从钼酸铵溶液中除去砷的研究
杨亮,赵中伟,陈爱良,刘旭恒,霍广生
(中南大学 冶金科学与工程学院,湖南 长沙,410083)
摘要:镍钼矿常压空气氧化浸出液经N235萃取、氨水反萃后得到含砷的钼酸铵溶液。溶液中钼质量浓度达96.8 g/L,砷质量浓度为8.75 g/L,加入氯化镁使砷形成砷酸铵镁沉淀而除去。实验结果表明:当氯化镁用量为理论量1.2倍,反应温度为297 K、反应时间为30 min时,溶液中残留砷质量浓度为46.7 mg/L,钼入渣率为0.34%,残余Mg2+质量浓度为0.52 g/L;除砷后的钼酸铵溶液用724弱酸性阳离子树脂吸附除镁;当料液pH为9.2、流速为1 mL/min、交换后液体积为树脂体积的17倍时,交换后液中镁质量浓度为72 mg/L,树脂操作质量浓度为6.58 g/L;负载镁的树脂用浓度为2 mol/L HCl溶液解吸,解吸率接近100%,并同时实现树脂再生。
关键词:镍钼矿;砷酸铵镁;除镁;离子交换
中图分类号:TF 803.25 文献标志码:A 文章编号:1672-7207(2011)08-2193-05
Research on arsenic removal from ammonium molybdate solution
YANG Liang, ZHAO Zhong-wei, CHEN Ai-liang, LIU Xu-heng, HUO Guang-sheng
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: The coarse ammonium molybdate solution containing Mo 96.8 g/L and As 8.75 g/L was obtained by extracting Mo with N235 from leaching solution of Ni-Mo ore. Arsenic was removed as MgNH4AsO4 after adding magnesium chloride into the solution. The results show that when the dosage of magnesium chloride is 1.2 times of stoichiometric quantity, the reaction temperature is 297 K and the reaction time is 30 min, 99.46% of arsenic is removed, 0.34% Mo is lost,and mass concentration of the residual Mg2+ in the solution is 0.52 g/L. Mg2+ is removed from the ammonium molybdate solution with weak acid resin 724. In the solution, the pH value is about 9.2, and mass concentration of Mg2+ is 72 mg/L in the effluent until effluent/resin volume ratio is over 17 at the flow rate 1 mL/min. In addition, the operating exchange capacity of the resin is 6.58 g/L. The loaded resin can be stripped and regenerated with 2 mol/L HCl solution, and the desorption ratio of Mg2+ is almost 100%.
Key words: Ni-Mo ore; MgNH4AsO4; Mg removal; ion exchange
