J. Cu(Ⅱ) and Ni(Ⅱ) activation in the flotation of quartz, lizardite and chlorite[J]. International Journal of Mineral Processing, 2005, 76(1): 75-81.
[8] MALYSIAK V, SHACKLETON N J, O’CONNOR C T. An investigation into the floatability of a pentlandite–pyroxene system[J]. International Journal of Mineral Processing, 2004, 74(1/4): 251-262.
[9] MALYSIAK V, O’CONNOR C T, RALSTON J, GERSON A R, COETZER L P, BRADSHAW D J. Pentlandite-feldspar interaction and its effect on separation by flotation[J]. International Journal of Mineral Processing, 2002, 66(1/4): 89-106.
[10] BREMMELL E, FORNASIERO D, RALSTON J. Pentlandite– lizardite interactions and implications for their separation by flotation[J]. Colloids and Surfaces A, 2005, 252(2/3): 207-212.
[11] SHACKLETON N J, MALYSIAK V, O’CONNOR C T. The use of amine complexes in managing inadvertent activation of pyroxene in a pentlandite-pyroxene flotation system[J]. Minerals Engineering, 2003, 16(9): 849-856.
[12] KELEBEK S, TUKEL C. The effect of sodium metabisulfite and triethylenetetramine system on pentlandite–pyrrhotite separation[J]. International Journal of Mineral Processing, 1999, 57(2): 135-152.
[13] SUI C, FINCH J A, XU Z. Effect of diethylenetriamine on xanthate interaction with Pb-contaminated Pyrite[J]. Minerals Engineering, 1998, 11(7): 639-649.
[14] WANG Xiang-huai. Interfacial electrochemistry of pyrite oxidation and flotation Ⅱ. FTIR studies of xanthate adsorption on pyrite surfaces in neutral pH solutions[J]. Journal of Colloid and Interface Science, 1995, 171(2): 413-428.
[15] HU Yue-hua, SUN Wei, WANG Dian-zuo. Electrochemistry of flotation of sulphide minerals[M]. Beijing: Tsinghua University Press, 2009: 99-102.
[16] ZHANG Ya-hui, CAO Zhao, SUN Chuan-yao, CAO Yong-dan. FTIR studies of xanthate adsorption on chalcopyrite, pentlandite and pyrite surfaces[J]. Journal of Molecular Structure, 2013, 1048(9): 434-440.
(编辑 何学锋)
基金项目:国家自然科学基金资助项目( 51174154);内蒙古科技大学创新基金资助项目(2012NCL012,2012NCL029)
收稿日期:2013-04-07;修订日期:2013-07-24
通信作者:张亚辉,教授,博士;电话:13504009158;E-mail: zhangyahui@mail.neu.edu.cn