近年来,镍钼矿作为一种新型钼矿资源引起了众多研究者们的关注[1-6]。镍钼矿是复杂多金属矿物,约含有钼4%(质量分数,下同),镍3%和砷0.8%等[7]。矿中的钼主要以无定形的胶硫化钼存在[8],因此,镍钼矿具有晶化程度低、化学活性高的特点。鉴于此,Zhao等[9]提出了碱性常压空气氧化法浸出镍钼矿。在浸出过程中,钼、砷、钨、钒一起进入浸出液,镍、铜、锌、镁等则进入渣中。在后续N235萃取提钼过程中,砷与钼一起进入有机相,氨水反萃时,又一起进入反萃液中。从钼酸盐溶液中除砷的方法主要有沉淀法、吸附法、萃取法以及离子交换法等。采用铁盐吸附法[10-11]除砷时,钼损失较大且吸附剂的再生及回收较为困难。采用离子交换法[12]难以处理砷含量较高以及成分复杂的溶液;采用伯铵和TBP萃取除砷[13]则存在砷萃取率不高且钼损失较大的缺点。本文作者采用铵镁盐沉淀法[14]除砷。反萃液经双氧水氧化后,加入氯化镁,砷以砷酸铵镁沉淀形式除去。除砷后的钼酸铵溶液中残留一定量的镁。采用阳离子交换[15]除去溶液中过量的镁后,可使钼酸铵产品中镁含量不 超标。
1 实验
1.1 实验原料
实验原料为六水合氯化镁、724弱酸性阳离子树脂、732强酸性阳离子树脂(使用前均转为铵型)、浓盐酸、氨水以及处理镍钼矿所得的钼酸铵溶液。钼酸铵溶液主要成分(质量浓度)如表1所示。
表1 钼酸铵溶液主要成分
Table 1 Chemical composition of ammonium molybdate solution g/L

1.2 实验步骤
取一定体积的钼酸铵溶液,然后加入300 g/L氯化镁溶液,恒温反应一定时间后,抽滤。滤渣用蒸馏水洗涤。除砷后的钼酸铵溶液用盐酸或氨水调节pH,再流入装有30 mL离子交换树脂的交换柱中动态吸附除镁(离子交换柱直径和高分别为1.0 cm和40 cm),收集交后液并分析镁浓度。负载树脂经蒸馏水洗涤后,用一定浓度的盐酸溶液解析,并分析解吸液中镁浓度。砷和镁用ICP-AES检测,钼用硫氰酸盐分光光度法检测。
2 结果与讨论
2.1 铵镁盐沉淀法除砷
2.1.1 氯化镁用量对除砷的影响
取100 mL钼酸铵溶液,其pH为9.3,分别加入氯化镁理论量的1.0,1.2,1.4,1.6,1.8和2.0倍。在温度为297 K、反应时间为30 min的条件下,氯化镁用量(即氯化镁加入量与其理论量之比)对除砷的影响如图1所示。
图1表明:氯化镁用量越大,则钼酸铵溶液中残留的砷质量浓度越小;当氯化镁用量为理论量的1.2倍时,溶液中残留的砷质量浓度为46.7 mg/L,经分析,钼损失率为0.34%,溶液中残留的镁质量浓度为0.52 g/L;继续增大氯化镁用量,溶液中砷含量下降逐渐趋于平缓,但溶液中残留的镁则几乎呈直线上升;当氯化镁用量为1.6倍理论量时,镁质量浓度达1.8 g/L左右,对后续除镁工序不利。因此,选择加入1.2倍理论量的氯化镁。
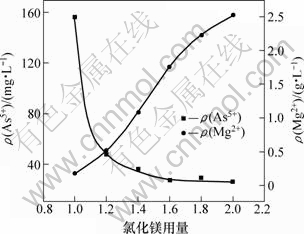
图1 氯化镁用量对溶液砷和镁质量浓度的影响
Fig.1 Influence of magnesium chloride dosage on mass concentration of arsenic and Mg
2.1.2 反应时间对砷的影响
取100 mL的钼酸铵溶液,其pH为9.3,加入氯化镁理论值的1.2倍。在温度为297 K时,反应时间对砷质量浓度的影响如图2所示。
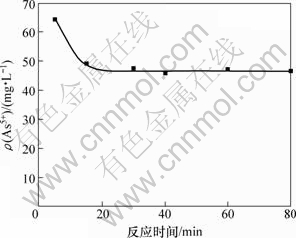
图2 反应时间对溶液砷质量浓度的影响
Fig.2 Influence of reaction time on arsenic mass concentration
由图2可知:镁盐沉淀法除砷的反应速度很快;当反应时间为15 min时,溶液中砷质量浓度下降至50 mg/L左右;继续延长反应时间,砷含量变化不大。为了确保除砷的效果,本实验选取反应时间为30 min。
2.1.3 反应温度对除砷的影响
取100 mL的钼酸铵溶液,其pH为9.3,加入1.2倍理论量的氯化镁。在反应时间30 min时,温度对除砷的影响如图3所示。
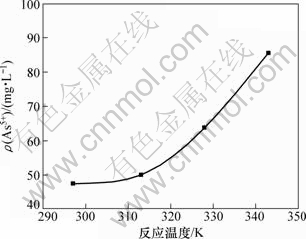
图3 反应温度对溶液砷质量浓度的影响
Fig.3 Influence of reaction temperature on arsenic mass concentration
从图3可知:钼酸铵溶液中残留砷的量随反应温度的升高而增大;当反应温度由297 K升高343 K时,溶液中残留砷质量浓度达到由47.6 mg/L增大至85.6 mg/L;温度越高,氨气挥发加剧。因此,反应选择在297 K下进行较好。
2.2 离子交换法除镁
由上述结果可知:加入氯化镁理论量的1.2倍除砷后的钼酸铵溶液中,镁残留质量浓度为0.52 g/L。溶液中镁质量浓度过高,在酸沉结晶钼酸铵时,会造成产品中镁质量浓度超标。因此,本研究选用离子交换法除去钼酸铵溶液中过量的镁。
2.2.1 树脂的筛选
本实验选用732强酸性阳离子交换树脂和724弱酸性阳离子交换树脂进行吸附除镁实验。钼酸铵料液中,Mo和Mg质量浓度分别为92和0.5 g/L,pH为9.2。2种树脂体积均为30 mL,料液流速为1.0 mL/min。2种树脂吸附镁的流出曲线如图4所示。
由图4可知:732强酸树脂吸附镁的效果远不如724弱酸树脂的效果好。因此,后续离子交换吸附除镁时全部选用724弱酸性树脂进行实验。
2.2.2 料液流速对吸附镁效果的影响
在钼酸铵料液中,Mo和Mg质量浓度分别为92和0.5 g/L,pH为9.2,树脂体积为30 mL,料液流速分别为3.0,1.5和1.0 mL/min,实验结果如图5所示。
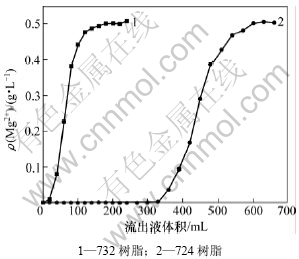
图4 2种树脂的镁流出曲线
Fig.4 Adsorption curves of two resins for Mg
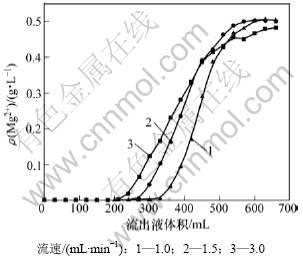
图5 不同料液流速时镁流出曲线
Fig.5 Adsorption curves of different flow rates for Mg
从图5可见:料液流速越慢即料液与树脂接触时间越长,则吸附镁的效果越好;料液流速过快,则溶液中的镁离子与树脂上的离子还来不及交换完全,便流入下一层树脂中,从而容易造成过早穿漏。取交后液中镁质量浓度为72 mg/L为穿漏点,则当料液流速分别为1.0,1.5和3.0 mL/min时,树脂的操作交换容量分别6.58,5.72和5.61 g/L。本实验选取流速为1.0 mL/min。
2.2.3 料液pH对吸附镁效果的影响
钼酸铵料液中,Mo和Mg质量浓度分别为92和0.5 g/L,树脂体积为30 mL,料液流速为1 mL/min,pH分别为9.2,8.3和7.2,料液pH对镁吸附效果的影响如图6所示。
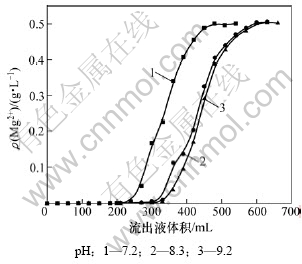
图6 不同pH时镁流出曲线
Fig.6 Adsorption curves of different pH values for Mg
由图6可知:随着溶液pH的减小,树脂吸附镁的效果变差。所用树脂为弱酸性树脂,溶液pH高则有利于树脂上可交换离子的电离,有利于镁的吸附;此外,pH越低,则溶液中竞争离子NH4+质量浓度越高,不利于镁的吸附。除砷后的钼酸铵溶液的pH为9.2左右,因此,选择溶液 pH=9.2时吸附镁效果较好。
2.2.4 HCl浓度对镁解吸效果的影响
吸附镁后的树脂经蒸馏水洗涤后,分别用浓度为0.5,1.0和2.0 mol/L的HCl溶液解吸,解吸液流速为1 mL/min,解吸曲线如图7所示。
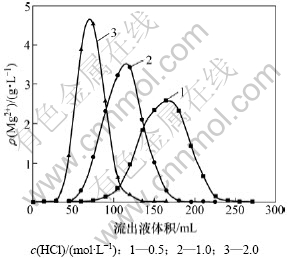
图7 不同浓度HCl时镁的解吸曲线
Fig.7 Desorption curves of different HCl concentrations for Mg
由图7可以看出:负载树脂中的镁用0.5 mol/L的盐酸就可以解吸完全,但解吸液体积较大,而且所得解吸液中镁质量浓度较低。解吸液质量浓度越高;解吸曲线峰越窄,所得高峰解吸液中镁质量浓度更高。但解吸剂质量浓度不宜过高,否则树脂容易发生严重缩变而破裂。用2 mol/L HCl解吸时,解吸液树脂体积的5倍可将吸附的镁几乎完全解析,并同时实现树脂的再生。
3 结论
(1) 采用铵镁盐沉淀法除去钼酸铵溶液中的砷是可行的。在氯化镁用量为理论量的1.2倍、反应时间为30 min、反应温度为297 K的条件下,钼酸铵溶液中的砷质量浓度由8.75 g/L降低至46.7 mg/L,溶液中残留的镁质量浓度为0.52 g/L,钼损失率为0.34%。
(2) 选用724弱酸性阳离子交换树脂吸附除砷后溶液中残留的Mg2+。当溶液pH为9.2、镁质量浓度为0.5 g/L,料液流速为1 mL/min,交换后液体积为树脂体积的17倍时,交换后液中镁质量浓度为72 mg/L,树脂的操作交换容量为6.58 g/L湿树脂。负载镁的树脂,用2 mol/L HCl可将吸附的镁几乎完全解吸。
参考文献:
[1] 李青刚, 肖连生, 张贵清, 等. 镍钼矿生产钼酸铵全湿法生产工艺及实践[J]. 稀有金属, 2007, 31: 85-89.
LI Qing-gang, XIAO Lian-sheng, ZHANG Gui-qing, et al. Process and practice of ammonium molybdate production from Ni-Mo ore by hydrometallurgy[J]. Chinese Journal of Rare Metals, 2007, 31: 85-89.
[2] WANG Ming-yu, WANG Xue-wen, LIU Wan-li. A novel technology of molybdenum extraction from low grade Ni-Mo ore[J]. Hydrometallurgy, 2009, 97: 126-130.
[3] ZHAO Zhong-wei, LI Jiang-tao, CAO Cai-fang, et al. Recovery and purification of molybdenum from Ni-Mo ore by direct air oxidation in alkaline solution[J]. Hydrometallurgy, 2010, 103: 68-73.
[4] HOU Xiao-chuan, XIAO Lian-sheng, GAO Cong-jie, et al. Kinetics of leaching selenium from Ni-Mo ore smelter dust using sodium chlorate in a mixture of hydrochloric and sulfuric acids[J]. Hydrometallurgy, 2010, 104: 76-80.
[5] LI Min-ting, WEI Chang, FAN Gang, et al. Acid leaching of black shale for the extraction of vanadium[J]. Hydrometallurgy, 2010, 95: 62-67.
[6] Anjum F, Bhatti H N, Ghauri M A. Enhanced bioleaching of metals from black shale using ultrasonics[J]. Hydrometallurgy, 2010, 100: 122-128.
[7] 鲍正襄, 万榕江, 包觉敏. 湘西北镍钼矿床成矿特征与成因[J]. 湖北地矿, 2001, 15(1): 14-21.
BAO Zheng-xiang, WAN Rong-jiang, BAO Jue-min. Metallogenic characteristics and genesis of the Ni-Mo deposits in northwestern Hunan[J]. Hubei Geology & Mineral Resources, 2001, 15(1): 14-21.
[8] 张爱云, 伍大茂, 郭丽娜, 等. 海相黑色页岩建造地球化学与成矿意义[M]. 北京: 科学出版社, 1987: 172-180.
ZHANG Ai-yun, WU Da-mao, GUO Li-na, et al. The geochemistry of marine black shale formation and its metallogenic significance[M]. Beijing: Science Press, 1987: 172-180.
[9] ZHAO Zhong-wei, ZHANG Gang, HUO Guang-sheng, et al. Kinetics of atmospheric leaching molybdenum from metalliferous black shales by air oxidation in alkali solution[J]. Hydrometallurgy, 2009, 97: 233-236.
[10] Buswell A M, Gore R C, Hudson H E , et al. Water problem in analysis and treatment[J]. Water Works Assoc, 1943, 35: 1303-1311.
[11] 吕荧, 孙放. Fe(OH)3吸附法从高钨钼酸钠溶液中分离钨钼的研究[J]. 稀有金属与硬质合金, 2005, 33(3): 1-3.
L? Ying, SUN Fang. Study of separation of tungsten and molybdenum from high W-containing sodium molybdate solution by Fe(OH)3 adsorption[J]. Rare Metals and Cemented Carbides, 2005, 33(3): 1-3.
[12] Suzuki T M, Bomani J O, Matsunaga H, et al. Removal of As( Ⅲ) and As(V) by a porous spherical resin loaded with monoclinic hydrous zirconium oxide[J]. Chemistry Letters, 1997(1): 119-125.
[13] ZHAO You-cai, CHEN Jia-yong. Extraction of phosphorus, arsenic, and/or silica from sodium tungstate and molybdate solutions with primary amine and tributyl phosphate as solvents: I. Synergistic extraction and separation of phosphorus, arsenic and silica from tungstate and molybdate solutions[J]. Hydrometallurgy, 1996, 42: 313-324.
[14] 李洪桂. 稀有金属冶金学[M]. 北京: 冶金工业出版, 1993: 83-85.
LI Hong-gui. Metallurgy of rare metalls[M]. Beijing: Metallurgical Industry Press, 1993: 83-85.
[15] Huggins D K, Queneau P B, Ziegler R C, et al. Ion exchange purification of ammonium molybdate solutions[J]. Hydrometallurgy, 1983, 6: 63-73.
(编辑 陈灿华)
收稿日期:2010-08-15;修回日期:2010-10-20
基金项目:国家高技术研究发展计划(“863”计划)项目(2007AA06Z122);湖南省国土资源厅矿产资源保护与合理开发利用科研专项计划项目(2006K06);湖南有色集团-中南大学有色研究基金资助项目(Y2008-01-004)
通信作者:赵中伟(1966-),男,河北永年人,教授, 博士生导师,从事湿法冶金及功能材料研究;电话:0731-88830476;E-mail:zhongweizhao@hotmail.com